Assessment of dietary chaste tree (Vitex agnus-castus) fruit extract on growth performance, hemato-biochemical parameters, and mRNA levels of growth and appetite-related genes in gold fish (Carassius auratus)
Medeh Rshmeei, Seyed Pezhmn Hosseini Shekri,*, Mehdi Shmsie Mehrgn,**,Hmed Pknejd
aDepartment of Fisheries, Science and Research Branch, Islamic Azad University, Tehran, 1477893855, Iran
bDepartment of Fisheries, Faculty of Fisheries and Environmental Sciences, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, 4913815739, Iran
Keywords:
Gold fish
Hepatic enzymes
Hematology
Growth promoter
Herbal medicine
A B S T R A C T
In this study, the effect of oral administration of chaste tree fruit (CTF) extract was evaluated on growth performance, hematological indices, serum biochemical parameters, and the expression of growth and appetiterelated genes in gold fish (Carassius auratus). The fry (N =300; 2.40 ±0.12 g) were randomly dispersed into 12 aquaria and fed with different levels of CTF extract (0 (control), 0.5%, 1.0%, and 1.5%) for 56 days. At the end of the trial, the highest body weight gain and the lowest feed conversion ratio were observed in the fish fed with 1.5% CTF extract (P <0.05). The group fed with CTF extract at 1.5% showed the highest levels of red blood cell,hemoglobin, hematocrit, and serum albumin (P <0.05), while no significant changes were observed for mean corpuscular volume, mean hemoglobin, and mean corpuscular hemoglobin concentration among all groups (P >0.05). The levels of aspartate transaminase and lactate dehydrogenase were decreased by increasing the supplementation levels of CTF extract and the lowest values were obtained in 1.5% CTF extract diet (P <0.05). The expression of growth hormone, insulin-like growth factor 1, and ghrelin genes was significantly up-regulated in the fish fed with 1.5% CTF extract compared to other treatments (P <0.05). Overall, the enrichment of gold fish diet with 1.5% CTF extract could interfere positively in the growth-related traits and hematological parameters.
1.Introduction
For a long time, antibiotics were widely used as a feed supplement for improving growth performance and controlling microbial infectious diseases in commercial aquaculture (Faggio et al., 2015). However, the emergence and spread of antibiotic-resistant pathogens are one of the most important side effects of the chemotherapy that has persuaded researchers to look for efficient alternatives such as prebiotics, probiotics, traditional medicinal plants, and other natural feed supplements(Van Doan et al., 2019). However, medicinal herbs have been used for various purposes; for example, cosmetics, drugs, natural preservatives,food, and feed supplements (Kelen & Tepe, 2008). In this regard, the genusVitex(Lamiaceae) includes almost 250 species and some of them have been widely used for medicinal purposes around the world(Kosovac et al., 2016). Chaste tree (Vitex agnus-castus) grows in different parts of the world, ranging from the Mediterranean regions to Central Asia and Southern Europe (Celep, Kahraman, & Do˘gan, 2011).V. agnus-castusis considered a traditional medicine across the globe because of having many important bioactive compounds such as iridoids, flavonoids, diterpenoids, and ketosteroids (Gomaa, Elmoghazy,Halim, & Elsayyad, 1978; Hoberg, Orjala, Meier, & Sticher, 1999; Kuruüzüm-Uz, Güvenalp, Ströch, Demirezer, & Zeeck, 2008; ˇSaden, Krehula, Kuˇstrak, & Blaˇzevic, 1990). Also, chaste tree fruit or chasteberry has been used traditionally to treat menstrual disorders, premenstrual syndrome, corpus luteum insuf ficiency, hyperprolactinemia, inflammatory bowel disease, menopause, and disrupted lactation (Daniele,Coon, Pittler, & Ernst, 2005). Along with its desirable effects, one of the side effects ofV. agnus-castusfruit is weight gain in consumers by stimulating the production of progesterone and reducing the level of prolactin (Ahangarpour, Najimi, & Farbood, 2016; Verkaik, Kamperman, van Westrhenen, & Schulte, 2017).
Medicinal herbs in aquafeed can be considered as a multifunctional additive and show their main properties like antioxidants, antiinflammatory and antibacterial agents, growth promoters, appetite stimulators, and immunostimulants (Dügenci et al., 2003). Moreover,plant extracts are often locally available, inexpensive, act against a broad range of pathogens, and biodegradable (Harikrishnan, Balasundaram, & Heo, 2011). Several studies have shown the positive effects of different medicinal plants extract as a feed additive in gold fish diet to boost up the growth performance such as Indian lilac (Azadirachta indica) (Kumar et al., 2013), pear tree (Utleria salicifolia) (Harikrishnan et al., 2009), garlic (Allium sativum), tangerine peel (Citrus tangerina),pine needle (Pinus densi flora), and clove (Syzygium aromaticum) (Ji, Li,Zhou, & Liu, 2008), ashwagandha (Withania somnifera), and tulsi (Ocimum sanctum) (Ahilan & Nithiyapriyatharshini, 2015).In-vitrostudies revealed that the medical parts (fruits and leaves) ofV. agnus-castushave effective antifungal (ˇSvecov´a, Proietti, Caruso, Colla, & Crin`o, 2013),free radical-scavenging (Pío-Le´on, Montes-Avila, Díaz-Camacho,Delgado-Vargas, & Gupta, 2014), antimicrobial (Maltas¸, Uysal, Yildiz, &Durak, 2010) and antimutagenic (Sarac, Ugur, & Sen, 2015) activities due to the presence of some valuable phytochemical compounds such as triterpenes, iridoids, diterpenoids, tannins, flavonoids, and alkaloids(Zahid, Rizwani, & Ishaqe, 2016). In our recently published paper, the supplementation of gold fish diet with chaste tree fruit extract had positive effects on the humoral and skin mucosal immune parameters as well as disease resistance (Rashmeei, Shekarabi, Mehrgan, & Paknejad,2020). However, to the best of our knowledge, there is a lack of information about the effectiveness of dietary chaste tree fruit extract on growth performance, feed utilization, and hematology of aquatic animals.
Gold fish,Carassius auratus(Cyprinidae), is one of the well-known teleost fish species which has been used as a model in numerous biological studies (Harikrishnan, Balasundaram, & Heo, 2010; Talaat,Reimschuessel, Wasserman, & Trucksis, 1998). Moreover, gold fish is the most popular and common freshwater ornamental fish due to several reasons such as the variety of skin colors, high adaptability to artificial diets, and high tolerance to environmental conditions in captivity(Gumus, Aydin, & Kanyilmaz, 2016). Since gold fish make a significant contribution to the global ornamental fish trade, raising healthy and fast-growing fish is vital to meet this demand. In this regard, using natural feed additives such as medicinal herbs and their derivatives instead of using synthetic supplements to boost up the growth performance and health status of fish can be efficient and environmentally friendly. Therefore, this study aimed to investigate the effects of chaste tree fruit hydro-ethanolic extract on growth performance,hemato-biochemical parameters, and mRNA transcription levels of growth and appetite-related genes in gold fish.
2.Materials and methods
2.1.Chaste tree fruit (CTF) extraction
The CTF was obtained from the Agricultural Sciences and Natural Resources Research Center of Gorgan University (Golestan Province,Iran). After washing, the whole ripe fruits ofV. agnus-castuswere dried at room temperature (26-28 ℃) for 72 h without direct exposure to the heat and grounded into the fine powder. The aqueous-alcoholic extraction was performed by adding 20 g of CTF powder and 400 mL of 70% (v/v) methanol. The mixture was stirred for 48 h at room temperature and then the solid particles were removed from the solution by aspirating through clean Whatman No. 1 filter paper. The solvent was vaporized by a rotary evaporator (Heidolph Instruments, Germany).Subsequently, the concentrated extract was lyophilized by a laboratory freeze-drier (Alpha 1—2 LDplus, Martin Christ Gefriertrocknungsanlagen, Osterode, Germany) at −55 ℃ and 0.47 mbar for 24 h and then powdered. The powder was kept in a refrigerator in dark and sealed containers away from light and moisture until use.
2.2.Experimental design
Comet-tailed gold fish fry (initial average weight of 2.40 ±0.12 g)were obtained from a local farm that had the health certification from Gorgan Veterinary Of fice (Golestan Province, Iran). Three hundred fry fish were randomly dispersed in 12 glass aquaria (60 × 30 × 40 cm3)with a static-water culture system. Fish were fed three times a day to visual satiety with a basal diet (commercial common carp pelleted feed;Kimiyagaran Taghziyeh, Shahrekord, Iran) for two weeks before the beginning of the experiment to adapt to the experimental condition. The proximate composition (% in dry weight) of the basal diet was: crude protein 39%, fat 7%, ash 9%, and fiber 7%. The physicochemical parameters of water were: temperature 26 ± 2 ℃, ammonia 0.013 ± 0.002 mg/L, nitrite 0.002 ± 0.001 mg/L, nitrate 0.410 ± 0.080 mg/L, water hardness 286.0 ± 1.2 mg/L total calcium carbonate, pH 7.2 ± 0.3, 12 h light:12 h dark photoperiodic regimen, and dissolved oxygen was in the saturated level. The water exchange rate was 30% daily to remove feces and debris.
The CTF extract powder was sprayed on the pelleted basal diet (2 mm diameter) by gelatin solution (4% w/v) at 1:500 (gelatin solution: feed,v/w) and left at room temperature to dry. Also, the gelatin solution was sprayed to the basal diet without CTF extract and considered as a control diet. Treatments were introduced with triplicates as T1: Control group(basal diet), T2: Basal diet +0.5% CTF extract, T3: Basal diet +1% CTF extract, and T4: Basal diet +1.5% CTF extract. The experimental diets were prepared weekly and stored in a refrigerator (−18 ± 1 ℃) until use.The fish were fedad libitumwith the feeding frequency of three times a day (8:00 a.m., 1:00 p.m., and 6:00 p.m.) for 56 days.
2.3.Measurement of growth performance
In order to evaluate the effect of different levels of CTF extract on the growth performance of gold fish, fish were deprived of food for 24 h,anesthetized in a solution containing 25 mL/L clove essential oil (80%eugenol, Barij Essence Company, Kashan, Iran) and then weighed to calculate the growth indices according to the following formulas(Ricker, 1979):

2.4.Hematological and serum biochemical analysis
At the end of the trial, fish were fasted for 24 h. Then, six fish from each tank were randomly sampled and anesthetized in a solution containing 25 mL/L clove essential oil. The blood samples were collected by cutting off the caudal stem. The blood of three fish (~2 mL) was transferred into non-coagulated tubes for 2 h in the refrigerator and then centrifuged (650 ×gfor 15 min at 4 ℃). After centrifugation, the serum samples were collected and stored at −80 ℃ until blood biochemical tests. The blood of another three fish was transferred to heparinized vials(10 IU Li-Heparin per 0.5 mL of blood) for analyzing hematological indices. Red blood cells (RBC, ×106/mm3) were counted by hemocytometer according to Thompson, Tollit, Corpe, Reid, and Ross (1997).Hematocrit (Hct, %) was analyzed by the method of Blaxhall and Daisley(1973). Hemoglobin (Hb, g/dl) concentration was determined based on the Pars Azmoon® kit (Pars Azmoon Co., Alborz, Iran) instruction. Mean corpuscular volume (MCV, fl), mean corpuscular Hb (MCH, pg/cell),and mean corpuscular Hb concentration (MCHC, g/dl) were calculated with the following formulas (Briggs & Bain, 2016);
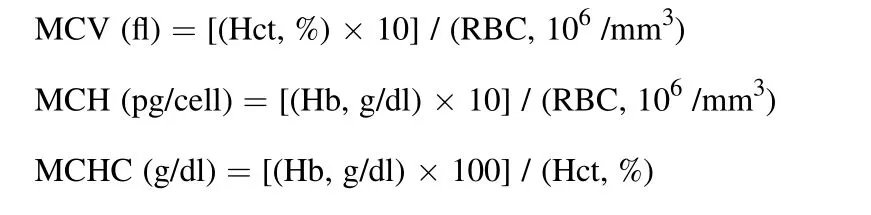
Alanine amino-transferase (ALT), aspartate amino-transferase (AST),albumin, glucose, and lactate dehydrogenase (LDH) were assessed in the sera samples according to the instructions of Pars Azmoon® kit (Tehran,Iran) using a Prestige 24i blood biochemical auto-analyzer (Tokyo Boeki Medical System, Tokyo, Japan).
2.5.Gene expression studies
2.5.1.Sample preparation
After 8 weeks of the feeding trial, three fish from each treatment (one individual from each tank) were euthanized with clove oil (40 mL/L)immersion bath and dissected for brain, liver, and intestine to evaluate growth and appetite-related gene expression. These samples were immediately immersed in liquid nitrogen (−196 ℃) and frozen. After freezing, the samples were stored at −80 ℃ until RNA extraction.
2.5.2.RNA extraction and cDNA synthesis
The sampled tissues (1 g) were individually smashed in a laboratory porcelain mortar using liquid nitrogen. One mL RNAxPlus reagent(Sinaclon; Iran) was added to 50—100 mg of each sample and homogenized. The homogenate solvent was left at room temperature for 15 min.Two hundred μL chloroform was added and then centrifuged at 10000 ×gfor 15 min at 4 ℃. The supernatant was discarded and equal volume isopropanol was added. The homogenate was left to incubate at room temperature for 15 min and centrifuged at 10000 ×gfor 15 min at 4 ℃.One mL of 75% ethanol was added and centrifuged at 4100 ×gfor 8 min at 4 ℃. The RNA pellet was dissolved in deionized double-distilled water. Total RNA was isolated according to the method described by Taghavizadeh, Shekarabi, Mehrgan, and Islami (2020). The purity of total extracted RNA was assessed by a spectrophotometer (Biochrom WPA Biowave II, Cambridge, UK) at the wavelengths of 260/280 and 260/230 ratios. The quality of RNA was measured by electrophoresis on a 1% agarose and staining with ethidium bromide. Samples (200 ng)were reverse transcribed according to Genet Bio cDNA® synthesis kit(Korea) and oligo dT primers to obtain the first-strand cDNA. Reverse transcription PCR reactions were run in triplicate and using a standard protocol (initial denaturation at 95 ℃ for 10 min, 40 cycles of denaturation, annealing, and extension at 95 ℃ for 15s).
2.5.3.Primer design
Based on the nucleotide sequence of the selected genes, the specific primers were designed using primer3 software (http://bioinfo.ut.ee/pri mer3-0.4.0/primer3/). The primers for insulin-like growth factor 1 (IGF-1), growth hormone (GH), andGhrelingenes are shown in Table 1. In addition, β-actin was considered as a housekeeping gene because of its stable expression level in gold fish (Sinha et al., 2012).
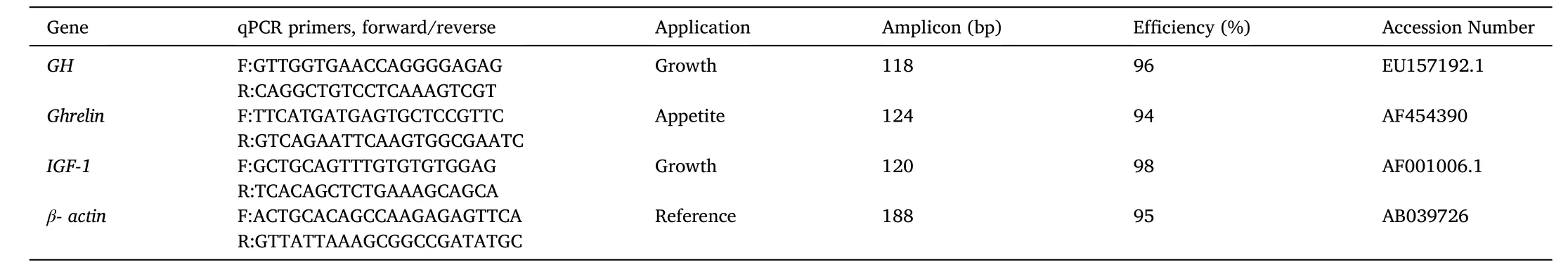
Table 1Primers sequences for the study of IGF, GH, and Ghrelin genes expression in gold fish.
2.5.4.Quantitative real-time PCR
Real-time PCR quantification was performed forIGF-1,GH, andGhrelingenes expression from the sampled tissues of the liver, brain, and intestine, respectively. The qPCR method was run according to the protocol described by Miandare et al. (2013). Serial dilutions of pooled cDNA (including seven dilutions from 1:10 to 1:2000) were used for obtaining the standard curves. The PCR efficiency of each gene was evaluated according to equation E (%) =(101/slope−1) × 100 (Radoni´c et al., 2004). The fold change in the relative mRNA expression of the genes was calculated by the 2−ΔΔCtmethod. The data were analyzed using the iQ5 optical system software 2.0 (Bio-Rad).
2.6.Statistical analysis
Statistical analyses were subjected using SPSS statistical package version 22.0 (SPSS Inc., Chicago IL, USA). Normal distribution of the data was tested using the Kolmogorov—Smirnov test and Levene’s test was used to test homogeneity of variance. The data were analyzed statistically using a one-way analysis of variance (ANOVA) and followed by Duncan’s multiple range test to determine significant differences among means at 95% (P <0.05). Results were reported as the mean ±standard deviation (SD). Figures were drawn by Microsoft Excel software version 2013.
3.Results
3.1.Growth performance and feed utilization
Results of growth indices and feed efficiency of gold fish fed with different levels of CTF extract are presented in Table 2. Based on the results, WG, BWI, SGR, and FI parameters showed a significant increase in 1.5% CTF extract compared to the control group and other treatments(P <0.05). However, the lowest value of FCR was obtained in the fish fed with CTF extract at 1.5% (P <0.05). Condition factor was signi ficantly enhanced by increasing CTF extract, from 0.75% in the control group to 0.94% in 1.5% CTF extract diet (P <0.05). Also, there was no significant difference in survival rate between different experimental treatments and no mortality was observed among different groups (P >0.05).
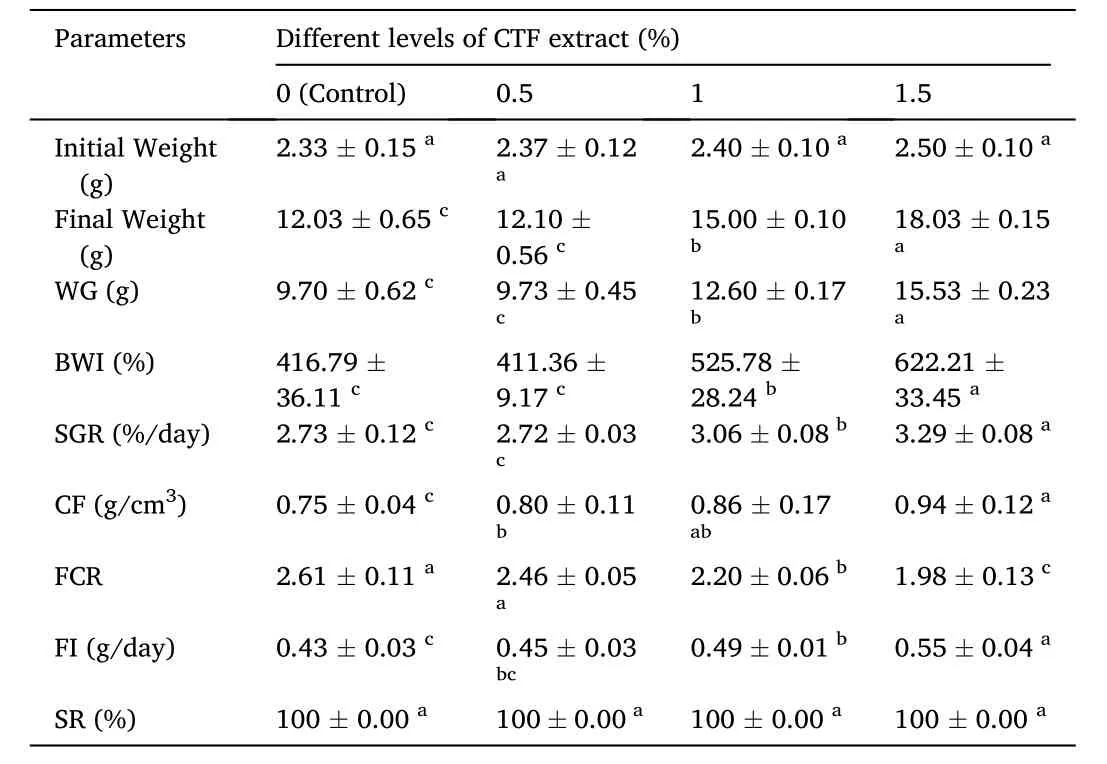
Table 2Growth performance and survival rate of gold fish fed with different dietary levels of chaste tree fruit (CTF) extract for 56 days.
3.2.Hematological and serum biochemical parameters
The effects of different levels of CTF extract on hematological and some serum biochemical parameters of gold fish are shown in Table 3.The number of RBC in the fish fed with different levels of CTF extract was significantly increased, while the lowest count was observed in the control group (P <0.05). The highest values of Hb and Hct were measured in 1.5% of dietary CTF extract (P <0.05), while no significant differences were seen between other groups (P >0.05). However, the addition of CTF extract to the basal diet of gold fish did not significantly affect the values of MCV, MCH, and MCHC (P >0.05).
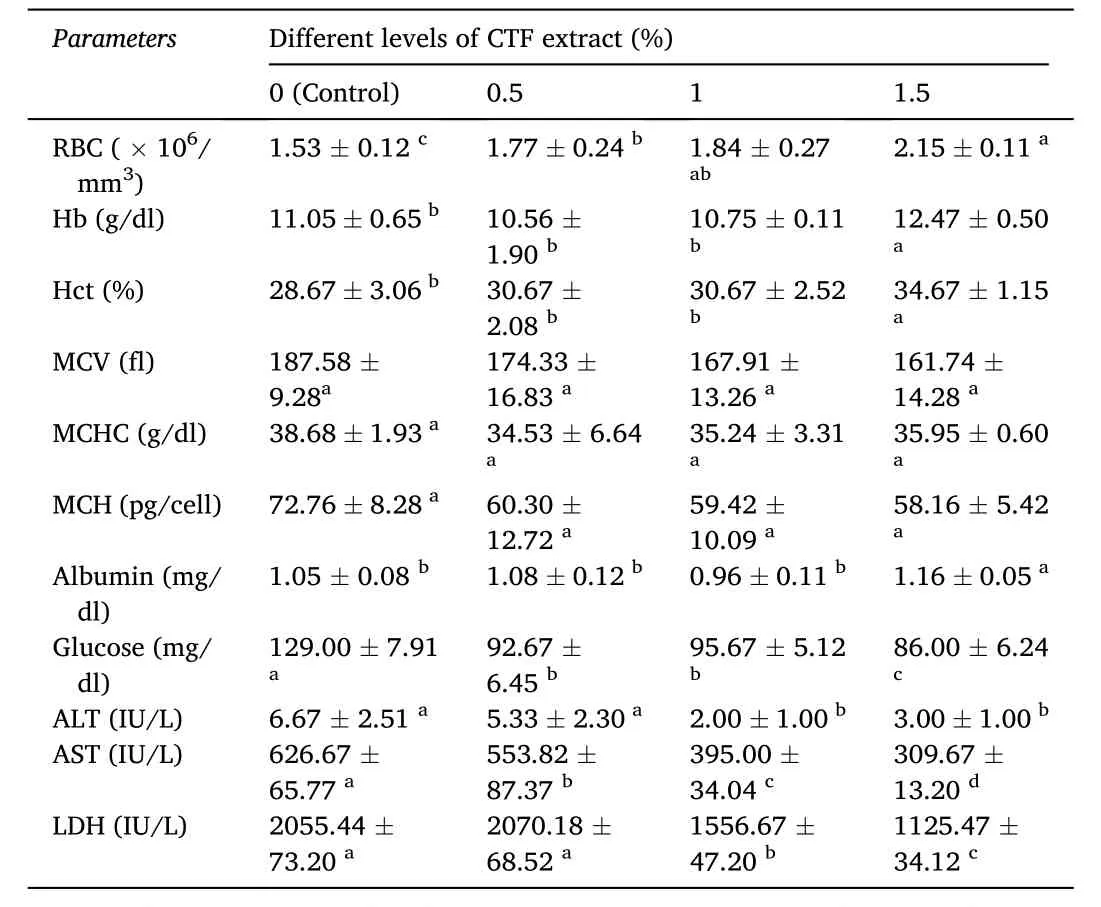
Table 3Hematological and biochemical parameters of gold fish fed with different dietary levels of chaste tree fruit (CTF) extract for 56 days.
The highest content of albumin was recorded in 1.5% CTF extract compared to other treatments (P <0.05; Table 3). However, the lowest level of blood glucose was seen in 1.5% CTF extract (P <0.05). As shown in Table 3, the oral administration of 0.5% CTF extract had no signi ficant effect on ALT level compared to the control group (P >0.05), while higher dietary levels of CTF extract (1% and 1.5%) could decrease the level of ALT, significantly (P <0.05). Also, the level of AST was significantly decreased by increasing in CTF extract concentration in the diets, from 626.67 IU/L to 309.67 IU/L. Fish fed with 0.5% CTF extract had no significant difference with the control group in LDH enzyme activity, while 1.5% CTF extract caused a significant decrease in the level of LDH to 1125.47 IU/L (P <0.05; Table 3).
3.3.Gene expression
The results ofGH,IGF-1,andGhrelingene expression are shown in Fig. 1A, B, and 1C, respectively.
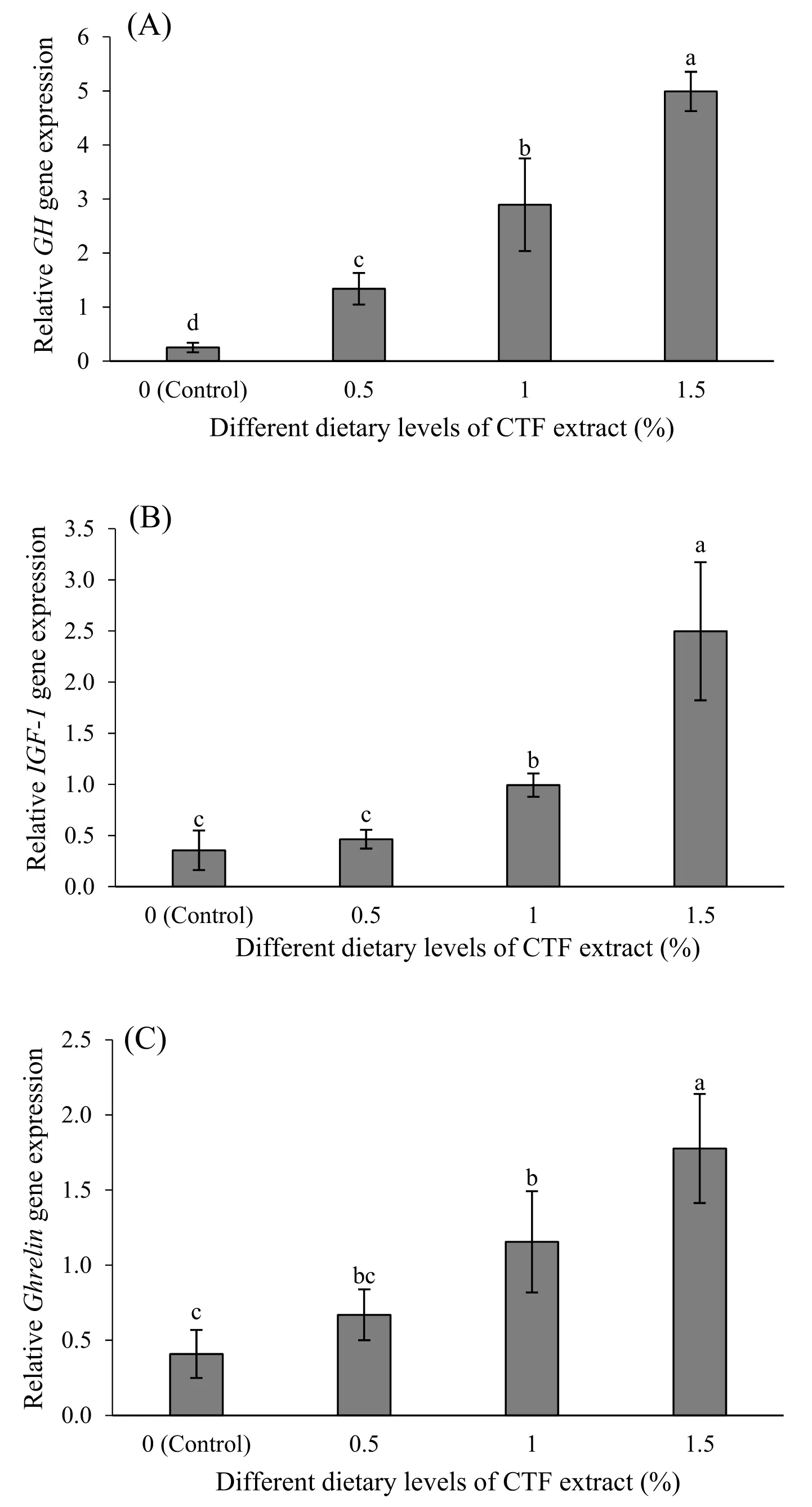
Fig. 1.Growth hormone (GH) (A), Insulin-like growth factor 1 (IGF-1) (B) and Ghrelin (C) genes relative expression levels by real-time PCR in gold fish fed with different levels of chaste tree fruit (CTF) extract for 8 weeks. Values are presented as the mean ±SD (n =3). The bars assigned with different letters denote significant difference between treatments (P <0.05).
The relativeGHgene expression was up-regulated by increase supplementation level of CTF extract and the highest value was obtained in 1.5% CTF extract (P <0.05; Fig. 1A).
The expression ofIGF-1andGhrelingenes in the fish fed with 1.5%CTF extract was significantly elevated compared to other treatments (P<0.05; Fig. 1B and C), while no statistically significant differences were recorded between the control and 0.5% CTF extract diets (P >0.05).
4.Discussion
Aquaculture production has become more efficient and using medicinal plants in the development of aquafeed is highly recommended due to their unique properties including anti-stress, growth promotion,appetite stimulation, enhancement of immune responses, aphrodisiacs,and anti-pathogenic properties (Abdel-Tawwab, Adeshina, Jenyo-Oni,Ajani, & Emikpe, 2018,b; Hoseinifar et al., 2017; Farsani et al., 2019;Tan et al., 2017; Karimi, Paknejad, Hoseinifar, Shabani, & Mozanzadeh,2020; Adel, Dawood, Sha fiei, Sakhaie, & Shekarabi, 2020). The results of this study showed that all of the growth indices in gold fish fed with different levels of CTF extracts were improved in comparison with the control group and the weight gain of the fish fed with the highest amount of CTF extracts was approximately 1.5 times higher than the control group. It can be assumed that the presence of beneficial phytochemical compounds in CTF extract is the main reason for elevating digestive enzyme activity, appetite, and the population of vitamins producing microbiota in the gut (Dawood et al., 2014, 2016). However,further research about the gut microbiota of fish fed with dietary CTF is needed. In agreement with our results, Enayat Gholampour, Jafari,Imanpour, and Kolangi Miandare (2017) showed that zebra fish (Danio rerio) fed with a diet containing 15 g/kg CTF extract had the lowest FCR and highest SGR compared to the control group. Our results also showed that the addition of CTF extract at 1.5% in gold fish diet could cause a significant increase in the feed palatability due to the higher FI.
Since CTF is a medicinal plant, it has some valuable bioactive compounds including total flavonoids (14.6—19.04 mg/g) and phenolics(5.72—7.19 mg/g gallic acid equivalents) (Latoui et al., 2012; Pío-Le´on et al., 2014). In addition, anthocyanins are reported as a major pigment in CTF which are also considered as a potential antioxidant agent to scavenge free radicals and reduce oxidative damages to cells (Kong,Chia, Goh, Chia, & Brouillard, 2003; Pío-Le´on et al., 2014). Many investigators pointed out that there is a positive correlation between antioxidant status and growth performance in fish (Rempel & Schlenk,2008; Taghavizadeh et al., 2020, p. 735315). The outcomes of the present study showed that the dietary supplementation of CTF extract could enhance the fish growth indices and feed utilization which are similar to the positive impacts of other herbal medicine extracts on fish growth performance such as peppermint in Asian seabass (Lates calcarifer)(Talpur, 2014), dandelion and hawthorn in golden pompano (Trachinotus ovatus) (Tan et al., 2017), medlar leaves in common carp (Cyprinus carpio) (Hoseinifar et al., 2017), wood betony and coriander in rainbow trout (Oncorhynchus mykiss) (Farsani et al., 2019; Moghanlou, Isfahani,Dorafshan, Tukmechi, & Aramli, 2018), green tea in olive flounder(Paralichthys olivaceus) (Cho et al., 2007), clove basil and lotus in African cat fish (Clarias gariepinus) (Abdel-Tawwab, Adeshina, et al., 2018;Munglue, 2016), ginger in Nile tilapia (Oreochromis niloticus) (Brum et al., 2017), and fenugreek in gilthead seabream (Sparus aurata) (Awad,Cerezuela, & Esteban, 2015).
Although hematological parameters can be altered by infectious diseases, inflammation, stress, temperature, nutrition, age, sex, and changes in hormone levels (Grant, 2015), they are commonly used as a bio-indicator to assess the general health condition of fish. In this research, the recorded blood indices of gold fish are within the acceptable limits for teleost fish (Satheeshkumar, Ananthan, Kumar, & Jagadeesan, 2012). The highest values of RBC, hematocrit, and hemoglobin were obtained from treated fish fed with 1.5% CTF extract. There is a direct relationship between RBC and anemia in vertebrates including fish (Iheanacho et al., 2017). From our data, dietary CTF extract had a positive influence on Hb and therefore, it can be considered as a hematopoietic accelerator in gold fish diet, but further research about explaining the physiological mechanisms of CTF extract on the fish hematopoietic stem cells is needed to be clarified. In agreement with our findings, Manaf et al. (2016) reported that supplementation of red hybrid tilapia (Oreochromissp.) diet with chaste tree leaves (Vitex trifolia) andAloe veracould significantly increase the levels of RBC and Hb by stimulating the hematopoietic stem cells in the kidney of fish(Kobayashi, Katakura, & Moritomo, 2016). Similarly, Paknejad et al.(2020) reported higher levels of Hb and Hct in the Caspian roach (Rutilus caspicus) fed with dietary peppermint powder compared to the basal diet.
Red blood cells indices such as MCV, MCH, and MCHC indicate the size and hemoglobin content of erythrocytes as well as re flecting the functions of hemoglobin in transporting oxygen (Iheanacho et al.,2017). In our study, dietary CTF extract did not significantly alter these indices in gold fish. Similar results have been reported by Yonar, Yonar,˙Ispir, and Ural (2019) in rainbow trout fed with a diet containing curcumin. Consistent with our results, Ghosal, Mukherjee, and Chakraborty(2020) reported no significant differences in MCH and MCV values of Nile tilapia fed with four different herbal extracts (i.e. Basella alba,Tribulus terrestris,Mucuna pruriens, andAsparagus racemosus). In addition, Karimi Pashaki, Ghasemi, Sharif Rohani, and Hosseini (2018) and Ali, Ambasankar, Musthafa, and Harikrishnan (2017) observed no significant changes in the blood indices of common carp and Asian seabass treated with garlic extract and earth apple (Jerusalem artichoke),respectively. Since stressful conditions cause significant changes in blood indices, our results in line with previous studies that CTF extract as a natural feed additive did not impose any kind of stress in gold fish(Bahrami Babaheydari, Paykan Heyrati, Dorafshan, Mahboobi Soo fiani,& Vahabi, 2015).
Carbohydrates are the main source of energy in many organisms and their reserve is used to meet energy demand in stress conditions.Therefore, serum glucose concentration is an important stress indicator for fish (Brum et al., 2018). In the present study, blood glucose concentration was decreased significantly when the highest levels of CTF extract were added into the gold fish diet. This probably occurs due to the reduction of cortisol-induced gluconeogenesis and changes in tissues’ glycogen to glucose (Banaee, Sureda, Mirvaghe fi, & Rafei, 2011).Serum albumin as a major type of globular blood protein is a multi-functional protein that has a crucial role in the immune system and also carries various biological substances throughout fish bodies,including hormones, vitamins, and enzymes (Punitha et al., 2008). Albumin content was significantly increased in gold fish fed with dietary levels of CTF extracts which suggests the enhancement of the non-specific immune response of fish (Brum et al., 2018). Some primary factors can affect the rate of albumin synthesis in the fish liver such as nutritional habits, species, reproductive cycle, and health status (Charoo, Chalkoo, & Qureshi, 2013).
From the results, the oral administration of 1% and 1.5% CTF extract in the diet could decrease the ALT activity, significantly. The AST was decreased significantly by increasing in CTF extract concentration in the diet of gold fish, from 626.67 IU/L to 309.67 IU/L. Also, LDH activity had a similar trend. LDH releases from various body tissues, such as gills,heart, liver, brain, skeletal muscle, erythrocyte, and kidney in response to tissue damage (Rao, 2006). Therefore, ALT, AST, and LDH enzymes are present at low concentrations in serum and increase in the blood due to the development of tissue damages, particularly hepatic tissues(Dixon, Hodson, & Kaiser, 1987; Mozhdeganloo, Jafari, Koohi, & Heidarpour, 2015). To confirm our result, many documents declare herbal medicines have been used to medicate liver diseases by their antioxidant properties such as scavenging free radicals, chelating metal ions,inhibiting lipid peroxidation, protecting the membrane permeability properties, and preventing liver glutathione depletion (Alqasoumi,Al-Howiriny, & Abdel-Kader, 2008; Banaee et al., 2011; Kumar et al.,2005; Palanisamy, Kannan, & Bhojraj, 2007; Premalakshmi & Thenmozhi, 2011).
Somatic growth is controlled through the regular release of hormones from the neuroendocrine system, which are influenced by both external factors and the internal state of the animal. The primary mechanism to regulate growth in all vertebrates, including fish, is the growth hormone/insulin-like growth factor axis (Duan, 1997; Kelley et al., 2002).GHacts both directly through its own receptors and indirectly by stimulating the liver to produceIGF-1(Reinecke et al., 2005).The nutritional status of animals can greatly influence theGH/IGF-1axis(Duan, 1997).GHandIGF-1are used as endocrine bioindicators that can re flect and predict growth rates in fish subjected to various biotic and abiotic manipulations (Tan et al., 2017). In our study,GHandIGF-1genes expression were upregulated in the fish fed with CTF extract at 1.5%. Similar results were observed in theghrelingene expression in the present study.Ghrelinalso functions primarily as aGH-releasing hormone and plays a key role in appetite, metabolism, gastrointestinal function, cardiovascular performance, and immune responses (Ghigo et al., 2005; Kojima & Kangawa, 2005). In addition to stimulatingGHrelease,ghrelinact as a regulator in neuroendocrine and metabolic responses to starvation in mammals (Chen et al., 2004; Horvath, Diano,Sotonyi, Heiman, & Tschöp, 2001) as well as fish (Riley, Fox, Kaiya,Hirano, & Grau, 2005; Unniappan, Canosa, & Peter, 2004). The improvement of fish digestion system causes the gastrointestinal tract to empty faster, which ultimately increases the appetite gene expression(Zhou et al., 2016). To our best knowledge, our study is the first report to focus on the effect of CTF extract in fish growth and appetite-related gene expression, so further studies in other fish species are needed to compare with our results.
From the obtained results, the supplementation of the diet with chaste tree fruit extract especially at 1.5% can improve growth rate and feed utilization in gold fish. Our results also documented the modulatory effect of CTF on the expression of growth and appetite-related genes.Furthermore, the present results suggested that the supplemented feeds are responsible for maintaining the hematological and serum biochemical parameters to normal levels. Therefore, using dietary CTF in a dose of 15 g/kg is a suitable and cost-effective option to reduce fish farming costs and it is also a suitable alternative to synthetic feed supplements.However, this preliminary study encourages other researchers to evaluate the effect of dietary chaste tree fruit extract higher than 1.5% in a dose-response manner.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-pro fit sectors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Maedeh Rashmeei: Visualization, Investigation. Seyed Pezhman Hosseini Shekarabi: Supervision, Writing - original draft. Mehdi Shamsaie Mehrgan: Supervision, Validation, Writing - review & editing. Hamed Paknejad: Formal analysis.
Acknowledgements
The authors would like to deeply acknowledge the expert staff at Fisheries and Genetic laboratories of Zakariya Razi Laboratory Complex(Science and Research Branch University, Tehran, Iran) for their valuable assistance during the project.
 Aquaculture and Fisheries2022年3期
Aquaculture and Fisheries2022年3期
- Aquaculture and Fisheries的其它文章
- Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review
- Recent advances in application of moving bed bioreactors for wastewater treatment from recirculating aquaculture systems: A review
- Characterization and expression analysis of gonad specific igf3 in the medaka ovary
- Liver DNA methylation and transcriptome between 1- and 3-year-old grass carp
- Screening and validation of reference genes for qPCR analysis in gonads and embryos of Takifugu bimaculatus
- Assessment of genetic diversity, detection of strain-specific single nucleotide polymorphisms and identification of the Bangladesh and Vietnam strain of Channa striata by PCR-RFLP analysis of the mitochondrial COI gene fragment
