Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review
Muziri Mugwny, Mhmood A.O. Dwood,b,*, Fhd Kimer, Hni Sewilm,c,**
aCenter for Applied Research on the Environment and Sustainability, The American University in Cairo, New Cairo, 11835, Egypt
bDepartment of Animal Production, Faculty of Agriculture Kafrelsheikh University, Kafrelsheikh, 33516, Egypt
cDepartment of Engineering Hydrology, The RWTH Aachen University, Aachen, 52074, Germany
Keywords:
Global warming
Aquaculture development
Sustainability
Production
Food safety
Environmental stress
A B S T R A C T
The reliance of aquaculture production on the ambient environment suggests its vulnerability to climate change.Global warming has led to the increase in water temperatures hence exerting a direct and indirect effect on several aquaculture species. Temperature is one of the major abiotic driving factors of the growth and survival of aquatic organisms. Extreme temperatures above their thermal threshold tend to lower their performance, health,and productivity. This paper, therefore, aims to give a detailed and comprehensive review on the influence of anthropogenic temperature increase on the general animal physiology, growth performance, survival, reproduction, digestive enzyme activity, immunity, differential expression of microRNAs and apoptosis-associated genes as well as gut and skin microbiome of aquaculture species. Likewise, the impacts of increasing water temperature on aquatic ecosystems with regards to pathogen-associated disease outbreaks, aquatic parasites,vaccine efficacy as well as toxicity, and uptake of heavy metals and pesticides are presented. To provide examples of how increasing water temperatures will impact the global trends of aquaculture production, a couple of freshwater, euryhaline, and marine species, as well as cold-water and warm water species, have been chosen to give a broader perspective on how different aquaculture species respond to temperature fluctuations above their thermal threshold. In the same regard, mitigation strategies for climate change adaptation in the context of global warming such as nutrition, genetics, selective breeding, aquaculture biotechnology, aquaculture nanotechnology, and bacteriophage therapy as well as management and husbandry practices are discussed to ensure a sustainable and continued aquaculture productivity.
1.Introduction
Aquaculture is the fastest-growing food production sector around the world contributing approximately 50% of animal protein for human consumption (Dawood, Noreldin, & Sewilam, 2021; Fazio, 2019). According to the Food and Agriculture Organization (FAO), aquaculture production is estimated to grow to 53% by 2030 (FAO, 2020). However,the current global trends of aquaculture production are threatened by climate change. Anthropogenic global warming is already changing our environment with devastating consequences. Although its implications have been previously focused on terrestrial organisms, less attention has been given to its effect on aquaculture species (Reid et al., 2019). Fish and crustaceans are ectothermic organisms whose body temperature fluctuates according to changes in the environmental temperature(Alfonso, Gesto, & Sadoul, 2021). Temperature is the major driving factor of the growth and survival of aquatic organisms as it directly affects their immunity, metabolic rate, and oxygen demand. Changes in the environmental temperature above the organism’s thermal threshold will not only increase the oxygen demand coupled with metabolic shifts but also reduce the feed intake (FI) and suppress their immunity(Dawood, 2021). This is because increasing water temperatures affect the solubility and availability of oxygen which negatively affects aerobic metabolism (Cheng, Ye, Guo, & Wang, 2017; Cook, Folli, Klinck, Ford, &Miller, 1998; Martins, Xu, Shoemaker, & Klesius, 2011; Roth, Kurtz, &Reusch, 2010; Seppälä & Jokela, 2011; Sun, Zhao, et al., 2019; Vargas-Chacoff, Regish, Weinstock, & McCormick, 2018; Wang et al., 2020).
According to the Intergovernmental Panel on Climate Change report(IPCC 2001), southern Europe is among the regions expected to have the most severe global warming with air temperatures ranging 4–7 ℃ by 2100 in the Iberian Peninsula (Madeira, Vinagre, & Diniz, 2016). More specifically, climate change models have predicted an increase in tropical water temperatures reaching 3 ℃ for the coming century (Campos,Braz-Mota, Val, & Almeida-Val, 2019; Ganachaud et al., 2011), 3–4 ℃ in the Mediterranean (Fischer & Schär, 2010; Madeira et al., 2016), and 2–3 ℃ in the Portuguese waters (Madeira et al., 2016). As such, with increasing intensity, frequency, and duration of heatwaves, species inhabiting these warm regions will experience higher water temperatures at a greater magnitude (IPCC, 2013, p. 1535). Over 90% of energy in the climate system has been absorbed by the ocean hence resulting in a 0.11 ℃ decade−1(1971–2010) temperature increase. Moreover,end-of-century prediction models have indicated temperature increases ranging from (mean ± SD) 0.71 ± 0.45 ℃ (Representative Concentration Pathway [RCP] 4.5) to 2.73 ± 0.72 ℃ (RCP 8.5) (Howes, Joos,Eakin, & Gattuso, 2015; Reid et al., 2019). Other end-of-century projections (RCP 8.5) suggest sharp temperature rises in the Tropics, Arctic,and North Pacific regions, over 4 ℃ increase (Howes et al., 2015). Rivers are of great interest, given that large aquaculture production is concentrated in the deltas. According to Van Vliet et al. (2013), the global average river temperatures are expected to increase by 0.8–1.6 ℃(under the IPCC Special Report on Emission Scenarios B1−A2 scenario)by end of the century compared to 1971–2000 with the highest warming projected for Europe, United States, eastern China, some parts of Southern Africa and Australia. Therefore, in contrast to subtropical and temperate species, it is expected that tropical species will be more susceptible to warming due to their narrower thermal range and survival closer to their thermal limits. Hence, even small temperature changes could cause a significant suppression in either their individual or population performance (Stillman, 2003; Tewksbury, Huey, & Deutsch,2008). Anthropogenic induced temperature fluctuations have the potential to influence aquaculture production in several dimensions namely; metabolic rate (Islam, Kunzmann, & Slater, 2021; Yanik &Aslan, 2018), oxygen demand and consumption (Islam et al., 2021), the solubility of oxygen (Cox & Whitehead, 2009; Ficklin, Stewart, &Maurer, 2013; Matear, Hirst, & McNeil, 2000; Missaghi, Hondzo, &Herb, 2017; Zhang et al., 2015), thermal stress tolerance (Dominguez,Takemura, Tsuchiya, & Nakamura, 2004; Liu et al., 2019a;P´erez-Casanova et al., 2008), tolerance to environmental pollutants(Brown, Jordan, & Tiller, 1967; Gluth & Hanke, 1983; Li, Li, & Wu,2021) immune response (Bowden, Thompson, Morgan, Gratacap, &Nikoskelainen, 2007; Buchtíkov´a et al., 2011; Burge et al., 2014;Chiaramonte, Munson, & Trushenski, 2016), reproductive performance(Islam et al., 2021), growth and growth performance (Iwama & Tautz,1981; Reid et al., 2019), nutrition and feeding (Huguet, Norambuena,Emery, Hermon, & Turchini, 2015; Khan, Pether, Bruce, Walker, &Herbert, 2014; Remen, Sievers, Torgersen, & Oppedal, 2016; Siikavuopio et al., 2012), disease outbreaks and vaccine efficacy (Kim, Kim, &Kim, 2020).
This paper, therefore, aims to give a detailed and comprehensive review on the influence of anthropogenic temperature increase on the general animal physiology, growth, growth performance, survival,reproduction, digestive enzyme activity, immunity, differential expression of microRNAs, and apoptosis-associated genes as well as gut and skin microbiome of aquaculture species. Likewise, the impacts of increasing water temperatures on aquatic ecosystems with regards to pathogens and aquatic parasites, toxicity, and uptake of heavy metals and pesticides as well as vaccine efficacy are presented. To provide examples of how increasing water temperatures will impact the global trends of aquaculture production, a couple of freshwater, euryhaline,and marine species, as well as coldwater and warm water species, have been chosen to give a broader perspective on how different aquaculture species respond to temperature fluctuations. In the same regard, mitigation strategies for climate change adaptation in the context of global warming are discussed to ensure sustainable and continued aquaculture productivity.
2.Marine species vs freshwater species: impact of fluctuating water temperatures
In the past 50 years, a continuous trend in warming in the large part of the world’s oceans has been observed although regional differences such as cooling have also been identified (Frost et al., 2012). For example, Levitus, Antonov, and Boyer (2005) have previously reported a 1 ℃ increase in the sea-surface temperature in the Arctic basin over the past 20 years. Similarly, Frost et al. (2012), reported increasing sea-surface temperatures in the tropical Pacific accompanied by increasing precipitation. In general, climate change will negatively impact the production of marine species in comparison to freshwater species (Frost et al., 2012). This is because increasing temperatures not only lower the concentration of dissolved oxygen but also reduce the water salinity. The latter is a result of increased precipitation in warming regions which negatively affect stock distribution, reproductive capacity, growth, and survival of marine species (Cravatte, Delcroix, Zhang,McPhaden, & Leloup, 2009; Durack, Wijffels, & Matear, 2012; Frost et al., 2012; Stenevik & Sundby, 2007). However, there is merit to increasing precipitation for freshwater species. Frost et al. (2012), reported that increased hydrological cycles in the tropical Pacific region will enhance river flow thus resulting in channel expansion and floodplain habitats in Melanesia which will ameliorate the potential negative effects of increasing water temperatures, lowered dissolved oxygen levels, and sea-level rise on freshwater fish habitats associated with global warming. In this region, freshwater habitats were predicted to increase by 10% in 2023 and>20% in 2100 hence increasing the production of freshwater species by at least 2.5% and 7.5% in 2023 and 2100 respectively. However, it is imperative to note that freshwater species are more vulnerable to increasing environmental pollution under conditions of high water temperature (Frost et al., 2012; Patra,Chapman, Lim, & Gehrke, 2007). Therefore, if water pollution persists,the production of freshwater species will be affected. Marine, euryhaline, and freshwater species discussed in this review are Atlantic cod(Gadus morhua), Yellowtail king fish (Seriola lalandi), turbot (Scophthalmus maximus), Shrimp (Litopenaeus vannamei, Penaeus monodon,Palaemonetesspp.,Palaemon peringueyi, Palaemon elegans), olive flounder(Paralichthys olivaceus) Mozambique tilapia (Oreochromis mossambicus)and trout (Oncorhynchus mykiss, Oncorhynchus clarkii, Salmo trutta, Salmo gairdneri. R, Salvelinus confluentus, Salvelinus fontinalis), carp (Cyprinus carpio,Ctenopharyngodon idellus,Hippelates nobilis,Hypophthalmichthys molitrix,Hypseleotris klunzingeri, Labeo rohita), salmon (Salmo salar,Oncorhynchus nerka,Oncorhynchus kisutch,Oncorhynchus gorbuscha,Oncorhynchus tshawytscha), Nile tilapia (Oreochromis niloticus, Oreochromis niloticusGIFT), cat fish (Silurus meridionalis,Clarias batrachus,Horabagrus brachysoma,Ictalurus punctatus, Ompok bimaculatus, Pangasianodon hypophthalmus), respectively.
3.Coldwater species vs warm-water species: impact of fluctuating water temperatures
With temperature fluctuations bending more to the increasing temperature trend, it is indeed clear that coldwater aquaculture species will be more affected than warm-water species. The historically cooler climates of certain regions will exhibit changes in their biodiversity which might affect the abundance and distribution of indigenous species.Bricknell et al. (2021) have recently reported that the Maine coast is under threat from the bio-invasion of nonindigenous aquatic species that are establishing dominance in the ecosystem as a result of increasing water temperatures. This in turn is affecting the survival and distribution of coldwater species that historically inhabited these ecosystems.Singh et al. (2015) have also reported that climate change, particularly temperature and rainfall patterns will, directly and indirectly, influence the survival of coldwater species in the Himalayan region of India.However, McKelvey and Buotte (2018) have predicted the persistence of certain temperature-sensitive salmonid species such as Bull trout(Salvelinus confluentus) inhabiting the Northern Rockies due to the projected retainment of certain environmental factors that will favor the continued survival of this species. Generally, however, coldwater species such as salmon, chars, steelhead, white fishes, and trout will be negatively affected in the Northwest and Northern Rockies due to the predicted increase in stream temperatures to approximately 2.4 ℃ by the mid-century (Williams, Isaak, Imhof, Hendrickson, & McMillan,2015). Coldwater and warm water species discussed in this review are;trout (Oncorhynchus mykiss, Oncorhynchus clarkii, Salmo trutta, Salmo gairdneri. R, Salvelinus confluentus, Salvelinus fontinalis), carp (Cyprinus carpio,Ctenopharyngodon idellus,Hippelates nobilis,Hypophthalmichthys molitrix,Hypseleotris klunzingeri), salmon (Salmo. salar,Oncorhynchus nerka,Oncorhynchus kisutch,Oncorhynchus gorbuscha,Oncorhynchus tshawytscha), turbot (Scophthalmus maximus), yellowtail king fish (Seriola lalandi), Atlantic cod (Gadus morhua), and Nile tilapia (Oreochromis niloticus, Oreochromis niloticusGIFT), cat fish (Silurus meridionalis,Clarias batrachus,Horabagrus brachysoma,Ictalurus punctatus), olive flounder(Paralichthys olivaceus), shrimp (Litopenaeus vannamei, Penaeus monodon,Palaemonetesspp.,Palaemon peringueyi, Palaemon elegans) respectively.
4.Impact of high-water temperature on the general animal physiology, reproductive capacity, growth performance,immunity, and aquatic eco-systems of aquaculture species
4.1.Impact on metabolic rate, oxygen consumption, and demand
The metabolic rate in ectotherms increases two – to threefold for every 10 ℃ increase in water temperature (Islam et al., 2021). However,once the temperature increases beyond the thermal threshold of a certain aquaculture species, its metabolic rate declines due to physiological stress. Fig. 1 shows the maximum thermal threshold of aquaculture species mentioned in this review. The higher the water temperature, the higher the metabolic rate. However, hyper-thermal stress triggers the reallocation of metabolic energy required for the maintenance of growth and reproduction to the restoration of physiological homeostasis (Islam et al., 2021). During this state, the fish stop feeding until temperatures return to their optimum thermal range.Studies on the effect of increasing water temperature on the metabolic rate have been conducted on several species such as), sockeye salmon(Brett, 1971), juvenile common carp (Zeng, Fu, & Fu, 2018), Nile tilapia(Caulton, 1978; Tuker, 2011), juvenile southern cat fish (Pang, Cao,Peng, & Fu, 2010), Turbot (Mallekh & Lagard`ere, 2002), yellowtail king fish (Pirozzi & Booth, 2009), Atlantic cod juveniles (Tirsgaard,Behrens, & Steffensen, 2015), olive flounder (Kim, Kim, & Kim, 2020;Oh, Jang, Park, Choi, & Kim, 2012), and shrimp (Allan, Froneman, &Hodgson, 2006; Dalla Via, 1985).

Fig. 1.Maximum temperature limit for aquaculture species.
High temperatures lower the solubility and availability of oxygen(hypoxia) hence affecting ATP production to accommodate the metabolic shifts (Dalla Via, Villani, Gasteiger, & Niederstätter, 1998;Dong, Dong, Tian, Wang, & Zhang, 2006; Siikavuopio, Mortensen, &Christiansen, 2008). Likewise, exposure of fish to high water temperatures increases their oxygen demand which results in high respiration rates and reduced feed intake under conditions of low dissolved oxygen(DO). Oliva-Teles et al. (1990) investigated the effect of temperature on utilization of endogenous energy reserves during embryonic development of diploid and triploid rainbow trout and observed a direct correlation between ammonia excretion, oxygen consumption, and temperature. Oxygen consumption was high at high water temperatures and vice versa in all the developmental stages with the greatest temperature effect on the respiratory metabolism observed in eggs. Hogue and Pegg (2009) also observed an increase in oxygen consumption with an increase in water temperature in two Asian carp species (H. nobilisandH. molitrix) at juvenile and adult stage. There was a correlation between body mass and oxygen consumption. A study by Lee et al.(2003) on different adult stocks of salmon (O. nerkaandO. kisutch)obtained from various field and laboratory locations showed that oxygen consumption increases with increasing water temperature up to the maximum thermal limit of the species and then declines. This correlated with the critical swimming speeds (Ucrit) of both species sinceUcrittended to decrease over time at high water temperatures above their thermal threshold. It has been previously reported that hypoxia as a result of certain factors such as high water temperature hinders aerobic swimming performance in fish by suppressing their aerobic metabolic scope (Domenici, Herbert, Lefrancois, Steffensen, & McKenzie, 2013). It is thus not surprising that theUcritof salmon decreased under such conditions. Salmonids are easily affected by combined effects of hypoxia and increasing water temperature challenges hence necessitating critical temperature monitoring during salmonid aquaculture. By experimenting on more partial pressure of oxygen (pO2) resistant and high-temperature tolerant Tabasco-line of the Nile tilapia, Burggren et al. (2019) observed increasing oxygen consumption with increasing water temperature in all developmental stages of fish. Even at extreme temperatures as high as 35 ℃ and very low DO levels, no behavioral changes were noticed in this line. Zeng et al. (2018) investigated the influence of temperature acclimation on resting oxygen consumption rate (MO2rest) and excess post-exercise oxygen consumption after exhaustive exercise (MO2peak) in juvenile southern cat fish. The authors reported that MO2restincreased linearly with increasing water temperatures whereas MO2peakinitially increased with increasing water temperatures and then plateaued. Recovery time was slower due to lower energy utilization efficiency during swimming and the post-exercise recovery process. Studies on turbot and Atlantic cod have shown increased oxygen consumption with increasing temperature (Gollock,Currie, Petersen, & Gamperl, 2006; Imsland, Folkvord, & Stefansson,1995).
4.2.Impact on osmoregulation
Aquaculture species such as juveniles of Atlantic salmon migrate from freshwater to seawater as part of their anadromous life history.This is facilitated by prior physiological, morphological, and behavioral changes necessary for adaptation to changing environments (McCormick, 1993; Vargas-Chacoff et al., 2018). Gills play a vital role in the maintenance of osmotic balance during this transition. The specialized cells, ionocytes, located in the gills facilitate the uptake of sodium (Na+)and chloride (Cl−) ions in freshwater and their secretion in seawater.Secretion of these ions is aided by an ion transport protein,Na+–K+-ATPase (Evans, Piermarini, & Choe, 2005; Vargas-Chacoff et al., 2018). The activity of this protein is temperature-dependent and any thermal stress might interfere with its activity (Islam et al., 2021).Fluctuations in water temperature reduce the influx of ions in freshwater species resulting in net loss unlike in marine species. And as such, fish increase the Na+–K+-ATPase and Na +-K +-Cl-cotransporter (NKCC)activities in gills, intestine, and kidney to compensate for ion loss and ion gain in freshwater and marine species respectively. Moreover, the epithelium permeability in freshwater species decreases to slow down the ion loss whereas it increases in marine species to speed up the ion ef flux (Islam et al., 2021; Lorin-Nebel, Boulo, Bodinier, & Charmantier,2006; Vargas-Chacoff et al., 2020). Vargas-Chacoff et al. (2018) investigated the effect of high water temperatures and salinity on osmoregulation and stress responses on Atlantic salmon molts in reared in both freshwater and seawater. It was found that fish exposed to 24 ℃ in seawater exhibited the highest mortality rate (100%) and the poorest ion regulation with or without prior acclimation. Elevated temperatures also led to a decrease in Na+-K+-ATPase activity in both seawater and freshwater. Likewise, Teffer et al. (2019) observed that exposure of Chilliwack River Coho salmon to elevated water temperatures (15 ℃)resulted in increased immune response and osmoregulatory impairment.Bernard, Mandiki, Duchatel, Rollin, and Kestemont (2019) also reported decreased activity of Na +-K +ATPase and concentrations of plasma Na+, Cl−in Atlantic salmon reared at 15 and 20 ℃ for 90 days compared to fish reared at 5 and 8 ℃. Turbot and sole (Solea senegalensis) acclimatized at 11 and 18 ℃ for 21 days showed a significant decrease in plasma Na+and Cl−ion concentrations compared to fish reared at lower temperatures (4 and 0 ℃) (Foss et al., 2019). However, previous studies on Mozambique tilapia and turbot juveniles have shown increasing Na+-K+-ATPase activity with increasing water temperature (Fiess et al.,2007; Imsland, Gunnarsson, Foss, & Stefansson, 2003). Likewise, previous studies in channel and tra cat fish have shown that the interaction of salinity and high temperatures do not affect Na+-K+-ATPase activity(Phuc, Mather, & Hurwood, 2017; Stewart, Aboagye, Ramee, & Allen,2019). The difference in results is attributed to a difference in species,body weight, duration of exposure, salinity levels, and thermal tolerance limits.
Overall, hyper-thermal stress increases osmotic stress and lowers salinity tolerance in aquaculture species. Species exposed to both hyperthermal stress and osmotic stress suffer excessively and in most cases death is inevitable. With the current threat of heatwaves and global warming, more studies are needed to elucidate detailed mechanisms underlying osmoregulation in aquaculture species along with other physiological parameters.
4.3.Impact on reproductive capacity
4.3.1.Reproduction
Several abiotic factors including water temperature have been previously reported to influence the reproductive capacity (i.e. spawning,reproduction, phenology, sexual maturity, and differentiation) of aquaculture species by interfering with the gametogenesis and steroidogenesis processes (Islam et al., 2021; Seebacher & Post, 2015;Servili, Canario, Mouchel, & Muñoz-Cueto, 2020). Moreover, the sensitivity to temperature fluctuations differs among different stages of the fish’s life cycle, and as such, the reproductive performance of fishes is expected to be affected by anthropogenic-induced temperature fluctuations (Islam et al., 2021; Miranda, Chalde, Elisio, & Strüssmann,2013; Pankhurst & King, 2010; Servili et al., 2020). Fluctuations in the water temperature either enhance or suppress sexual maturity and gametogenesis through disruption of the endocrine system (Islam et al.,2021; Zanuy & Carrillo, 1985). Lahnsteiner and Mansour (2012) reported that rearing brown trout at ≥ 8 ℃ resulted in the highest sperm motility. However, at ≥ 12 ℃, a decline in Mg2+-ATPase, adenylate kinase, pyruvate kinase, and malate dehydrogenase enzymatic activities were noted. Similar results have been reported in other studies (Manning & Kime, 1985; Vladic & Järvi, 1997). Manning and Kime (1985),showed that testicular steroid production in rainbow trout was inhibited at higher water temperatures (17 ℃) bothin vivoandin vitro. Dadras,Dzyuba, Cosson, Golpour, and Dzyuba (2016) conducted anin vitrostudy to elucidate the effect of different thermal regimes (4, 14, and 24 ℃) on the motility and antioxidant response in common carp spermatozoa. It was observed that spermatozoa motility and swimming velocity significantly declined at 24 ℃. Unexpectedly, thiobarbituric acid-reactive substance production was recorded at 14 and 24 ℃ thus indicating the inability of antioxidant enzymes to confer protection to cells against the negative impacts of antioxidant stress induced by high temperatures. In another study, Dadras et al. (2017) observed that higher temperatures (24 ℃) led to reduced lipid content, increased sperm curvilinear velocity, and decreased duration of the period over which motility is sustained in common carp. Glasser, Mikolajczyk,Jalabert, Baroiller, and Breton (2004) also observed that the ovulation rate and response of ovaries to gonadotropin by maturation-inducing steroid (MIS) synthesis in common carp was arrested at 28 ℃. De Alvarenga and De França (2009) investigated the effect of temperature(20, 25, 30, and 35 ℃) on testis structure and function (somatic cells) in sexually mature Nile tilapia. The authors observed that the optimum proliferation index of Sertoli cells, spermatogonia, and Leydig cells occurred at 20 ℃ and significantly declined at higher temperatures (30 and 35 ℃) Fig. 2. Exposure of Nile tilapia to temperatures above their critical thermal threshold (37 ℃) has been shown to cause histological changes in the testis which not only negatively impact germ cell survival and milt quality but also suppress the expression of germline-specific genes(piwil1, piwil2, nanos2, bcl-xLb, baxa, nanos3, and gfra1)(Jin,Davie, & Migaud, 2019). Pandit, Bhandari, Kobayashi, and Nakamura(2015) also observed a possible irreversible complete loss of germ cells in female Nile tilapia reared at 37 ℃ for 45–60 days.
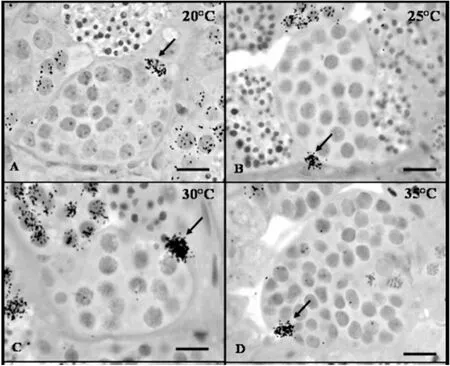
Fig. 2.Labeled testicular somatic cells (arrows) 2 h after 3H-thymidine injection in adult Nile tilapia. A–D) Labeled Sertoli cells associated with different spermatogenic cysts in fish kept at 20 (A), 25 (B), 30 (C), and 35 ℃ (D). Image obtained from (De Alvarenga & De França, 2009).
4.3.2.Sexual maturity and differentiation
As discussed earlier, fluctuations in water temperature affect the sexual maturity and differentiation of fish. High water temperatures disrupt the endocrine system thus causing hormonal imbalances that could lead to changes in the organism’s sex. For instance, Jonsson,Jonsson, and Finstad (2013) conducted an elegant study to investigate the effect of diet and temperature on sexual differentiation in Atlantic salmon. It was reported that exposing Atlantic salmon to high water temperatures and a high feed quality diet led to an increased proportion of mature males. However, females were older than males at maturity when exposed to high water temperatures and high-quality feed.Feeding high-quality feed and rearing immature fish in high water temperatures led to an increased growth rate (mean mass). Jeffries et al.(2012) observed a decline in the plasma sex steroids of female sockeye salmon and pink salmon reared at 19 ℃ and that female pink salmon were less likely to reach maturity at 19 ℃. Wang et al. (2017) demonstrated that exposure of 8 months old female tilapia to high water temperatures (28 and 36 ℃) influenced the epigenetic control ofcyp19a1apromoter expression which might play a role in promoting gonadal differentiation in females under high-temperature stress towards masculinization. As such, males exhibited highercyp19a1apromoter DNA methylation levels (50.33 ± 7.38% to 51.66 ± 4.75%) than females (30.87 ± 4.56% to 48.34 ± 0.92%). Proteomics studies revealed that females had lower levels of cyp19a1a than males after exposure to high temperatures. The authors further suggested that lower levels of cyp19a1a protein expression as a result of high-temperature stress stimulate masculinization. Another study by Sun et al. (2016) investigated global DNA methylation changes in Nile tilapia gonads during high temperature-induced masculinization. The authors found that a larger number of genes exhibited differentially methylated regions(DMRs) in high temperature-induced males and females compared to the control. Moreover, expression of gonadotropin-releasing hormone receptor (GnRH receptor type 2), gonadotropin receptor II(gth-rII),and G protein-coupled receptor (gpr54)genes were noted in high temperature-induced groups hence their possible role in sex hormone production during high temperature-induced masculinization in Nile tilapia.
4.3.3.Sensitivity and stage of the life cycle
Certain stages of the fishes’ life cycles are more sensitive to increased water temperatures than others. This could impact their behavior and survival. For example, Wagner, Arndt, and Roubidoux (2006) observed that rearing cutthroat trout at higher temperatures (13 ℃) resulted in high egg mortality before attainment of the eye stage. Ojanguren and Braña (2003) also observed a decline in maximum survival of fertilized eggs of brown trout exposed to 16 and 18 ℃. However, Oliva-Teles et al.(1990) found that yolk-sac larvae of both triploid and diploid rainbow trout were more sensitive to high water temperatures compared to eggs.Marine and Cech (2004) showed that rearing chinook salmon juveniles and adults at higher temperatures (21–24 ℃) led to increased predation vulnerability as well as decreased growth rates and impaired smolti fication indices. Nissling, Johansson, and Jacobsson (2006) observed reduced egg survival of turbot when exposed to 19 ℃.
Therefore, from the above discussion, it can be anticipated that the reproductive capacity of aquaculture species reared under captivity and in the wild will be affected by the anthropogenic-induced temperature increase in aquatic environments. More studies are however needed to elucidate the combined effect of increasing water temperatures and other abiotic factors on the reproductive capacity of aquaculture species.
4.4.Impact on growth performance
In ectothermic animals, environmental temperature fluctuations influence the feed intake (FI), digestion, absorption, and assimilation of food nutrients (Fu, Fu, Qin, Bai, & Fu, 2018; Islam et al., 2021; Volkoff &Rønnestad, 2020). Moreover, under extreme temperature events, fish utilize higher amounts of energy in regaining homeostasis. In other words, fish will redirect the energy required for growth, development,immunity, and reproduction towards the cellular repair of damaged tissues hence suppressing growth performance and immunity (Islam et al., 2021). For certain fish species, however, increasing water temperatures within their thermal range might enhance metabolism thus improving growth (Islam et al., 2021; Sotoyama et al., 2018). In contrast, anthropogenic induced temperature increase studies have shown that extreme temperatures above the desirable thermal range of certain aquaculture species negatively impact their growth performance(i.e. FI, Food conversion ratio (FCR), and specific growth rate (SGR))(Islam et al., 2020, 2021; Neuheimer, Thresher, Lyle, & Semmens,2011). However, this depends on other factors such as the stage of the organism’s life cycle, sex, body size, nutrition, ploidy, and species. For example, Brown et al. (1967) investigated the interactive effect of increasing water temperatures and body size on the general performance of cutthroat trout juveniles. The authors observed less sensitivity of smaller juveniles to different thermal regimes compared to larger fish.The authors suggest that this could have been due to decoupled growth rates and metabolism which enabled smaller fish to meet growth requirements under different thermal regimes. As such, larger fish tended to feed less which resulted in retarded growth. However, Xu, Letcher,and Nislow (2010) reported a uniform suppression of survival rates in brook trout despite their different body sizes. Alanärä (1994) investigated the effect of high temperatures on the appetite of rainbow trout,initial size 30–120 g. It was found that bite activity declined at higher temperatures (15 ℃) hence lower growth and FCR. Selong and McMahon (2000) observed a decline in the food consumption rate of bull trout reared at ≥ 22 ℃. Feed, lipid, and protein efficiencies declined signi ficantly at 20 ℃ hence indicating that this North American salmonid species exhibits the lowest thermal limits and growth optima in both in field and laboratory temperature regimes. A recent study by Ignatz et al. (2020a) has shown that rearing female triploid Atlantic salmon at high temperatures (16 ℃) leads to huge economic losses as revealed by high a FI but low feed utilization rates and lower growth rates as indicated by the thermal-unit growth coefficient. Pandit and Nakamura(2010) found a significant decline in growth performance (mean survival, total weight, total length, daily growth rate, and food conversion ratio) in the female Nile tilapia (9 and 50 days after hatching) at 35–37 ℃ compared to the male species. In olive flounder, Hur, Lim, and Chang (2008) observed a reduction in growth performance (FI, body length, and body weight) upon exposure of fish to high water temperatures (>25 ℃). High temperatures (>30 ℃) have been reported to suppress growth performance in shrimp (Coman, Crocos, Preston, &Fielder, 2002; Duan, Zhang, Dong, Wang, & Zhang, 2017; Zhou et al.,2010). Ilham and Fotedar (2016) also reported that yellowtail king fish exposed to 26 ℃ exhibited a reduction in growth performance. This was not in agreement with results obtained by Abbink et al. (2011) under the same temperature conditions. The contradicting results of the two studies could be attributed to differences in the body size of the fish.
4.5.Impact on digestive enzyme activity, gut, and skin microbiome
4.5.1.Digestive enzyme activity
High temperatures above the desirable ranges of the aquaculture species decrease FI thus resulting in lower nutrient retention which affects enzyme accumulation and activity in the gut. Furthermore, high temperatures result in changes in the pH and ion concentration in the gut which suppress digestive enzyme activities and normal metabolism(Solovyev & Izvekova, 2016). It is worth noting that enzymes work at optimum temperatures above or below which their activity is suppressed. At temperatures above their optimum range, enzyme-catalyzed reactions are slowed or stopped due to enzyme denaturation whereas, at temperatures below the critical minimum, they are inactivated(Mazumder, Das, Rahim, & Ghaffar, 2018). Endogenous digestive enzymes such as lipases, phosphatases, amylases, maltase, trypsin, and carboxypeptidases are commonly found in most fish and their pro files and activities differ between species depending on food preferences and habitat (Gioda et al., 2017; Jan & Ahmed, 2016; Volkoff & Rønnestad,2020). For instance, herbivorous and omnivorous fish have higher levels of carbohydrate enzymes (amylases and maltase) whereas carnivorous species have higher levels of proteolytic enzyme activities. The influence of water temperature on digestive enzyme activity seems to be species-specific since the optimum temperature for enzyme activity often falls within the host’s optimum thermal range (Volkoff &Rønnestad, 2020). Ahmad, Singh, Khangembam, Sharma, and Chakrabarti (2014) investigated the effect of varying temperatures on food consumption and digestive activity in cat fish. The authors observed no changes in food consumption at 25, 30, and 35 ℃ but the enzymatic activity of protease, trypsin, and chymotrypsin significantly decreased at 30 and 35 ℃. However, lipase activity increased at 30 ℃ thus indicating a metabolic shift under high thermal stress. Similarly, Zhao, Han, Xie,Zhu, and Yang (2009) observed a decline in enzyme activity of trypsin,pepsin, and lipase in cat fish reared at 32 ℃ compared to those reared at 24 and 28 ℃. Chen, Li, Wang, and Li (2022) reported that rearing common carp under high-temperature stress (30 ℃) and feeding with two meals every single day led to a decline in digestive enzyme activity(Lipase and amylase). In rainbow trout, Jiang et al. (2021) has recently reported that rearing fish at temperatures above their thermal threshold(21 ℃) reduces the activity of digestive enzymes (trypsin, lipase, and amylase). Similarly, Guerreiro, Enes, Rodiles, Merrifield, and Oliva-Teles (2016) showed that rearing turbot juveniles at higher temperatures (20 ℃) suppressed the activity of digestive enzymes (lipases,amylase, and total alkaline protease) hence indicating that optimum digestive enzyme activity is species-specific.
Fish also possess exogenous digestive enzymes released by gut microbiota and these include amylase, cellulase, chitinase, phytase,lipase, and proteases. In addition to theBacillusgenera, other enzymeproducing microbiotas belonging to several microbial genera such asPseudomonas, Flavobacterium, Vibrio,Microbacterium, Aeromonas, Photobacterium, Staphylococcus, Micrococcus, Acinetobacter,and yeasts have been identified and isolated (Ray, Ghosh, & Ringø, 2012). The optimum temperature of most of these exogenous digestive enzymes ranges between 25 and 70 ℃ (Champasri, Phetlum, & Pornchoo, 2021) hence their potential use in the aquaculture industry under hyper-thermal stress. Only a few studies have so far reported the effect of temperatures on exogenous digestive enzyme activity. For example, Lazaddo et al. (2012) conducted anin vitrostudy to investigate the effect of temperature on digestive enzyme activity of bacterial gut enzymes released by two bacterial species GP21;Pseudomonassp. and GP12;Psychrobactersp., isolated from the gastrointestinal tract of Atlantic cod.The authors observed that the optimum activity of amylase, cellulase,and protease seemed to be around 20 ℃ and this declined with increasing temperature. Champasri et al. (2021) investigated the influence of temperature and pH on digestive enzyme activities of microbial enzymes (amylase and alkaline protease) isolated from six freshwater species;O. niloticus,Ompok bimaculatus,Puntius gonionotus,Puntioplites proctozysron,Kryptopterus geminus, andHemibagrus spilopterus. Results of thein vitroanalysis showed that the optimum temperatures for amylase were 45–60 ℃ and alkaline protease were 50–55 ℃ with a decline in activity of both enzymes at temperatures above their optimum thermal conditions. Therefore, the stability of microbial digestive enzymes at temperatures above the thermal threshold of their hosts makes them good candidates for aquafeed additive enzymes.
4.5.2.Gut and skin microbiome
Aquatic organisms are surrounded by a plethora of microorganisms present in their medium, food, and sediments. Gut microorganisms constitute part of the animal’s digestive system many of which have been found to release exogenous digestive enzymes (Lazado, Caipang, &Kiron, 2012) and confer protection to their host through suppressing the growth and multiplication of pathogenic microbes (Li et al., 2018;Wang, Wei, Chang, & Ding, 2021). Global climate change is affecting the diversity and abundance of gut microbiota, their function, and community dynamics in aquatic ecosystems thus a shift in host-microbe interactions (Li et al., 2018). Changes in the gut and skin microbial diversity or abundance increase the organisms’ susceptibility to disease and previous studies have shown increasing disease outbreaks in aquaculture due to increasing water temperatures among other factors (Wang et al., 2021; Zhang, Zeng, et al., 2016; Zhang, Zeng, et al., 2016). Several studies have reported the influence of increasing water temperatures on gut and skin microbial diversity and abundance. For example, Pelusio et al. (2020) have recently investigated the combined effects of temperature and dietary organic acid and nature-identical compounds on the gut microbiota and expression of intestinal immune genes in rainbow trout. The authors observed a reduction in the gut microbiota diversity particularly of the bacterial generaLeuconostocin fish reared at 23 ℃ with or without dietary supplements. In Atlantic salmon, studies by Neuman et al. (2016) and Ciric, Waite, Draper, and Jones (2019)have reported a temperature-dependent increase in the abundance of potentially pathogenic bacteria ofVibriospp. in the fish guts which suppress the growth of potentially beneficial lactic acid bacteria (LAB).Likewise, Huyben et al. (2018) observed a reduction in the abundance of gut LAB (i.e.Lactobacillus reuteriand species belonging to familyLeuconostocaceae) in rainbow trout reared at 18 ℃. These bacteria have been previously reported to play a vital role in the digestion and immunity of fish and crustaceans (Ringø et al., 2010, 2018, 2020) hence their reduction in abundance could indicate poor fish health. Similar results have been reported in Atlantic salmon (Hovda, Fontanillas,Mcgurk, Obach, & Rosnes, 2012; Neuman et al., 2016). In contrast,Soriano et al. (2018) observed enhanced gut microbial diversity and abundance in yellowtail king fish with increasing water temperature with optimum microbial species diversity and abundance recorded at 26 ℃ (i.e. Operational taxonomic Units (OTUs) identified at this temperature belonged to families Microbacteriaceae, Pseudomonadacea,andAlcaligenaceae) compared to that at 20 ℃. The discrepancy in results could be attributed to differences in species hence changes in microbial diversity and abundance as a result of temperature fluctuations are species dependent.
Just like the gut microbiome, the skin microbiome of fish is also affected by the ambient water temperature and is species-dependent(Rosado et al., 2021; Sehnal et al., 2021). Furthermore, the duration of exposure (days and months) to hyper-thermal stress determines changes in microbial richness, structure, and function (Rosado et al.,2021). This is because changes in water temperature impact the adhesion and growth of fish skin microbial species. Seasonal temperature fluctuations, as well as geographical location, impact microbial diversity and OTU composition. For example, Wilson, Danilowicz, and Meijer(2008) observed that Atlantic cod sampled from three sites (Baltic,Icelandic, and North Seas) during spring/fall 2002 and spring 2003 exhibited stability in skin microbial diversity and OTUs related to Cytophaga–Flavobacterium–Bacteroides (CFB) groups and γ-proteobacteria in samples obtained from Baltic and Icelandic sites in the three seasons whereas those from the North Sea exhibited changes in microbial abundance as indicated by the reduced and increased abundance ofAcinetobacterspp. andBacteroidesspp. in the autumn of 2002 season respectively. However, in the spring seasons, the authors observed an increased abundance ofPhotobacteriumspp. andPsychrobacterspp. on fish skin from the North Sea compared to fish samples obtained from Baltic and Icelandic sites.
High water temperatures can favor the growth of skin pathogenic microbes such asVibriospp. which suppress the immunity of fish(Larsen, Bullard, Womble, & Arias, 2015). For instance, Rosado et al.(2021) observed increased abundance of several potentially pathogenic genera (Vibrio,Pseudomonas,Aliivibrio, andPhotobacterium) in European seabass (Dicentrarchus labrax) reared in an outdoor exterior pond with water temperatures reaching 25 ℃. Likewise, Horlick, Booth, and Tetu(2020) observed a reduced abundance of microbial species belonging to the family Alteromonadaceae on the skin of yellowtail king fish thus indicating a reduced health status and general wellbeing of fish. This family of bacterial species has been suggested as a potential health indicator in fish. Overall, skin microbiomes play a significant role in the fishes’ immunity and their microbial diversity and abundance differ between different species and habitats. It is imperative to characterize microbial taxa colonizing fish skin as these could be potential indicators of the health status of fish.
4.6.Impact on nonspecific immunity
The immunity of aquatic organisms plays a central role in conferring protection against biotic and abiotic stress. Several factors such as genetics, age, stage of the life cycle, weight, physiological status, and the external environment, contribute to the ability of the aquaculture species to trigger an immune response (Biller-Takahashi & Urbinati, 2014;Uribe, Folch, Enriquez, & Moran, 2011). In fish and crustaceans, the first line of defense against pathogens and abiotic stress is based on a broad range of nonspecific cellular and humoral immune mechanisms (Roy,Bossier, Norouzitallab, & Vanrompay, 2020; Uribe et al., 2011).Nonspecific immunity indicators include several hematological parameters and blood biochemical indices, antioxidant enzymes, metabolic enzymes, heat shock proteins, cytokines, and complement components.The aforementioned parameters are used to assess the health status of aquaculture species and hence, the influence of heat stress on these parameters is discussed below.
4.6.1.Hematological parameters and blood biochemical indices
Hematological analysis and evaluation of blood biochemical indices are some of the most important standardized, non-lethal, and fairly cheap techniques used to provide a reliable evaluation of the health status of aquaculture species. Examples of hematological parameters used in aquaculture include; red blood cells (RBCs), white blood cells(WBCs), hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and hemoglobin (Hb). Blood biochemical indices include serum glucose and serum cortisol. It is imperative to note, that a variation in concentration of these parameters from known reference ranges of different aquaculture species could indicate stress caused as a result of changes in environmental conditions,disease outbreaks, husbandry practices, changes in the life stage,behavior, and habitat (Fazio, 2019; Seibel, Baßmann, & Rebl, 2021;Witeska, Kondera, Ługowska, & Bojarski, 2022). Moreover, Hb, an oxygen-binding protein whose concentrations correlate with RBC count is also affected by fish physiology and metabolism (Harikrishnan, Kim,Kim, Balasundaram, & Heo, 2011). Temperature fluctuations above the thermal threshold decrease the concentrations of several hematological parameters and blood biochemical indices which weaken the immune system of the stressed organism. For example, concentrations of serum glucose and or serum cortisol increase with increasing rearing water temperature. This is due to the enhanced metabolism of both muscle and liver carbohydrate reserves aimed at providing more energy to the stressed organism (Bao et al., 2018; Dalvi et al., 2017; Kim, Kim, & Kim,2020; Lermen et al., 2004). However, depending on the species and duration of heat stress exposure, their concentrations later decrease hence reducing the organism’s adaptability and tolerance to stress(Ahmad, Shah, Bhat, Bhat, & Balkhi, 2011; Ashaf-Ud-Doulah et al.,2019; Bao et al., 2018; Dengiz Balta, Akhan, & Balta, 2017; Mahmoud,Sabry, Abdelaziz, & Shukry, 2020; Musa, Ramly, Abdul Manaf, Razzak,& Musa, 2017; Zutshi, Singh, & Dasgupta, 2020). Acute thermal stress tends to decrease the concentrations of several hematological parameters. For example, Qiang, Yang, Wang, Kpundeh, and Xu (2013)observed that high-temperature exposure (29 and 30 ℃) of Nile tilapia juveniles fed on 37.5% protein resulted in a reduction of RBC and WBC counts respectively. Likewise, Hb content decreased at temperatures above 29 ℃ regardless of the percentage of dietary protein content.Moreover, high mortality rates were recorded at temperatures above 28 ℃ underS. iniaeinfection challenge. Ndong, Chen, Lin, Vaseeharan,and Chen (2007) investigated the immune response in Mozambique tilapia reared under high temperatures (35 ℃) and observed decreased leukocyte count under conditions of acute heat stress. In rainbow trout,Lewis, Hori, Rise, Walsh, and Currie (2010) observed a reduction in the Ht levels in fish exposed to heat stress (25 ℃) at the 24 h blood sampling time-point. Ahmad et al. (2011) investigated the effect of acute thermal stress on thermal adaptability and disease association in common carp.The authors observed a decline in MCH in the blood of heat-stressed fish(32 ℃) which indicated poikilo-anisocytosis. Overall, a decline in hematological parameters under conditions of prolonged acute stress could impair the organism’s fitness to heat stress tolerance.
4.6.2.Antioxidant and hepatic enzyme activity
Both biotic and abiotic stress trigger irregular oxidative stress that induces the production of reactive oxygen species (ROS). Accumulation of ROS in tissues often leads to more devastating consequences such as protein carboxylation, lipid peroxidation, and DNA damage (Meng et al.,2017). Fish and crustaceans, therefore, have several antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione-s-transferase (GSTs), and glutathione peroxidase (GPx) whose role is to confer protection to cells against oxidative stress (Li, Wei, et al.,2016; Meng et al., 2017; Refaey & Li, 2018). Generally, increased antioxidant enzymatic activity (i.e. SOD, CAT, and GPx) has been noted across several aquaculture species under heat stress which is accompanied by a reduction in the concentration of malondialdehyde (MDA) (Liu et al., 2019a; Ming et al., 2012; Vinagre, Madeira, Narciso, Cabral, &Diniz, 2012). MDA is an indicator of cellular membrane damage caused by ROS and its accumulation is a result of lipid peroxidation (Abd El-Gawad & M Abd El-latif, 2016). Accumulation of MDA in tissues often leads to homeostasis impairment which suppresses the organism’s adaptability and tolerance to stress (Jia et al., 2020). Several studies have reported reduced antioxidant enzyme activity coupled with increased levels of MDA in aquaculture species reared under prolonged heat stress. For example, Musa et al. (2021) observed a reduction in antioxidant enzymatic activity (SOD, CAT, and GSTs) coupled with enhanced levels of MDA in liver tissues of red hybrid tilapia reared under prolonged heat stress (31 ℃). Likewise, Liu et al. (2019a) investigated the effect of heat stress (32 ℃) on nonspecific immunity in yellow cat fish fed with or without (control) dietary carotenoids. The authors observed a reduction in serum antioxidant enzymatic activity (CAT and SOD) and increased levels of MDA in the control group. This correlated with the fishes’ vulnerability toProteus mirabilisinfection challenge. In shrimp,Duan, Wang, Zhang, and Xiong (2018) observed that prolonged high-temperature stress impaired the activities of SOD, CAT, and GPx in the intestine, reaching their lowest levels at 48 h post-heat exposure.Increased MDA levels were noted after 6–72 h. Yu et al. (2020) conducted a comparative study on the nonspecific immune response among the three strains of koi carp under acute heat stress and observed enhanced levels of MDA in Showa Sanshoku and this correlated with SOD activity. Moreover, this suppressed the fishes’ immunity as indicated by reduced expression of complement 3. Jia et al. (2020) experimented on juvenile turbot and observed a reduced enzymatic activity of SOD, 24 h after heat stress (27 ℃). Likewise, enzymatic activities of CAT and GPx declined in a time-dependent manner under similar stress conditions hence leading to increasing levels of MDA.
Hepatic enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH), glucokinase(GK), glucose-6-phosphatase (G6Pase), and glucose-6-phosphate dehydrogenase (G6PD) are important indicators of the health status of the liver (Hemre, Mommsen, & Krogdahl, 2002). Since the liver is mainly involved in metabolism and is the most sensitive organ in the body of fishes, it is imperative to assess its health status under different environmental conditions. Acute temperature stress causes liver damage which often results in the leakage of these enzymes thus increasing their levels in blood or serum. Likewise, prolonged exposure to heat stress enhances their activity which serves as an indicator of tissue damage or organ dysfunction (Abarike et al., 2020; Abdel-Tawwab & Wafeek,2017). For instance, significant increases in AST, ALT, G6PD, and LDH activities and concentration under heat stress have been reported in the serum or blood of tilapia (Abarike et al., 2020; Abdel-Tawwab &Wafeek, 2017; Bao et al., 2018; Zeng et al., 2021), cat fish (Dalvi et al.,2017), olive flounder (Lim, Han, & Hur, 2021), and Atlantic cod (Larsen et al., 2018). However, certain studies have also reported a decline in the enzymatic activities and mRNA expression of G6Pase and GK under chronic hyper-thermal stress (Qiang et al., 2014; Shikata, Iwanaga, &Shimeno, 1995). Overall, the activity of these hepatic enzymes is not only temperature-dependent but also influenced by other factors such as species and type of feed.
4.6.3.Heat-shock proteins
Heat shock proteins (hsps) are highly conserved molecular chaperones involved in the functioning of cells under both normal and stressful conditions (Boone & Vijayan, 2002; Roberts, Agius, Saliba, Bossier, &Sung, 2010). The 70-kDa family (hsp70) is the most widely studied hsp commonly induced during thermal stress (Boone & Vijayan, 2002).Thermal stress negatively affects the protein machinery of cells and as such, hsps70 are induced as molecular chaperones to assist cells in stress recovery and promote cytoprotection (Shi et al., 2015). Other important hsps commonly studied include the hsp60 and hsp90 whose induction is triggered by thermal stress. The induction of hsp60 has been hypothesized as a “danger signal” due to its role in proinflammatory response in cells of the innate immune system (Shi et al., 2015). Despite the role of these hsps in aquaculture species under heat stress, a reduction in their level under extreme heat stress renders the organism incapable of tolerating stress. For example, Dalvi, Pal, Tiwari, and Baruah (2012)observed reduced expression of HSP70 in livers of cat fish reared at 38 ℃. Jia et al. (2020) observed down-regulation of hepatic hsp70 and hsp90 in turbot post 12 h exposure to heat stress (). Likewise, Kim, Kim,and Hur (2019) also reported that exposure of olive flounder to extreme heat stress (30 ℃) for 2 weeks under bio floc conditions led to a reduction in hsp70 levels in the liver. Therefore, this indicates that chronic hyper-thermal stress could interfere with the production of hsps thus lowering the organism’s ability to tolerate thermal stress.
4.6.4.Cytokines and complement components
The expression of immune-related genes is one of the several ways fish respond to environmental stress. Cytokines, which are low molecular weight proteins secreted by cells of the immune system are considered immune regulators in fish during stress (Kurata et al., 2010).Differential expression of cytokine genes (i.e.IL-1β, IL-6, IL-8, IL-10,IL-17, TNFα, TGFβ, CCL27,CCR7,CXCR4,CXCb2,CCL-C25) under high-temperature stress has been reported in several aquaculture species such as rainbow trout (Pelusio et al., 2020), grass carp (Yang, Yu, Li,Wang, & Yu, 2016), Nile tilapia (Kayansamruaj, Pirarat, Hirono, &Rodkhum, 2014), and turbot (Huang, Ma, & Wang, 2011), among others. On the other hand, complements are the main humoral components of the innate immune response which play a vital role in inflammatory reactions, phagocytosis, elimination of immune complexes,antibody protection, and microbial killing (Yang et al., 2016). Furthermore, the body’s ability to maintain homeostasis under stressful conditions is strengthened by complements and as such, their suppression weakens the immune system of the organism hence making it more susceptible to environmental stress (Liu et al., 2019b). For example, a transcriptomic pro file analysis on the spleens of grass carp reared at 34 ℃ showed downregulation in the expression of variation in factor B and complement component (Bf/C2), Complement component 1q (C1q),Complement component (C7), and Complement component 8 beta (C8β)genes (Yang et al., 2016). Likewise, a recent study on rainbow trout has indicated that chronic exposure of fish to heat stress (24 ℃) led to the down-regulation of complement proteins (i.e. complement 3 and complement 4) (Quan et al., 2021). Kang et al. (2019) also reported that short-term exposure of rainbow trout to heat stress (25 ℃) led to a downregulation of complement c3-like, complement factor I, and complement factor b-like which led to increased susceptibility to heat stress and pathogen infection. Overall, changes in the expression of the complement system are correlated with the duration and magnitude of stress experienced by an organism.
4.7.Impact on immunoglobulin production
Immunoglobulins M (IgM) in fishes are the only component of the specific humoral defense system that is affected by abiotic stress; mainly temperature and salinity (Dominguez et al., 2004; Kim et al., 2019).Several studies have indicated fluctuations in IgM production levels in heat-stressed fish and as such, their production in fish is temperature-dependent (Huang et al., 2011). High temperatures above a species thermal range could result in an increase or decrease in their production hence affecting the organism’s immunity and ability to tolerate stress (Dominguez et al., 2004; Liu et al., 2019b;P´erez-Casanova et al., 2008). For example, Dominguez et al. (2004)investigated the effect of different environmental factors on the concentrations of circulating IgM in Nile tilapia. Rearing fish at 33 ℃ resulted in a decline of IgM concentrations compared to those reared at 18.4, 23, and 28 ℃. Likewise, Kim et al. (2019) also observed that rearing the olive flounder under heat stress (28 and 30 ℃) resulted in reduced IgM concentrations after 1 and 2 weeks of culture. Lange and Webster (2017) reported that high rearing temperatures (30 ℃) suppressed the production of mucosal IgM in channel cat fish immunized with DNP-KLH antigens. However, increasing levels of IgM with increasing water temperature have been identified in turbot (Huang et al., 2011). This indicates that aquaculture species have a thermal range for the production of immune substances (Dominguez et al.,2004).
4.8.Impact on differential expression of MicroRNAs and apoptosisassociated genes
MicroRNAs (miRNAs) are largely involved in post-transcriptional gene regulation in living organisms including viruses (Buck et al.,2010; Cullen, 2006). They are endogenous and evolutionarily conserved(Buck et al., 2010; Forman, Legesse-Miller, & Coller, 2008; Zeng et al.,2021). The non-coding miRNAs are responsible for regulating gene expression through sequence-specific interactions with the 3′untranslated regions (UTRs) of mRNAs hence suppressing protein synthesis either through degradation of mRNA targets or inhibiting translation (Bao et al., 2018; Qiang et al., 2014; Zeng et al., 2021).However, certain miRNAs can stabilize mRNA hence regulating several cellular pathways linked to cellular stress response (CSR) such as proliferation, immunity, inflammation, apoptosis, and metabolism (Sun,Liu, et al., 2019; Zeng et al., 2021). Molecular biology techniques such as microarray and RNAseq technologies have recently been utilized to investigate the relationship between the environment and the organism’s transcriptome and to identify differential gene expression in aquaculture species exposed to low temperature and high-temperature stress (Sun, Liu, et al., 2019). Thermal stress alters the organism’s cellular metabolism and certain miRNAs are involved in either the organism’s adaptability or vulnerability to stress (Bizuayehu, Johansen,Puvanendran, Toften, & Babiak, 2015; Sun, Liu, et al., 2019). For instance, Zhou, Zhou, Ren, Cao, and Wang (2019) reported that heat stress (27 ℃) in rainbow trout enhances the upregulation of miR-133–3p and miR-133a-3p which might suppress the expression of MED16 and DECR2. The latter has been reported to play a vital role in the breakdown of unsaturated fatty acids whereas the former has been found to prevent oxidative damage in cells. Suppression of these genes might affect the fish’s ability to tolerate acute heat stress. For example, unsaturated fatty acids have been previously reported to be the dominant source of energy during high-temperature stress (Kayansamruaj et al.,2014). And as such, the downregulation of DECR2 could cause an imbalance in the fish’s energy requirements thus affecting its tolerance to high temperatures. In tilapia GIFT strain, Bao et al. (2018) reported that exposure of fish to chronic heat stress (35 ℃) triggered the upregulation of miR-122, miR-10c, and miR-1 which correlated with enhanced cell metabolism and liver damage. Likewise, Qiang et al.(2014) observed that exposure of tilapia GIFT strain to heat stress(37 ℃) resulted in the upregulation of miR-122 which the authors anticipated to suppress the expression of complement C3, the central complement of the immune system. Furthermore, two novel miRNAs,PC-5P-27517 and 3p-50929 were detected and these may mediate the expression ofAQP10athat codes for integral membrane proteins that function as water-permeable channels in the epithelia of organs that absorb and excrete water. Their upregulation in heat-stressed fish was anticipated to have suppressed the expression ofAQP10ahence indicating impaired cell membrane fluidity. Sun, Liu, et al. (2019) observed upregulation of liver microRNA molecules (miR-301a, miR-203b-5p,miR-210–3p, miR-9-5p, miR-27d, miR-92b-3p, and miR-155) involved in the insulin-signaling and glycerophospholipid metabolism pathways in common carp reared under hyper-thermal stress 30 ℃. In another study, Sun, Liu, et al. (2019) observed that rearing of common carp at high temperatures (30 ℃) resulted in the downregulation of miR-122 involved in aerobic glycolysis hence promoting lactic acid metabolism.Likewise, a down-regulation of miR-15b-5p involved cellular metabolism was noted hence indicating that temperature stress affects glucose metabolism. Besides cellular metabolism and immunity, several miRNAs have a phenotypic effect on organisms and, therefore, undergo differential expression at different stages of the organisms’ life cycles.Bizuayehu et al. (2015) investigated the influence of fluctuating water temperatures during the early development of Atlantic cod on miRNA expression and observed a low expression of miR-430, miR-130b, and miR-206 at high-temperature stress of 10 ℃. The authors reported that a low expression of miR-430 could partially explain the observed delay of epiboly and closure of blastopore in embryos reared at 10 ℃ compared to those reared at 4 and 7 ℃. Moreover, miR-130b influences cell proliferation which is a vital heterochronic process during the epiboly and before somite formation. Similarly, the authors attributed the observed shorter body axis of Atlantic cod embryos incubated at 10 ℃ to the low expression of miR-206 hence suggesting that miRNAs have a phenotypic effect on organisms.
Programmed cell death, also known as apoptosis, promotes the elimination of damaged cells through the activation of caspases (Li &Yuan, 2008). Any interference in this genetically programmed process will affect the fish’s ability to tolerate unfavorable environmental conditions. Li et al. (2017) reported that exposure of rainbow trout to moderate heat stress (24 ℃) resulted in the downregulation ofCAPN1,a ubiquitous calpain isoform and an upstream regulator of apoptosis.Anderson, Luckenbach, Yamamoto, and Elizur (2019) also observed a downregulation in apoptotic genes (casp3 and casp8) in hormone-treated ovarian follicles of coho salmon under heat stress (22 ℃). Likewise, Jia et al. (2020) reported that rearing juvenile turbot at 27 ℃ for 6 h resulted in a down-regulation of apoptosis-related genes (p53, caspase 3,bax, and bcl2).
4.9.Impact on disease outbreaks and vaccine efficacy
4.9.1.Disease outbreaks
According to the IPCC (2007), extreme weather changes are predicted to become more frequent in the future. This will in turn favor pathogen-associated disease outbreaks as a result of changes in temperature and precipitation (Leung & Bates, 2013). Increasing temperatures tend to favor the growth and transmission of pathogens hence an emerging threat to global aquaculture production. Moreover, increasing water temperatures enhance the severity of infectious diseases which if not properly managed could lead to significant economic losses. For example,Vibriooutbreaks have been previously reported in shrimp aquaculture as a result of increasing water temperatures (Chandrakala &Priya, 2017; Cheng, Wang, & Chen, 2005; Noriega-Orozco, Acedo-F´elix,Higuera-Ciapara, Jim´enez-Flores, & Cano, 2008; Pawar, Sanaye,Shyama, Sreepada, & Dake, 2020). Furthermore, several studies have also reported a correlation between the moderate increase in water temperatures and the rate of parasitic transmission in aquatic ecosystems (Baker, Freeman, Wong, Fogel, & Knowlton, 2018; Barber,Berkhout, & Ismail, 2016; Harvell et al., 2002; Lõhmus & Björklund,2015; Mouritsen, Sørensen, Poulin, & Fredensborg, 2018). Just like any other living organism, the survival of aquatic parasites depends on the ambient temperature. Optimal conditions favor and prolong the parasites’ infection cycle. Fluctuations in temperature above their optimal threshold could enhance their metabolic rate hence resulting in higher numbers of transmission stages (Lõhmus & Björklund, 2015). For instance, previous studies on salmon have indicated the increased spread of aquatic parasites such asLepeophtheirus salmonis, Caligusspp.,Neascus pyriformis, andParamoeba peruransunder conditions of increased water temperature (Cairns et al., 2005; Godwin, Krkosek,Reynolds, & Bateman, 2021; Hvas, Karlsbakk, Mæhle, Wright, &Oppedal, 2017). In crustaceans like shrimp (P. monodon), increased incidences of microspridian parasites and peritrichous ciliates have been reported in warming environments (Chakraborti & Bandyapadhyay,2011). Studies on freshwater species such as Nile tilapia have also indicated increased incidents of parasitic organisms (i.e.Centrocestus formosanus,Myxobolus tilapiae, andCichlidogyrus halli) in fish reared under increasingly warm environments (Eissa et al., 2021; Ojwala,Otachi, & Kitaka, 2018).
4.9.2.Vaccine efficacy
Vaccines are one of the most effective available tools used for combating the spread of infectious diseases. However, there is a high possibility of continued resurgence of vaccine-preventable diseases such as those caused byVibriosp.,Streptococcussp.,Aeromonassp.,Lactococcussp.,Edwardsiellasp., andPseudomonasspp. among others due to enhanced pathogenicity caused by the changing environment (Ahmadivand et al., 2017; Angulo et al., 2021; Ghasemi, Bouzari, & Emtiazi,2014; Kumar et al., 2020; Pal, 2015; Rajesh Kumar et al., 2008; Zhang,Zeng, et al., 2016). Therefore, understanding the influence of global warming on the future dynamics of these infectious diseases and the underlying control and prevention approaches (i.e., vaccination) to safeguard sustainable aquaculture production is pertinent. Vaccine efficacy in the context of global warming is a very crucial issue that needs more attention since few studies have so far been conducted. For example, disease outbreaks caused by thisStreptococcus agalactiaein tilapia mostly occur at temperatures above 26 ℃ which is a temperature range for optimum growth and survival of tilapia. However, high temperatures affect vaccine efficacy in fish (Mian et al., 2009; Wang et al.,2020). Wang et al. (2020) reported that vaccination of Nile tilapia againstS. agalactiaeand rearing them at high temperatures (29 and 33 ℃) arrested vaccine efficacy and resulted in suppressed immunity in fish. Similarly, the efficacy of vaccines in other fish species such as olive flounder, cat fish, Lump fish, and rainbow trout have been assessed under extreme temperature events (Erkinharju, Dalmo, Vågsnes, Hordvik, &Seternes, 2018; Kim, Kim, & Kim, 2020; Lange & Webster, 2017; Raida& Buchmann, 2008). However, Martins et al. (2011) observed no reduction in vaccine efficacy in Channel cat fish reared at high water temperatures (30 ℃) and recycling water temperatures (20–30 ℃). The discrepancy in results could be attributed to certain factors such as differences in species, culture conditions, type of vaccine, and method of vaccine administration.
4.10.Impact on the toxicity and uptake of environmental pollutants and adaptability to abiotic stress
High water temperatures lead to increased water vapor evaporation which in turn result in increased rainfall. The increase in rainfall patterns in certain locations increases the accumulation of heavy metals in aquatic environments as a result of increased resuspension of contaminated suspended sediments (Kibria, 2014). Todd et al. (2012) have previously reported direct correlations between increasing water temperatures coupled with changes in snowmelt timings and heavy metal toxicity in aquatic ecosystems of acidic mountain streams in the United States. And as such, organisms exposed to increasing water temperatures are more vulnerable to heavy metal toxicity which affects their survival.A recent study on grass carp by Li, Li, and Wu (2020) has shown that high temperatures can potentiate mercury (Hg2+) toxicity which in turn suppresses antioxidant enzymatic activities hence causing toxicological associated damages in fish. Waheed et al. (2020) have also shown that exposure of Nile tilapia to 34 ℃ increases its sensitivity to mercury chloride as indicated by reduced levels of antioxidant enzymes (i.e. SOD,CAT, and GPx) and significant injuries in the liver and kidney. In another study, Abdel-Tawwab and Wafeek (2017) investigated the effect of cadmium (Cd) on Nile tilapia under high water temperatures. The authors observed increased activity of hepatic enzymes in groups exposed to both Cd and high-water temperatures (32 ℃) thus indicating high Cd toxicity in fish. Jacquin et al. (2019) conducted an elegant study to investigate the synergistic effects of high temperatures and pesticide toxicity in gold fish(Carassius auratus). The authors observed that at high temperatures (32 ℃), there were stronger deleterious effects of pesticides on gills and liver structure of fish thus suggesting that high water temperatures could increase the negative effects of pesticides on freshwater species. In marine species such as grass shrimp, Breken-Folse et al.(1994) observed that exposing grass shrimp (Palaemonetesspp.) to high temperatures and organophosphates (OPs) suppressed their growth performance due to increased toxicity of OPs at high temperatures.Likewise, Pawar et al. (2020) reported increased toxicity of dimethoate(DMT) and chlorpyrifos (CPF) in whiteleg shrimp post-larvae and juveniles exposed to high rearing water temperatures of 34 ℃. As mentioned earlier, high water temperatures increase the metabolic rate of aquatic organisms. This enhances the water movement across the gills and thus increasing the uptake of environmental pollutants.
Adaptability to environmental stress is also influenced by temperature fluctuations in combination with environmental pollutants. Xenobiotics might cause a shift in the organism’s thermal tolerance making it more sensitive to temperatures close to its upper thermal limit. For example, Patra et al. (2007) reported that exposure of rainbow trout and western carp gudgeon to sublethal concentrations of endosulfan and chlorpyrifos reduces their critical thermal maximum 4.8 and 5.9 ℃(rainbow trout), 3.1 and 4.3 ℃ (western carp gudgeon) for endosulfan and chlorpyrifos respectively. A study by Tu et al. (2012) showed that antioxidant enzymatic activity of CAT in the liver is arrested in black tiger shrimp exposed to deltamethrin and reared under 34 ℃ and 25 ppt salinity. Moreover, acetylcholinesterase (AChE) activity in the muscles was strongly suppressed by deltamethrin at all tested temperatures (24,29, and 34 ℃) and salinity (15 and 25 ppt). Suppression of CAT activity and other antioxidant enzymes has been linked to increased concentrations of MDA in blood or serum which lowers the organism’s tolerance to heat stress (Jia et al., 2020; Liu et al., 2019b; Musa et al., 2017).
5.Mitigation of climate change effects in aquaculture:adaptation to hyper-thermal stress
5.1.Nutritional status
Feed quantity and quality are some of the widely used strategies in the adaptation of aquatic animals to changing water temperatures(Dawood et al., 2021b, 2021c, pp. 1–13). Exposure of fish to high temperatures has a direct impact on the metabolic rate. To satisfy this energy demand, fish must be in a good nutritional status to trigger stress tolerance against harsh environmental conditions. Research on the effect of feed supplements (i.e. probiotics, prebiotics, synbiotics, and medicinal plants) on the growth, growth performance, immunity, and survival of aquaculture species has recently gained momentum in aquaculture (Abarike et al., 2020; Mugwanya, Dawood, Kimera, &Sewilam, 2021b). And as such, several studies have been conducted to investigate the effects of several feed supplements on growth performance and immunity of several aquaculture species exposed to extreme temperature events (Abarike et al., 2020; Abass, Obirikorang, Campion,Edziyie, & Skov, 2018; Dawood et al., 2020; Ismail, 2019). Likewise,dietary supplementation of certain nutrients (i.e. vitamin C, E, selenium,amino acids, and polyunsaturated fatty acids), pigments (i.e. carotenoids and astaxanthin), essential oils (i.e. dietary oregano essential oil)microalgae, aquatic macroalgae, and algal extracts have been previously reported to curb temperature stress effects in aquaculture species(Cheng, Guo, Ye, & Wang, 2018; Corrˆea, Nobrega, Mattioni, Block, &Fracalossi, 2017; Camila Fernandes Corrˆea, Nobrega, Block, & Fracalossi, 2018; Elkatatny, El Nahas, Helal, Fahmy, & Tanekhy, 2020; Flores-Vergara, Cordero-Esquivel, Cer´on-Ortiz, & Arredondo-Vega, 2004;H´egaret et al., 2004; Ilham & Fotedar, 2016; Kamunde, Sappal, &Melegy, 2019; Liu et al., 2019b; Magouz et al., 2022; Norambuena,Rombenso, & Turchini, 2016). Table 1 shows the effect of several feed supplements on the growth performance and immune response of several aquaculture species reared under hyper-thermal stress conditions.
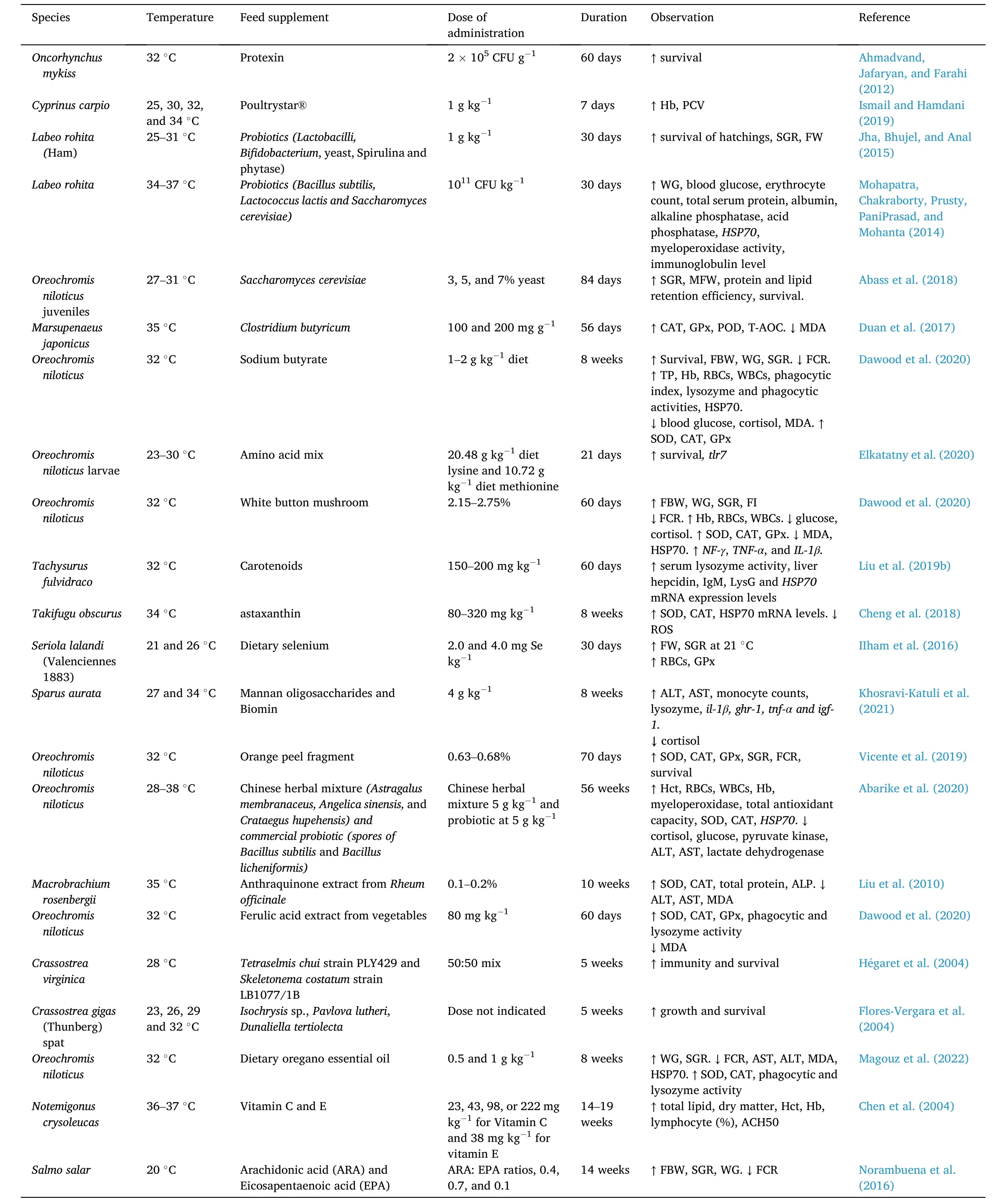
Table 1Effect of feed supplements on growth, survival, and immune response on several aquaculture species under heat stress.
5.2.Genetics, selective breeding, and aquaculture biotechnology
In every population, some individuals exhibit peculiar traits (physiological and phenotypical characteristics) compared to those expressed by other individuals of the same population. Thus, this means that within a given population, there might be individuals that respond better to environmental stress than others hence leading to natural selection. Early exposure of organisms to stress (acclimation) can sometimes amount to a plastic response: key changes in their animal physiology and phenotype to adapt to harsh conditions (Zambonino-Infante et al., 2013). Zambonino-Infante et al. (2013) noted an irreversible plastic response during early ontogenesis in the juvenile common sole (Solea solea) when exposed to warmer larval-rearing temperatures. There is a high likelihood of transgenerational plasticity since many desirable traits are acquired as a result of complex gene interactions and epigenetics.
Donelson, Munday, McCormick, and Pitcher (2012) were the first to observe complete amelioration of the negative effects of high temperatures in spiny damsel fish (Acanthochromis polyacanthus)when several generations were exposed to the same thermal stress. The spiny damsel fish is a model species used in the assessment of climate change effects in reeffish. Any temperature variations (1.5–3 ℃) within the range predicted by climate change models has highly significant effects on the reproduction, growth, and metabolic performance of this species(Munday, Kingsford, O’Callaghan, & Donelson, 2008; Rummer et al.,2014). In another study, Salinas and Munch (2012) found similar transgenerational plasticity inCyprinodon variegatusjuveniles whose parents were acclimated at high temperatures though these juveniles expressed a faster growth rate compared to those whose parents were acclimated to low temperatures. Shama, Strobel, Mark, and Wegner(2014) used a split-clutch design in which parents (Gasterosteus aculeatus) were acclimated to different thermal regimes and found a stronger maternal influence on the juveniles’ thermal adaptability. Moreover, the transgenerational plasticity was maintained for a longer period since F1 offspring exhibited similar thermal tolerances as their parents. Therefore, plastic responses during the early stages of the fish life cycle could trigger adaptive epigenetic responses necessary to confer stress tolerance and these could be inherited by succeeding generations.
Selective breeding programs for desirable traits are more practical in aquaculture and have been used to enhance global aquaculture production (N. H. Nguyen, 2016; Sardella, Cooper, Gonzalez, & Brauner,2004; Waldbusser, Bergschneider, & Green, 2010). In the commercial production of rainbow trout, farmers prefer culturing triploid species to prevent interbreeding of caged species with wild stocks. Additionally,female triploid species escape oocyte development which would channel all the metabolic energy from somatic growth to reproduction hence reducing flesh appearance and quality (Ojolick, Cusack, Benfey, & Kerr,1995). In salmonid aquaculture, triploid species are widely cultured mostly in Tasmania and Canada (Hansen et al., 2015). However, they are more sensitive to increasing water temperatures compared to diploid species. This is because at high temperatures, their metabolic rate declines which negatively impacts their fitness to tolerate hyper-thermal stress (Hansen et al., 2015; Ignatz et al., 2020b). Transferring stress tolerant genes into triploid species via genetic engineering could enhance production hence safe-guarding food security. However,several ethical considerations should be followed before releasing genetically modified aquaculture species on market for human consumption.
5.3.Aquaculture nanotechnology
In commercial aquaculture, feed additives and formulations are useful aspects of fish nutrition and growth. Nanoparticles can manipulate the feed flavor and color which increases feed intake (Sarkar et al.,2022). Moreover, the bioavailability of certain nutrients such as carotenoids and water-insoluble vitamins can be increased via an enhanced solubilization processing step with nanoparticles (Jampilek, Kos, &Kralova, 2019; Singha, Das, & Jha, 2017). Nutraceuticals such as Ubisol-Aqua™ (Vitamin A and B, squalene and fish oil [EPA/DHA], coenzyme Q10) ColloidAG Aqua (Nano silver), NannoCAL Aqua (Nano calcium), Fabgrow Aqua (Nano PUFA), NanoPHOS Aqua (Nano phosphorus), and Nanomin Aqua (Nano trace elements) are some of the aqua-feed Nano-based products available on market and used for improving fish growth (Sarkar et al., 2022). Dietary inclusion of green synthesized Nano-based feed additives in aquaculture under extreme temperature events and other abiotic and or biotic factors could be a sustainable approach in safe-guarding survival and productivity. For example, Kumar et al. (2020) investigated the mitigation potential of green synthesized selenium nanoparticles (Se-NPs) and ribo flavin (RF)against arsenic and hyper-thermal stress (34 ℃) inPangasianodon hypophthalmusfingerlings. The authors observed improved growth performance and immunity in fish supplemented with 5 mg kg−1RF and 0.5 mg kg−1Se-NPs. This concentration did not show any toxicity in fish hence safe for this fish species. Similarly, Kumar, Krishnani, and Singh(2018) also showed that dietary inclusion of green synthesized zinc nanoparticles at the rate of 10 mg kg−1not only enhances the growth performance and immunity ofP. hypophthalmusfingerlings but also reduces the bioaccumulation of lead (Pb) in muscle tissue of fish reared under hyper-thermal stress. Kumar, Krishnani, Gupta, and Singh (2017)also reported that dietary inclusion of 1 mg kg−1Se-NPs alone conferred protection against Pb and hyper-thermal stress inP. hypophthalmusfingerlings. In another study, Kumar, Singh, Bhushan, and Jamwal (2021)reported that dietary supplementation of green synthesized Se-NPs in combination with Omega-3-fatty acids (EPA and DHA) at the rate of 0.2 mg kg−1and 0.4% or 0.2% respectively, alleviates temperature stress,bacterial infection, and arsenic pollution effects inP. hypophthalmusfingerlings. Besides their application as nutraceuticals, nanoparticles could also be used as immunomodulators and vaccine delivery systems for the control of several viral and bacterial diseases in aquaculture(Nasr-Eldahan, Nabil-Adam, Shreadah, Maher, & El-Sayed Ali, 2021;Sarkar et al., 2022). Due to their small size similar to that of pathogens,nanoparticles can trigger cellular and humoral immune responses in aquaculture species (Angulo et al., 2021; Gheibi Hayat & Darroudi,2019; Nasr-Eldahan et al., 2021; Vinay et al., 2018). For instance, infectious pathogens which commonly spread under conditions of high-water temperatures such asVibriospp.,S. agalactiae, and infectious pancreatic necrosis virus (IPNV) can be effectively controlled via oral administration of Nano-vaccines. However, more studies are needed to understand the stability and immunomodulatory effects of Nano-vaccines under conditions of high-water temperature.
5.4.Bacteriophage therapy
With a recent ban on the use of antibiotics in aquaculture, one other alternative to control disease outbreaks in aquaculture amidst global warming is bacteriophage therapy (Kowalska et al., 2020; Seeley &Primrose, 1980; Silva et al., 2014). Bacteriophages (hereafter, phages)are viruses that infect and kill bacteria. Unlike antibiotics which are often broad-spectrum, phages infect and kill only the target bacteria(Peng, Borg, Dow, Pruitt, & Chen, 2020). Their mode of action differs from that of antibiotics thus able to act on bio films and even on bacterial species that have attained resistance against multiple antibiotics(Kowalska et al., 2020; Pal, 2015). Moreover, phages are known to coevolve with their target bacteria hence chances of bacterial resistance against them are extremely low (Kowalska et al., 2020). Interestingly,the development of bacterial-phage resistance is much slower in contrast to the development of antibiotic resistance. Likewise, phage-resistant bacteria do not have resistance to other phages possessing a similar target range (Ly-Chatain, 2014). The application of phages in aquatic environments to control bacterial diseases has already been demonstrated in several studies (Ghasemi et al., 2014; Imbeault, Parent,Lagac´e, Uhland, & Blais, 2006; Luo et al., 2018; Nakai & Park, 2002;Silva et al., 2014; Skurnik, Pajunen, & Kiljunen, 2007; Vinod et al.,2006). Nonetheless, the stability and efficiency of phages in aquatic environments are of great interest and this depends on several factors such as pH, temperature, salinity, formulation, stage of life of the host organism, dosage, time, and method of administration (Pal, 2015). Selection and isolation of phages that can maintain their stability and efficiency against bacterial pathogens in harsh aquatic environments are key in safe-guarding aquaculture productivity. Silva et al. (2014)investigated the influence of environmental variables (pH, temperature,salinity, and organic matter content) in the efficiency of phage therapy in aquaculture. The authors observed no significant effects in phage survival by variations in pH (6.5–7.4), temperature (10–25 ℃), and salinity (0–30 g L−1NaCl). However, the efficiency of phage therapy increased with increasing salinity and organic matter content. Tan et al.(2021) isolated and characterized sixVibrio parahaemolyticuslytic phages from seafood samples.In vitrostudies indicated that the stability and survival of phages (i.e., vp33, vp22, vp21, vp02, vp08, vp11) were not affected by temperatures; 20, 25, 37, and 50 ℃. However, at 70 ℃,all the phages were inactivated. Furthermore, the stability of the phages was maintained at a pH of 3–11. Stalin and Srinivasan (2017) also investigated the efficiency of potential phage cocktails againstVibrio harveyiand closely relatedVibriospecies isolated from shrimp aquaculture environment in the southeast coast of India. Results ofin vitrostudies indicated that the phage VVP1 was stable at temperatures 4–50 ℃ above which it was inactivated. The phages were able to maintain their stability at a pH of 2–11. A previous study by Luo et al.(2018) also showed that the bacteriophage HN48 that could potentially be used to controlS. agalactiaeinfections in Nile tilapia maintains its stability at temperatures below 60 ℃ and a pH range of 6–10. In another study, Ghasemi et al. (2014) reported that WWP-1 phage isolated fromLactococcus garvieaeremained stable at a temperature range of 15–30 ℃ hence suggesting its potential therapeutic use in the control ofL. garvieaeinfections in freshwater fish which occur at temperatures above 15 ℃.
Overall, the stability of certain phages at high temperatures and pH suggests their potential use under different environmental conditions in aquaculture. However, more studies are needed to compare the ef ficiency of phages under different methods of administration (i.e., injection, immersion, oral administration via feeds, and topical application)since few studies have so far been conducted. For instance, Li et al., 2016 compared different methods of phage administration (i.e. feeding, injection, and immersion) for the control ofVibrio cyclitrophicusin juvenile sea cucumbers (Holothuria edulis). The authors reported that feeding(phage freeze-dried powder) was more effective in the control ofV. cyclitrophicusfollowed by immersion and the least effective was injection. Khairnar, Raut, Chandekar, Sanmukh, and Paunikar (2013) reported that topical application was effective in the control of the antibiotic-resistant strains ofPseudomonas aeruginosain cat fish. However, Park, Shimamura, Fukunaga, Mori, and Nakai (2000) observed that oral administration of phages that targetPseudomonas plecoglossicidawas more effective in ayu fish (Plecoglossus altivelis). It is imperative to note, that determining the best method of phage administration under hyper-thermal stress and other abiotic factors is paramount for sustainable aquaculture production.
5.5.Management and aquaculture husbandry practices
5.5.1.Localized mitigation
Traditionally, inland aquaculture production has mainly been conducted in earthen ponds. Although ponds are exposed to the open environment, it is indeed possible to maintain good water quality parameters (i.e. temperature and DO) through protective cover and using aeration equipment to control levels of dissolved oxygen (Qayyum,Ayub, & Tabinda, 2005; Sultana, Haque, Salam, & Alam, 2017).Furthermore, recirculating aquaculture systems (RAS) could also be used in the production of sensitive aquaculture species at different stages of growth or manipulate rearing conditions to enhance an adaptive response (Abbink et al., 2011; Atse, Konan, Alla, & Pangini, 2009;Holan, Wold, & Leiknes, 2014; Ignatz et al., 2020b; Liu, Zheng, Fu,Simco, & Goudie, 2021). Similarly, indoor bio floc technology systems(BFT) could also be used to increase the production of endangered and highly sensitive aquaculture species (Azim & Little, 2008; Kamilya et al.,2017; Mugwanya, Dawood, Kimera, & Sewilam, 2021a; Van Doan et al.,2019). Just like RAS, rearing conditions in BFT systems can be manipulated to provide optimum conditions for the growth, survival, and intensive production of several aquaculture species. Several studies have shown improved survival, growth, growth performance and production of several species reared in BFT compared to those reared in RAS(Hisano, Barbosa, Hayd, & Mattioli, 2019; Luo et al., 2014; Nguyen et al., 2019; Vinatea et al., 2018). For the control of parasitic infections whose severity increases under increasing water temperatures, snorkel sea cage technology could be used to reduce sea louse (Lepeophtheirus salmonis) infestations in mariculture. Previous studies have shown a successful reduction of sea louse infestations in Atlantic salmon reared under snorkel sea cage technology (Geitung et al., 2019; Oppedal, Bui,Stien, Overton, & Dempster, 2019; Wright et al., 2017).
5.5.2.Polyculture
Polyculture of different aquaculture species comes with its benefits.One species could provide nutrients to the other species thus improving its tolerance and immunity against environmental stressors. For example, Anaya-Rosas, Rivas-Vega, Miranda-Baeza, Piña-Valdez, and Nieves-Soto (2019) demonstrated that co-culture of marine algae (Gracilaria vermiculophylla, Dictyota dichotoma, andUlva lactuca)with whiteleg shrimp provided the necessary nutrients which enhanced growth, survival, and immunity of shrimp under infection challenge withVibrio parahaemolyticus. Fern´andez, Leal, and Henríquez (2019) has also shown that co-culturing macroalgae with shell-forming molluscs improved calcification and other threatened physiological processes of calcifying organisms. This is because the macroalgae reduced excess carbon dioxide (CO2) and seawater pH which hinder calcification.Macrophytes (seaweeds) could also be beneficial in the mitigation of climate change-induced water temperature increase in aquaculture. This is because macrophytes could increase the concentrations of DO,sequester carbon and increase water pH. However, producers should put into consideration the seaweed species, nutrient load, culture system and water flow, irradiance, latitude, and production timing when planning the addition of macrophytes (Reid et al., 2019). Willis et al.(2017) has previously shown that macrophytes lower the water temperatures in streams throughout the Western USA. Co-culturing these macrophytes in traditional aquaculture production systems such as ponds could provide suitable conditions required by fish to survive.However, some macrophytes negatively affect fish swimming, lower concentrations of DO, and shifts in water pH thus choice of species matters. A methodological approach for choosing floating macrophytes for ecological intensification of small-scale fish farming in tropical areas has been demonstrated by Slembrouk et al. (2018).
5.5.3.Site relocation
According to FAO (2020), the inclusion of climate change and other risks into strategic planning and aquaculture zoning has been suggested for implementation as a mitigation factor against climate change effects.Relocating to regions that have been less impacted by climate change effects is a reasonable adaptation. Remote sensing tools such as GIS have been used to locate potential aquaculture sites or regions that would be suitable for aquaculture production (Reid et al., 2019). Relocating to regions with deeper ponds could offer protection against high water temperatures (the deeper the pond, the cooler the water) as well as provide a huge DO reservoir. Furthermore, relocating would break the disease cycle since new locations might not have newly emerged pathogens as a result of increasing water temperatures. However, site relocation might also be expensive and detrimental to certain life stages of fish. For example, B´aez, Aigo, and Cussac (2011) have advocated for separate site locations broodstock and grow-out of rainbow trout due to their different sensitivity to increasing temperature.
5.6.Climate-smart systems for epidemiological forecasting
Providing timely and real-time information on disastrous conditions as well as forecasting early warnings of future environmental harsh conditions promotes strategic planning and efficient management responses. The use of smart technology can provide aquaculturists with real-time information on water quality before the onset of clinical symptoms in susceptible aquaculture species. Currently, ocean condition monitoring networks such as Northwest Association of Networked Ocean Observing Systems and Integrated Ocean Observing System (Reid et al., 2019) are available to offer nearly real-time monitoring and information on the state of water quality in oceans. This enables aquaculturists to make prompt decisions on management strategies to counteract possible outcomes of reduced water quality.
Since increasing water temperatures have resulted in the emergence of exotic pathogens and new disease outbreaks, there is a need to develop epidemiology and risk assessment models that will help identify pathogens whose threat will intensify under established climate change scenarios. For example, in the United Kingdom, a risk assessment model developed for freshwater species has been applied and hence, the emergence of diseases caused by exotic pathogens such asAnphanomyces invadans and L. garvieae has been predicted to occur in the future as a result of increasing water temperatures (Marcos-L´opez, Gale, Oidtmann,& Peeler, 2010; Peeler & Taylor, 2011). Overall, such epidemiological predictions alert aquaculturists of future disease outbreaks which permits the necessary preparations required to minimize production losses.
6.Conclusion
Research on the effect of climate change on aquaculture and fisheries management is still in its infancy despite its devastating consequences on aquatic ecosystems. Both freshwater and marine species will be affected with more impact directed towards the latter due to the declining water quality caused by global warming. There is a chance for freshwater species to survive the changing toxic environment, but this will depend on the upper thermal limit of the organism. Likewise,coldwater and warm water species will most likely respond differently to increasing water temperatures. There is a high likelihood for the former to experience significant survival and growth challenges due to tissue damage and lower feed intake at temperatures beyond their thermal threshold. Therefore, aquaculturists must develop strategic risk plans for endangered species to prevent significant losses. Furthermore, there is a dire need for epidemiologists to keep track of the changing aquatic environment and predict the possibility of emerging alien pathogens and disease outbreaks. Environmentalists too should address the concerning issues of water pollution and their associated impacts on aquatic biodiversity failure of which, future production trends will be affected.On-going research is necessary particularly regarding feed supplements and their interaction with the immune system. However, more focus should be put on the effect of feed supplements on the survival, performance, and immunity of aquaculture species under a combination of environmental stress factors since ectotherms are exposed to concurrently multiple biotic and abiotic stress factors. Concerning the management of aquaculture disease outbreaks via administration of Nanovaccines and phage therapy, more studies are needed to elucidate their efficiency under high water temperatures. Moreover, the methods,doses, time, and duration of administration should be considered to determine the optimum conditions and concentrations that will confer better protection to aquaculture species under hyper-thermal stress.Lastly, there is an urgent need for governments, civil society, and international organizations to sensitize the public on the effects of climate change in the context of global warming and mitigation strategies for sustainable aquaculture productivity.
 Aquaculture and Fisheries2022年3期
Aquaculture and Fisheries2022年3期
- Aquaculture and Fisheries的其它文章
- Recent advances in application of moving bed bioreactors for wastewater treatment from recirculating aquaculture systems: A review
- Characterization and expression analysis of gonad specific igf3 in the medaka ovary
- Liver DNA methylation and transcriptome between 1- and 3-year-old grass carp
- Screening and validation of reference genes for qPCR analysis in gonads and embryos of Takifugu bimaculatus
- Assessment of genetic diversity, detection of strain-specific single nucleotide polymorphisms and identification of the Bangladesh and Vietnam strain of Channa striata by PCR-RFLP analysis of the mitochondrial COI gene fragment
- Assessment of dietary chaste tree (Vitex agnus-castus) fruit extract on growth performance, hemato-biochemical parameters, and mRNA levels of growth and appetite-related genes in gold fish (Carassius auratus)
