Recent advances in research on natural product inhibitors of SREBPs
Yi-Ping Zhang,Jing Jin,Ping Li,Hua Yang*,Zu-Guo Zheng*
1State Key Laboratory of Natural Medicines,Department of Pharmacognosy,School of Traditional Chinese Pharmacy,China Pharmaceutical University,Nanjing 210009,China.
Abstract Sterol regulatory element-binding proteins(SREBPs)are a class of cholesterol-sensitive transcription factors that play important roles in lipid metabolism.Natural product inhibitors of SREBPs have been shown to inhibit the synthesis of free fatty acids and cholesterol,promote the burning of brown fat,and relieve insulin resistance by inhibiting different links during the synthesis,cleavage,and transport of SREBPs,thereby improving obesity,diabetes,atherosclerosis,and other metabolic diseases and disorders.There are numerous Chinese herbal medicines with verified efficacy in the treatment of metabolic diseases,including Coptis chinensis Franch.and Pueraria peduncularis Grah.for diabetes,Pueraria peduncularis Grah.,Epimedium brevicornum Maxim.,and Panax ginseng C.A.Meyer for osteoporosis,and Nelumbo nucifera Gaertn.and Poria cocos(Schw.)Wolf.for obesity.Present-day research on the mechanisms underlying the activity of traditional Chinese medicine has indicated that certain chemical components of these traditional preparations can be used to treat metabolic diseases by inhibiting SREBPs.Progress in the research on natural product SREBP inhibitors is continuing apace,and the mechanisms of action of certain small molecules have become well established.In this study,we review recent progress in the research on natural small-molecule inhibitors of SREBPs,including flavonoids,saponins,triterpenoids,and alkaloids,which we hope will provide a useful reference for future research and development of drugs for the treatment of metabolic diseases.
Keywords:sterol-regulatory element-binding protein;lipid metabolism;metabolic disease;natural product;traditional Chinese medicine
Background
Sterol regulatory element-binding proteins(SREBPs),which play pivotal roles in lipid metabolism,are endoplasmic reticulum(ER)-bound transcription factors that control the expression of genes essential for lipid synthesis and uptake[1].Aberrant SREBPs activity has been linked to a range of metabolic diseases,including obesity,hepatic steatosis,insulin resistance,hyperlipidemia,atherosclerosis,and tumors[1-3].Given that such metabolic diseases are an important risk factor for the development of type 2 diabetes,cardiovascular diseases,and all-cause morbidity and mortality[4].The inactivation of SREBPs is considered a potential therapeutic approach towards treating these diseases.
However,despite the promise of this strategy,the currently reported SREBP inhibitors tend to have low specificity,and in addition to target SREBP pathways,these compounds may inadvertently affect other signaling pathways.For example,whereas 25-hydroxycholesterol inhibits SREBP activity,it may also activate liver X-activated receptors(LXR)transcriptional activity[5].Chinese herbal medicines constitute an extensive treasure house of lead compounds,comprising numerous natural small molecules with good biocompatibility,diverse structure,and valued medicinal properties.Traditional Chinese medicine has a long history of treatment of SREBP-related metabolic diseases stretching back thousands of years and is particularly noted for the mild durable preventative treatment and overall regulation of complications[6].Moreover,compared with chemically synthesized SREBP inhibitors,natural small-molecule inhibitors generally have fewer side effects.Consequently,it is highly desirable to identify novel natural product inhibitors of SREBPs from Chinese herbal medicines to enable the development of new therapeutic strategies for metabolic diseases.
Traditional Chinese medicinal preparations have widely acknowledged advantages in the clinical treatment of metabolic diseases,particularly with respect to the protection of important tissue and organ injuries.A notable example isFolium nelumbinis,derived from the dried leaves ofNelumbo nuciferaGaertn.,which is widely planted in Southeast Asia[7].In numerous ancient texts,it is recorded that this preparation can be effectively used to treat obesity,and indeed,modern medicine has provided evidence to indicate thatFolium nelumbiniscan be used to reduce blood lipid levels and contribute to weight loss.As recorded in theSupplement to Materia Medica,written in the Han Dynasty(202 B.C.E.-220 C.E.),Chen Zangqi believed that the consumption ofFolium nelumbinisfor a prolonged period would aid weight loss[8],and similarly,in the Ming Dynasty(1368 C.E.-1644 C.E.),Dai Yuanli recorded in theSecret Tips for Diagnosisthat consumingFolium nelumbiniswould contribute to the loss of weight[9].Modern pharmacological studies have indeed provided evidence to indicate that the total alkaloids inFolium nelumbinisplay roles in reducing blood lipid contents[10].A further notable example isRhizoma Coptidis,the dried rhizomes ofCoptis chinensisFranch.,Coptis deltoideaC.Y.Cheng et Hsiao,andCoptis teetaWall.Since ancient times,a large number of documents have recorded thatRhizoma Coptidiscan be used to treat diabetes.The earliest documentary reference in this regard can be found in theSupplementary Records of Famous Physiciansby Tao Hongjing compiled in the Wei and Jin Dynasties(220 C.E.-420 C.E.)[11].Similarly,theCompendium of Materia Medica,a Chinese herbology volume written by Li Shizhen during the Ming Dynasty(1368 C.E.-1644 C.E.),records thatRhizoma Coptidiscan be used to treat diabetic polyuria[12].In more recent times,berberine,the active component ofRhizoma Coptidis,has been demonstrated to reduce blood lipid levels by inhibiting SREBPs[13].
Traditional Chinese medicines constitute a rich source of natural products,many of which have been recorded in ancient texts and used to successfully treat metabolic diseases such as obesity and diabetes and disorders of glucose and lipid metabolism that have more recently been established to be closely associated with SREBPs.In this review,we summarize recent progress in research that has focused on the natural product inhibitors of SREBPs,thereby providing a reference for the development of drugs that can be used to treat metabolic diseases.Although several reviews have previously covered the classification and pharmacological effects of SREBP inhibitors,these have tended to focus on the inhibitors of SREBPs from the perspective of signal pathway classification.In this review,we instead describe the structure of natural compounds to provide an insight into the structural characteristics of inhibitors and examine the functions of their natural product analogues[14].In contrast to other review articles that have focused exclusively on progress in the pharmacological characterization of SREBP inhibitors,we also provide detailed descriptions of the mechanism of action of different natural small-molecule inhibitors,which we believe will provide a useful reference for those engaged in research on the medicinal properties of SREBP inhibitors[15,16].More importantly,we highlight the study of Chinese herbal medicines,and for each of the described inhibitors,we provide information on herbal source material.Accordingly,in this review,we provide a comprehensive introduction to natural product SREBP inhibitors,covering SREBP signaling pathways,and the origins,chemical structures,mechanisms of action,and target sites of these inhibitors.We hope that this information will provide a broad overview of recent progress in the related research,thereby stimulating further interest in these natural product SREBP inhibitors and promoting the development and therapeutic application of associated drugs.
SREBP signal pathways
SREBPs are members of the basic helix-loop-helix-leucine zipper family of transcription factors that play roles in the regulation of cholesterol absorption and fatty acid and triglyceride synthesis[17].Structurally,they consist of cytosolic NH2-terminal and COOHterminal domains separated by two transmembrane-spanning sequences and a short loop that projects into the lumen of the ER[18].In humans,the three recognized isoforms of SREBPs are encoded by the two genes sterol-regulatory element-binding transcription factor 1 and sterol-regulatory element-binding transcription factor 2.Among the different isoforms,SREBP-1a has been identified as a more potent transcription activator than SREBP-1c,which can be attributed to the longer transactivation domain in the N-terminal region.SREBP-1 is mainly distributed in the liver and pronephros,whereas SREBP-2 is more widely expressed[19,20].It has been reported that SREBP-1 is involved in energy metabolism,including fatty acid and glucose/insulin metabolism,whereas SREBP-2 appears to be specifically associated with cholesterol synthesis[21].SREBP cleavage-activating protein(SCAP),a receptor for intracellular sterol content located on the ER membrane,and insulin-induced genes(Insigs),which are intrinsic ER proteins comprising six transmembrane helical domains[22],have been shown to play roles in the regulation of SREBPs.
Activation of SREBPs is regulated by a negative feedback loop,in which sterols bind to SCAP or Insigs,thereby resulting in the sequestration of the SREBPs-SCAP-Insigs complex within the ER[23].In the absence of sterol cells,SCAP promotes the incorporation of SREBPs into the membrane of protein complex II(COPII)vesicles,thereby facilitating the transport of SREBPs from ER to the Golgi apparatus[24].Following the subsequent cleavage of full-length SREBPs via the activities of site 1 and site 2 proteases in the Golgi apparatus,SREBPs can be transferred to the nucleus,wherein they stimulate the transcription of genes involved in the synthesis and absorption of cholesterol and fatty acids by binding to sterol regulatory elements(SRE)[21,25,26].When cholesterol accumulates to a threshold level on the ER membrane,it binds to complexed SCAP and Insig,which has the effect of blocking the transport of the SCAP-SREBP complex to the Golgi apparatus[27].Furthermore,SREBP-2 has been shown to bind to the SRE of the lowdensity lipoprotein receptor(LDLR)promoter to enhance gene expression and the uptake of low-density lipoprotein cholesterol.In response to cholesterol depletion,protein phosphatase 2A promotes the binding of SREBP-2 to the LDLR promoter.Consequently,the phosphatase involved in regulating SREBP-2 is considered a novel pharmacological target for the treatment of hypercholesteremia[28].
The promoter of SREBP-1c genes has been found to contain response elements for insulin,glucagon,and LXR[20],and recent studies have revealed the presence of a phosphorylated sequence in the SREBP-1a protein that serves as a recognition motif for the complex of SKP1,CUL1 and F-box protein-F-box and WD repeat domain-containing 7 ubiquitin ligase.Fbw7 interacts with nuclear SREBP-1a and enhances its ubiquitination in a glycogen synthase kinase 3 phosphorylation-dependent manner at the T426 and S430 sites,and nuclear SREBP-1 and SREBP-2 are degraded via the Fbw7-dependent pathway[29].The adenosine 5ʹ-monophosphate(AMP)-activated protein kinase α(AMPKα)subunit binds preferentially to and phosphorylates SREBP-1c and SREBP-2,with the Ser372 residue of SREBP-1c serving as the main site of AMPK phosphorylation[30].miR-33 is an intronic microRNA encoding SREBP loci[31],and sequence prediction analysis and mechanistic studies have indicated that miR-33a targets mRNAs encoding adenosine triphosphate(ATP)-binding cassette transporters A1(ABCA1)and G1(ABCG1),both of which are ATP-dependent membrane cholesterol transporters that function in the control of cholesterol efflux.These observations thus indicate that miR-33a plays a key role in balancing the levels of intracellular cholesterol via mediating the co-expression of SREBP-2[32].
SREBPs are regulated via a range of signaling pathways,among which hepatic insulin signaling has been shown to be necessary for the accumulation of nuclear SREBP-1c protein.In this pathway,insulin activates mechanistic target of rapamycin complex 1(mTORC1)via the Akt-mediated phosphorylation of Thr308 and Ser473 residues[33].In response to insulin stimulation,activated Akt can also phosphorylate SREBP-1,thereby promoting an association with COPII vesicles and subsequent transport to the Golgi apparatus[34].In contrast,insulin signaling appears to have little effect on the interaction between SREBP-2 and COPII vesicles.In sebaceous cells,insulin-like growth factor-1 has been demonstrated to enhance the mRNA and protein expression of SREBP-1 in a phosphoinositide 3-kinase(PI3K)-dependent manner,thereby promoting a corresponding up-regulated expression of the SREBP-1 target genes.It has been established that the PI3K-AKT-mTORC1-p70S6K signaling pathway drives the feed-forward expression of SREBP-1c[35],whereas insulin-like growth factor-1 has also been shown to induce the transport of SCAP from the ER to Golgi apparatus,which is inhibited by PI3K and dominant negative Akt.Although this work has tended to focus on SREBP-2,these findings can be extended to SREBP-1a/c,given that SCAP mediates the conveyance of all SREBP subtypes to the Golgi.This is indeed highlighted by observations of the reduced expression of SREBP-1c target genes following PI3K/Akt inhibition[36].Furthermore,it has been found that mTORC1 promotes the retention of Lipin-1,a phosphatidic acid phosphatase,within the cytoplasm,thereby inducing an up-regulated expression of SREBP-2[25].In addition,Lin28A/B has been observed to enhance the translation and maturation of SREBP-1,and it protects cancer cells from lipotoxicity.Conversely,Lin28A/B-stimulated tumor growth is abrogated by the inhibition of SREBP-1 and impairment of the RNA-binding properties of Lin28A/B[20].
In further studies,the overexpression of SCAP has been demonstrated to increase the formation and size of lipid drops[37],whereas in contrast,a deficiency in SCAP was found to prevent steatosis in mice fed high-fat diets.Moreover,in animals,it has been established that the SCAP/SREBP pathway is essential for the development of diabetic fatty liver and carbohydrate-induced hypertriglyceridemia.Furthermore,studies have indicated that whereas specific knockout of SREBP-2 results in embryonic lethality,a proportion of fetuses survive following the deletion of SREBP-1a,and a lack of SREBP-1c appears to be of little consequence[38].However,knockout mice deficient in all SREBP types die at an early stage of embryonic development.The overexpression of SREBP-1c in mouse liver has,however,been found to promote the activation of transcription factors and genes related to triglyceride synthesis[39],and similarly,overexpression of the minor nSREBP-1a isoform in mouse liver induces the activation of genes involved in both fatty acid and cholesterol biosynthesis.As a consequence of such enhancements inde novolipogenesis,mice develop fatty liver.
Metabolic disease,a cluster of metabolic abnormalities linked to insulin resistance and abdominal obesity,is associated with an increased risk of type II diabetes mellitus and cardiovascular disease[40],and given the estimation that approximately 20% to 25% of the world’s adult population are afflicted by such abnormalities,it is thus considered essential to identify the underlying pathophysiologies and seek new pharmacological targets(Figure 1)[41].
Classification of natural product SREBP inhibitors
To date,scientific and systematic quality evaluations of traditional Chinese medicine have generally focused on chemical components.However,more recently there has been an increasing tendency to explore the natural products of traditional Chinese medicine,which is accordingly beginning to reveal the considerable potential of natural products in the treatment of a diverse range of diseases.Notable in this regard are the small-molecule natural product inhibitors of SREBP,which,as described in the preceding section,have been reported to be associated with the development of metabolic diseases.In this section,we describe some of the different classes of natural product inhibitors of SREBPs,primarily saponins,flavonoids,alkaloids,and terpenoids(Figure 2,Table 1).
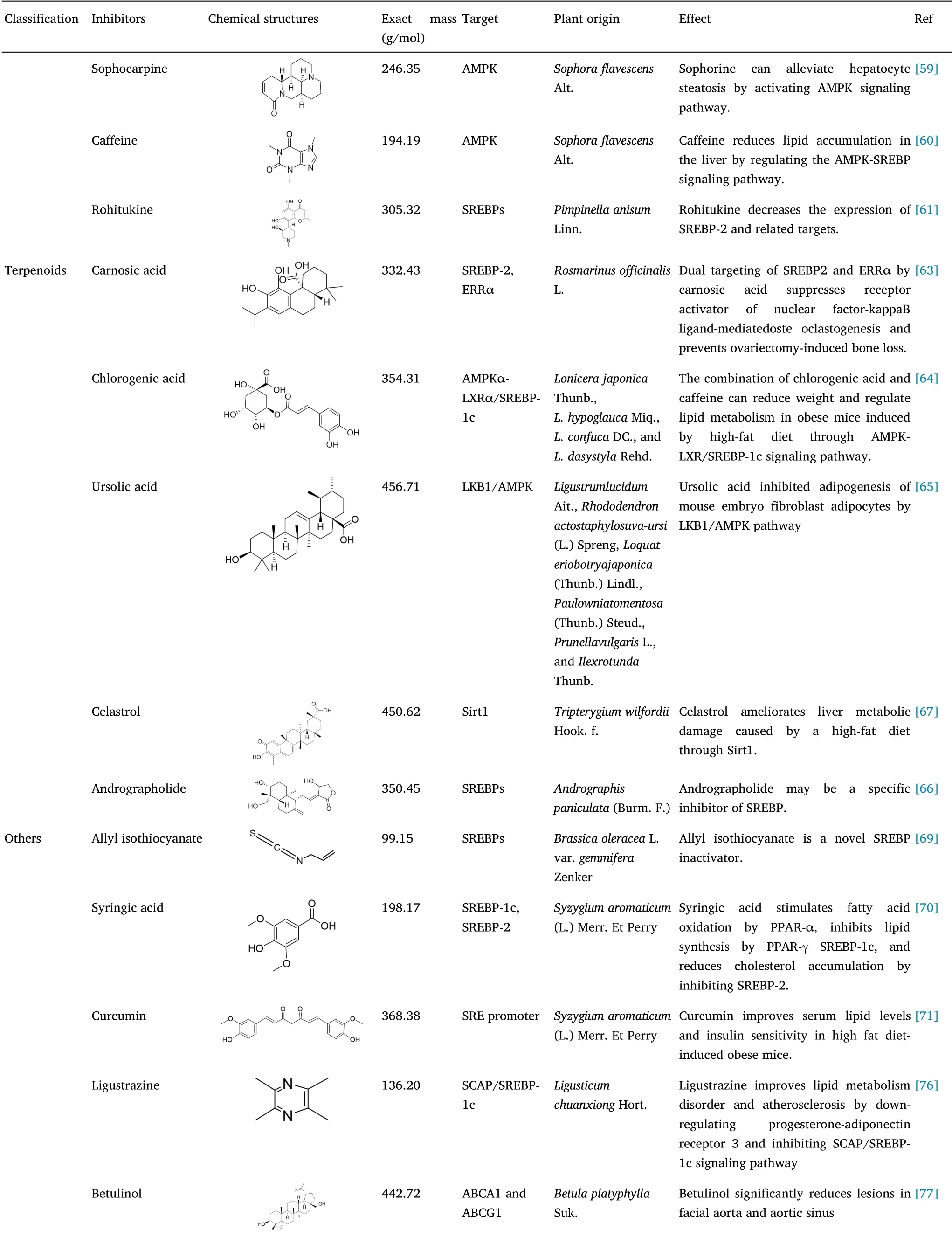
Table 1 Classification of small molecule inhibitors of natural products of SREBPs(Continued)
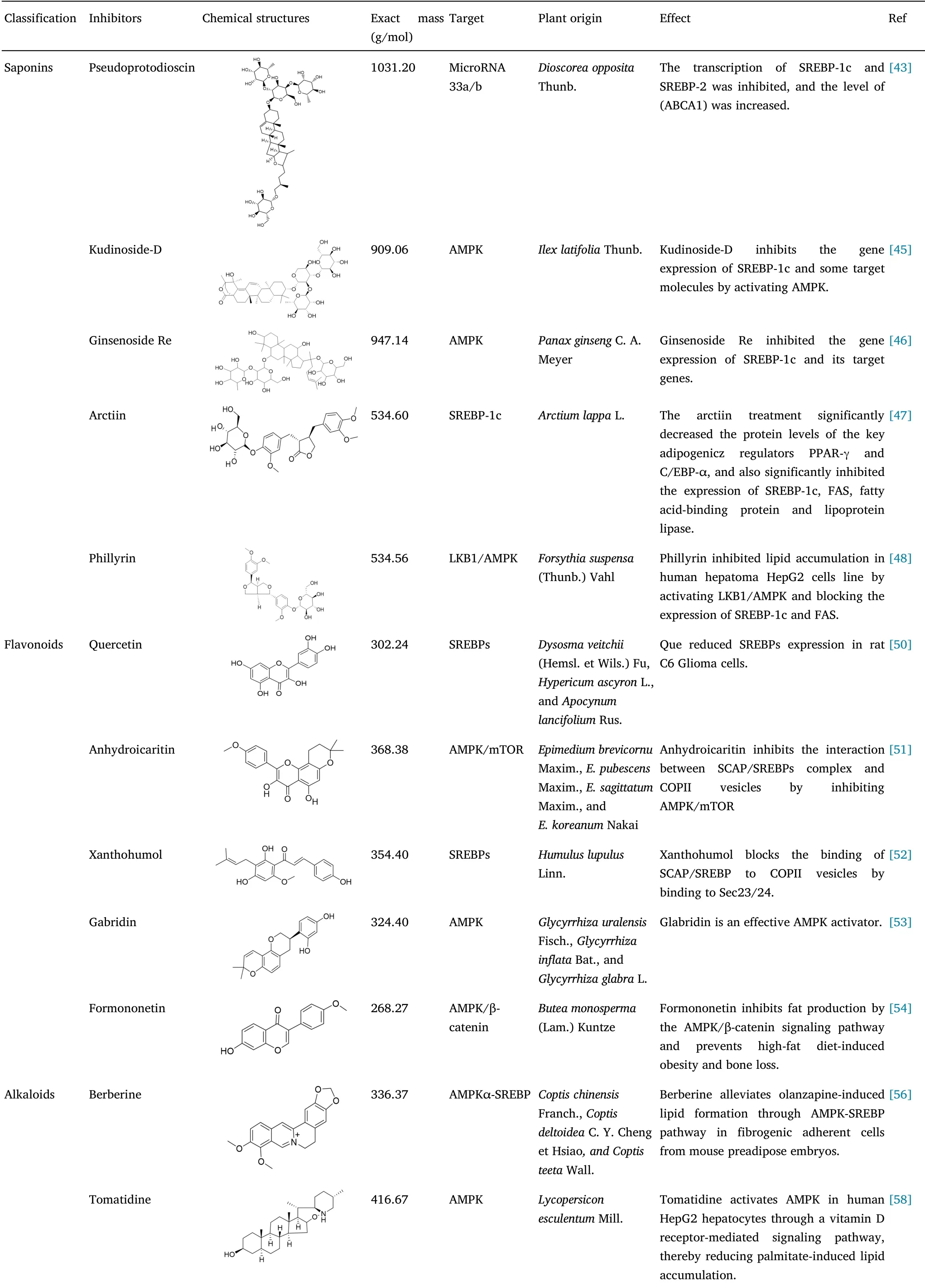
Table 1 Classification of small molecule inhibitors of natural products of SREBPs
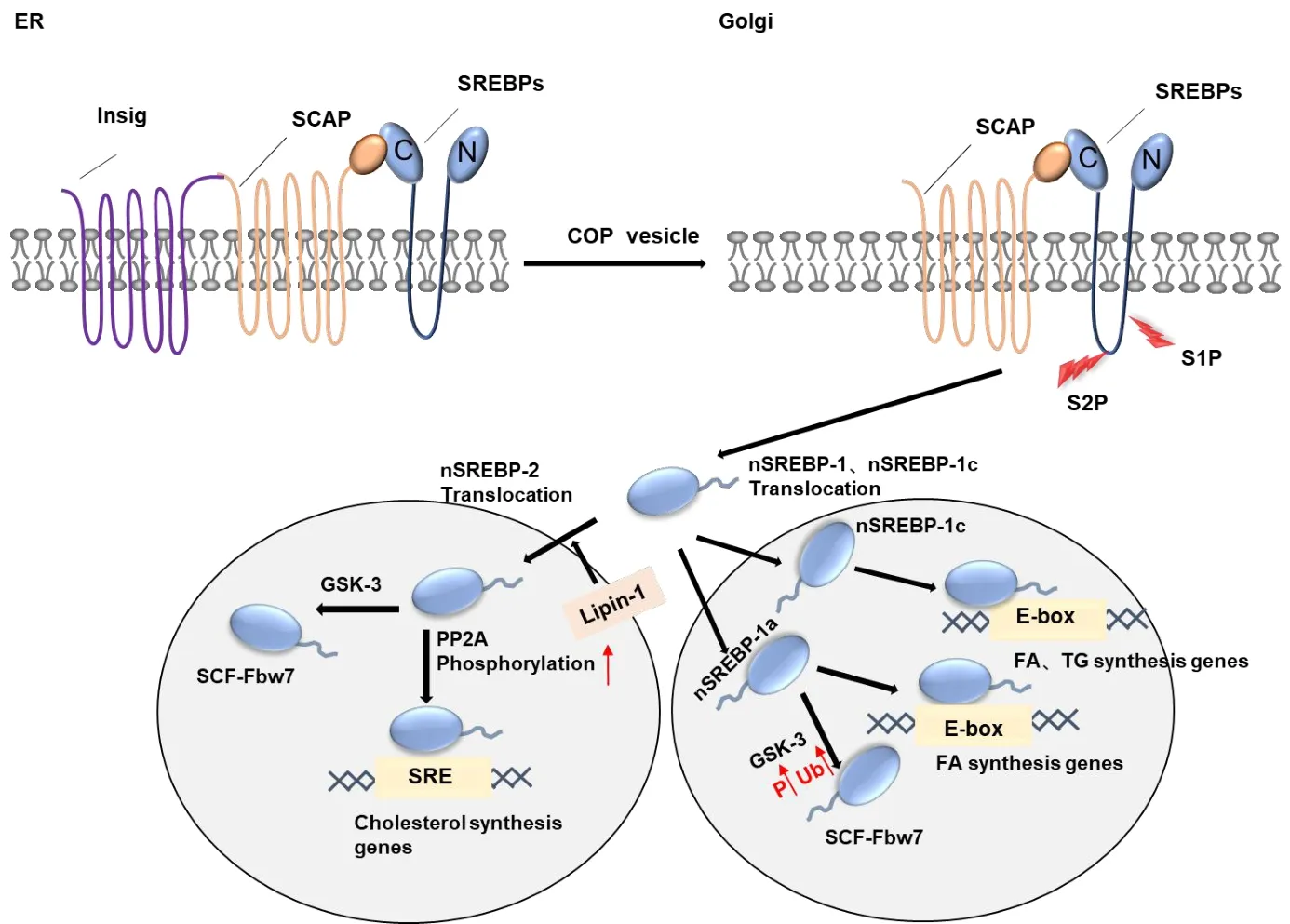
Figure 1 The SREBP signaling pathway.ER,endoplasmic reticulum;Insigs,insulin-induced genes;SCAP,SREBP cleavage-activating protein;SREBPs,sterol regulatory element-binding proteins;COP,complex;Golgi,Golgi apparatus;S1P,site 1 protease;S2P,site 2 protease;GSK-3,glycogen synthase kinase 3;SCF-Fbw7,the complex of SKP1,CUL1 and F-box protein-F-box and WD repeat domain-containing 7;PP2A,protein phosphatase 2A;SRE,sterol-regulatory element;FA,fatty acid;TG,triglyceride.
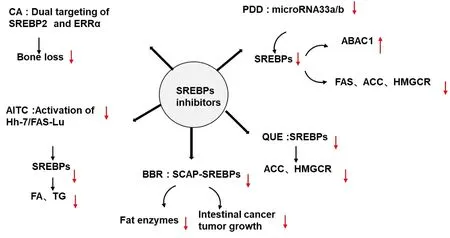
Figure 2 Classification of SREBP inhibitors.CA,carnosic acid;SREBPs,sterol regulatory element-binding proteins;ERRα,estrogen-related receptor α;AITC,allyl isothiocyanate;FAS,fatty acid synthase;FA,fatty acid;TG,triglyceride;BBR,berberine;SCAP,SREBP cleavage-activating protein;PDD,pseudoprotodioscin;ABCA1,adenosine triphosphate-binding cassette transporters A1;ACC,acetyl-CoA carboxylase;HMGCR,3-hydroxy-3-methylglutaryl-CoA reductase;QUE,quercetin.
Saponins
Saponins are a class of glycosides,the aglycones of which are triterpenes or helicosteranes,and have been identified as the main active constituents in numerous traditional Chinese medicines,includingRadix Ginseng,Platycodon grandiflorus(Jacq.)A.DC.,Glycyrrhiza uralensisFisch.,andBupleurum chinenseDC.A 70%ethanol extract of the rhizomes ofDioscorea zingiberensisC.H.Wright has been found to have therapeutic efficacy in the treatment of cardiovascular disease,and steroidal saponins,among which pseudoprotodioscin has anti-inflammatory and anti-lipogenesis capacities[42],have been shown to have considerable therapeutic potential.Pseudodiosgenin,which has been extracted from the rhizomes ofDioscorea panthaicaPramn et Burk,inhibits SREBP-1c and SREBP-2 transcription by reducing the levels of microRNA 33a/b,thereby down-regulating the expression of genes involved in cholesterol and triglyceride synthesis[43].Ilex latifoliaThunb.is noted for its weight reduction and anti-diabetic properties,and crude preparations of triterpenoid saponins extracted from the leaves of this plant by boiling in water for 2 h have been observed to improve insulin resistance and liver injury in mice fed a high-fat diet.Studies have revealed that crude triterpenoid saponins activates AMPKα phosphorylation,reduces the expression of SREBP-1c,fatty acid synthase(FAS),stearoyl-CoA desaturase(SCD1),and acetyl-CoA carboxylase(ACC),and also inhibits the cholesterol biosynthesisassociated expression of SREBP-2.Moreover,by promoting the upregulated activity of AMPKα and inhibiting SREBPs and target gene transcription,crude triterpenoid saponins can also contribute to inhibit fat synthesis and improve insulin resistance and non-alcoholic fatty liver disease in high-fat diet-fed mice[44].
Kudinoside-D,identified as the main natural constituent of crude triterpenoid saponins,has been shown to inhibit the accumulation of lipids in mouse embryo fibroblast adipocytes,mainly by inhibiting peroxisomeproliferator-activatedreceptor(PPAR)-γ,CCAAT/enhancer-binding protein(C/EBP)-α,SREBP-1c,and their respective target genes[45].Ginsenoside Re fromPanax ginsengC.A.Meyer,a protopanaxatriol-type saponin with anti-diabetic and hypolipidemic properties,has been found to inhibit the expression of SREBP-1c and its target genes by stimulating AMPK phosphorylation,thereby eliminating the excess accumulation of fat in the liver of C57BL/6J mice[46].Arctiin,extracted fromArctium lappaL.,is characterized by a range of biological activities,including anti-tumor and anti-inflammatory effects,and in recent years has been found to dose-dependently inhibit adipogenesis in fibrogenic adherent cells derived from mouse pre-adipose embryos.Treatment based on arctiin is associated with a significant inhibition of SREBP-1c and lipase expression and also promotes increases in the phosphorylation of AMPK and its downstream target protein ACC.Accordingly,these findings would tend to indicate that arctiin may have potential therapeutic application in the prevention of obesity[47].Phillyrin,extracted fromForsythia suspensa(Thunb.)Vahl.,has similar antiobesity effects in vivo by inhibiting the accumulation of lipids induced by high glucose in human hepatocellular carcinoma(HepG2)cells.These effects appear to involve the strong inhibition of FAS expression induced by high glucose,along with regulation of SREBP-1c activation.This phillyrin-mediated inhibition of SREBP-1c occurs via AMPKα-mediated phosphorylation of the Thr172 residue,which in turn causes inactivation of ACC as a consequence of the phosphorylation at Ser79,thereby inhibiting the proximal and ratelimiting steps of adipogenesis.It is also predicted that a reduction in synthesis of the ACC product malonyl coenzyme A also contributes to reducing the inhibition of carnitine palmityl transferase 1,thereby promoting an increase in the oxidation of fatty acids.It is believed that the effects attributed to phillyrin may contribute to the inhibition of lipid accumulation observed in human HepG2 hepatocytes[48].
The nuclear transcription factor SREBP-2 is a major regulator of cholesterol metabolism and is also involved in osteoclast formation.Studies have shown that astragaloside IV derived fromAstragalus membranaceus(Fisch.)Bunge.can contribute to a reduction in insulin resistance and the accumulation of lipids in HepG2 cells by regulating the AMPK-dependent phosphorylation of SREBP-1c at the Ser372 residue,thereby indicating the potential application of this saponin as a drug for the treatment of hepatic steatosis[49].SREBPs are key transcription factors implicated in the regulation of liver lipid metabolism,and a number of saponins can be used as inhibitors of SREBP pathways for the treatment of metabolic diseases such as insulin resistance and obesity.Insulin resistance is associated with an increase in free fatty acids in the liver,with excess fatty acids being converted to triglycerides,which are stored within the cytoplasm,leading to steatosis.Insulin resistance affects SREBP-1c activity and further increases liver steatosis and plays a unique role in the dynamic balance of liver lipids and glucose metabolism[23].In this context,a notable therapeutic limitation of the SREBP inhibitors applied to improve liver steatosis is that SREBP-1c has both hypoglycemic-and hypotensive-associated effects.Therefore,related pharmacological approaches need to be carefully assessed in order to identify and suitably address the likelihood of any adverse effects.
Flavonoids
Flavonoids are the most abundant phenolic compounds in plants,with proven antioxidant,anti-inflammatory,and immunomodulatory properties[2],among which quercetin,an antioxidative flavonoid extracted fromDysosma veitchii(Hemsl.et Wils.)Fu,Hypericum ascyronL.,andApocynum lancifoliumRus.,is widely found in items of the human diet.A study examining the effects of quercetin on lipid synthesis in C6 glioma cells revealed that quercetin acts at the transcription level and reduces the mRNA abundance and protein contents of ACC1 and 3-hydroxy-3-methylglutaryl-CoA reductase(HMGCR).Quercetin has also been shown to reduce the expression of SREBP1 and SREBP-2,which play pivotal roles as regulators of thede novosynthesis of fatty acids and cholesterol,respectively.Accordingly,this flavonoid has a direct and rapid down-regulatory effect on the synthesis of cholesterol and new fatty acids in C6 cells[50].A further flavonoid,anhydroicaritin,extracted fromEpimedium brevicornuMaxim.,Epimedium pubescensMaxim.,Epimedium sagittatumMaxim.,andEpimedium koreanumNakai,has been characterized as an inhibitor of the interaction between SCAP/SREBP complexes and COPⅡvesicles by suppressing the activity of AMPK/mTOR,which is considered to be unrelated to the phosphorylation of SREBP-1 at Ser372,thereby indicating the likelihood of alternative phosphorylation sites in SREBPs associated with the binding affinity for COPⅡvesicles.Anhydroicaritin inhibits the activation of SREBPs,which has the effects of disrupting thede novosynthesis of fatty acids and cholesterol,thus reducing lipid levels,and can accordingly be used as a main compound in drugs used to control metabolic diseases[51].
Xanthohumol,extracted fromHumulus lupulusLinn.,is a recently identified SREBP-inactivating agent,which has been shown to inhibit triglyceride synthesis and apo B secretion,thereby reducing the synthesis of fatty acids and cholesterol.It has been established that xanthohumol independently inhibits the maturation of insulininduced SREBPs in a manner distinct from that of sterols,which involves inhibiting the binding of SCAP/SREBP to COPII vesicles by binding to Sec23/24.In diet-induced obese mice,dietary xanthohumol has been observed to inhibit the expression of SREBP-1 target genes in the liver,accompanied by a reduction in the maturation of liver SREBP-1,thereby preventing the development of obesity and liver steatosis.Xanthohumol is thus considered to have therapeutic utility in the treatment of metabolic disease,either as a dietary supplement or a dedicated pharmacological preparation[52].
It has been reported that licorice extract has properties associated with the regulation of metabolism which,in part at least,is believed to be associated with glabridin,a flavonoid extracted from the roots and rhizomes ofGlycyrrhiza uralensisFisch.,Glycyrrhiza inflataBat.,andGlycyrrhiza glabraL.Glabridin has been characterized as an effective AMPK activator,which can reduce obesity and generally improve lipid disorders and insulin resistance,and has been shown to promote the activation of AMPK and thus the oxidation of fatty acids.Specifically,upon activation of AMPK,fatty acid oxidation is enhanced in response to a rapid phosphorylation of ACC and is further maintained by the expression of certain oxidation-associated genes.In addition,glabridin inhibits the expression of adipogenic genes,including SREBP-1c,FAS,ACC,and SCD1,in white adipose tissue and liver and has been demonstrated to reduce weight,obesity,fatty liver,and hyperlipidemia by activating AMPK,thereby improving blood glucose levels and lipid homeostasis[53].
Formononetin,an isoflavone extracted from the rhizomes ofButea monosperma(Lam.)Kuntze,stimulates the formation of osteoblasts and prevents post-menopausal bone loss.The inverse effects on osteoblasts and adipocytes led researchers to analyze the effects of formononetin on adipogenesis and bone loss in vivo associated with high-fat diet-induced obesity.The anti-obesity effects and mechanism of this isoflavone were examined in mouse embryonic fibroblasts and high-fat diet-induced obese male mice,which revealed that formononetin inhibits the lipogenic differentiation of mouse embryonic fibroblasts by down-regulating key lipid-forming markers,such as PPAR-γ,(C/EBP)-α,and SREBP,and inhibits intracellular triacylglycerol accumulation.Moreover,in vivo,mice treated with formononetin for 12 weeks were found to show improvements in the development of obesity in terms of reductions in high-fat dietinduced weight gain and visceral fat accumulation,and this antiobesity effect was established to be associated with an increase in energy expenditure[54].
Alkaloids
Alkaloids are a class of physiologically active compounds,among which,12,000 known alkaloids are used as drugs,stimulants,and anesthetics[55].Berberine,an alkaloid extracted from the rhizomes ofCoptis chinensisFranch.,Coptis deltoideaC.Y.Cheng et Hsiao,andCoptis teetaWall.,has been characterized as an inhibitor of SREBPs and demonstrated to alleviate olanzapine-induced adipogenesis via the AMPKα-SREBP pathway in fibrogenic adherent cells derived from mouse pre-adipose embryos.Berberine has also shown utility as a potential adjuvant that can be applied to prevent dyslipidemia and obesity associated with the use of second-generation anti-psychotic medication[56].Importantly,berberine has been found to inhibit SREBP activation and SCAP expression,which has the effects of down-regulating these lipogenic enzymes,whereas knockdown of SCAP via shRNA treatment abolishes the effect of berberine on SREBP-1 activation.Furthermore,berberine has been shown to suppress the growth and lipogenesis of colon cancer xenografts in a SCAP-dependent manner.Collectively,these findings thus tend to indicate that berberine may serve as a candidate inhibitor of tumor growth in colon cancer,partially by targeting SCAP/SREBP-1 pathway-driven lipogenesis[57].Tomatidine,a tomatine glycoside isolated fromLycopersicon esculentumMill.,can be used to significantly inhibit palmitic acid accumulation and has been shown to stimulate the phosphorylation of AMPK and ACC1 in human liver cells.This alkaloid also has the effect of promoting triglyceride turnover and reducing fat generation by up-regulating fatty triglyceride lipase and down-regulating FAS by regulating AMPK signal dependence of the transcription factors SREBP-1c and FOXO1 and is accordingly considered to have potential efficacy in the prevention and treatment of obesity-related fatty liver disease[58].Sophora alkaloid is an alkaloid extracted from the dried roots,foliage,and fruits ofSophora flavescensAlt.,using ethanol and other organic solvents,and has been reported to reduce non-alcoholic steatohepatitis in rats and influences the synthesis of adipocytokines.In model systems,steatosis is induced by incubating primary hepatocytes isolated from pathogen-free male Sprague Dawley rats with 200 µmol/L oleic acid for 24 h.Using such models,it has been established that AMPK can down-regulate the enzymes involved in fatty acid synthesis and gluconeogenesis by inhibiting the transcription factors SREBP-1c,hepatocyte nuclear factor-4A,and ACC and that by significantly up-regulating AMPK phosphorylation,Sophoraalkaloid can be used to alleviate hepatocyte steatosis[59].
Coffee,produced from the fruits ofCoffea arabicaL.,contains a complex mixture of more than thousand substances,including caffeine(primary source),phenolic compounds(chlorogenic acid and quinides-primary source),minerals and vitamins(magnesium,potassium,manganese,chromium,and niacin),and fiber,a number of which are believed to play roles in lipid and glucose metabolism.The finding that caffeine reduces lipid accumulation in mouse embryonic fibroblasts has stimulated further research to establish whether caffeine affects lipid metabolism in HepG2 cells.Treatment of these liver cancer cells with caffeine has been found to promote a significant reduction in the accumulation of liver lipids,including triglycerides and cholesterol.Moreover,caffeine was observed to reduce mRNA levels of fat-producing genes(SREBP-1c,SREBP-2,FAS,SCD1,HMGR,and LDLR)and increase the levels of CD36,a gene associated with lipid uptake and catabolism.Further investigations examining the effects of caffeine on AMPK signaling pathways revealed significant increases in phosphorylation of AMPK and ACC in cells exposed to caffeine for 24 h,which were suppressed in the presence of AMPK inhibitors.These observations thus indicate that caffeine has the valuable effect of reducing lipid accumulation in the liver by regulating the AMPK-SREBP signaling pathway,and accordingly,either caffeine per se or caffeinated beverages may represent promising dietary supplements for the prevention of fatty liver disease and hypercholesterolemia[60].A further alkaloid,rohitukine,isolated from the bark ofPimpinella anisumLinn.,is noted for its anti-cancer properties.Rohitukine has been reported to inhibit lipid accumulation and differentiation in 3T3-L1 and C3H10T1/2 cells in both concentration-and time-dependent manner,downregulates the expression of PPAR-γ,C/EBP,Pα aP2,FAS,and glucose transporter type 4,and also inhibits the mRNA expression of LPL,SREBP-1c,FAS,and aP2,which are downstream targets of PPAR-γ.Moreover,rohitukine has been shown to increase the expression of LXR in the liver,reduce the expression of SREBP-2 and related targets,reduce the accumulation of lipids in the liver and gonads,and significantly improve dyslipidemia[61].
Terpenoids
Terpenoids are a class of secondary metabolites widely distributed among the plants used in traditional Chinese medicine,with wideranging pharmacological activities,such as antibacterial,antiinflammatory,anti-tumor,and neuroprotective effects,which accordingly highlights their potential therapeutic value[3].Among these carnosic acid,extracted fromRosmarinus officinalisL.,is recognized as one of the most effective antioxidants that can be applied for the prevention of degenerative and chronic diseases[62].Zheng et al.[63]established that carnosic acid can be used to suppress bone loss via the dual targeting of SREBP-2 and estrogenrelated receptor α(ERRα).Mechanistically,carnosic acid has been established to reduce the nuclear localization of mature SREBP-2 and suppresses thede novobiogenesis of cholesterol,subsequently promoting a reduction in the interaction between ERRα and PPAR-γ coactivator 1-β,thereby down-regulating the transcriptional activity of ERRα and target gene expression.Carnosic acid appears to mediate these processes by binding directly to the ligand-binding domain of ERRα,thereby significantly promoting its ubiquitination and proteasomal degradation,for which STUB1,identified as the E3 ligase of ERRα,and the lysine residues K51 and K68 have been established to be essential.In conclusion,by dual targeting SREBP-2 and ERRα,carnosic acid inhibits the receptor activator of nuclear factor-kappaB ligand-induced osteoclast formation and improves ovariectomy-induced bone loss,and may accordingly serve as a lead compound for pharmacological control of osteoporosis.
Chlorogenic acid is the main active component ofLonicera japonicaThunb.,Lonicera hypoglaucaMiq.,Lonicera confucaDC.,andLonicera dasystylaRehd.In previous studies that have investigated the mechanisms underlying the combined effects of chlorogenic acid and caffeine on lipid metabolism in obese mice induced by a high-fat diet,80 female Institute of Cancer Research mice were randomly divided into eight groups and fed a high-fat diet with or without chlorogenic acid or caffeine supplementation for 14 weeks.The combination of chlorogenic acid and caffeine was duly found to effectively reduced weight gain;intraperitoneal adipose tissue weight;serum low-density lipoprotein cholesterol,free fatty acid,total cholesterol,triglyceride,leptin,interleukin-6 concentrations;and hepatic triglyceride and total cholesterol levels,and increase the levels of serum adiponectin levels.The same combined treatment was also found to promote the phosphorylation of AMPK,inhibit the expression of SREBP-1c and LXR,reduce the expression of FAS and HMGR,and increase the expression of acetyl-coenzyme A,adipose triglyceride lipase,and heat shock proteins.Collectively,these findings indicate that by regulating lipid metabolism,treatments based on the combined application of chlorogenic acid and caffeine could be effective in reducing the weight of obese mice,induced by high-fat diets,through their effects on the AMPKα-LXRα/SREBP-1c signaling pathway[64].
A further terpenoid,ursolic acid,occurs in a wide range of plant species,although is found mainly in the leaves ofLigustrum lucidumAit.,Actostaphylos uva-ursi(L.)Spreng,Eriobotrya japonica(Thunb.)Lindl.,Paulownia tomentosa(Thunb.)Steud.,andIlex rotundaThunb.,and the whole plant ofPrunella vulgarisL.In studies that have examined the effects and underlying mechanisms of ursolic acid on adipogenesis in 3T3-L1 pre-adipocytes,it has been shown that this terpenoid dose-dependently attenuates fat formation and reduces the expression of C/EBP-b,PPAR-c,C/EBP-a,and SREBP-1c proteins.Moreover,ursolic acid was found to increase the protein expression of ACC,carnitine palmitoyltransferase 1(CPT1),AMPK,and Sirt1 but reduce that of FAS and fatty acid binding protein 4.Further studies revealed that the anti-lipid-forming effect of ursolic acid could be reversed by AMPK siRNA,although not by niacinamide,a Sirt1 inhibitor.Additionally,live kinase B1(LKB1),the upstream kinase of AMPK has been shown to be up-regulated by ursolic acid,whereas knockdown of LKB1 attenuates the effects of ursolic acid on AMPK activation.Ursolic acid has also been demonstrated to inhibit the differentiation and adipogenesis of 3T3-L1 pre-adipocytes via the LKB1/AMPK pathway,although appears to have no appreciable effects on pre-adipocyte number,cell cycle,or apoptosis.On the basis of these observations,ursolic acid is considered a promising natural therapeutic agent that has potential application in the prevention and treatment of obesity[65].
Among other terpenoids,andrographolide,a natural compound derived fromAndrographis paniculata(Burm.F.),may function as a specific inhibitor of SREBPs.Studies have revealed the weight loss effect of androandrosterone in obese C57BL/6 mice fed a high-fat diet.The application of andrographolide has been shown to downregulate the expression of SREBP target genes and reduce cellular lipid accumulation in vitro,although does not appear to affect LXR target genes,including ABCA1,ABCG5,and ABCG8,which thus tends to indicate that this terpenoid may specifically inhibit the activity of SREBPs.Furthermore,the administration of andrographis(100 mg/kg per day)has been found to reduce weight gain and fat accumulation in the liver or adipose tissues and to improve lipid levels and insulin or glucose sensitivity in diet-induced obesity mice.Androandroactone has also been proved to be effective in inhibiting respiration rate,energy consumption,and oxygen consumption,which may be one of the factors contributing to the observed weight gains and losses observed in diet-induced obesity mice.In liver or brown adipose tissues,andrographolide has been reported to regulate SREBP target genes and metabolism-related genes,which is plausibly the main mechanism underlying the aforementioned reduction in lipid levels and increased insulin sensitivity.Given these beneficial effects of andrographolide in enhancing lipid metabolism and glucose utilization in high-fat diet-induced obese mice,this terpenoid could potentially serve as the main pharmacological control compound in the treatment of obesity and metabolic diseases,particularly type 2 diabetes[66].
As a final example of therapeutic terpenoids,celastrol,a pentacyclic triterpenoid extracted from the roots ofTripterygium wilfordiiHook.f.,has been proved to have wide-ranging pharmacologicaleffects,includinganti-inflammatory,immunosuppressive,anti-obesity,and anti-tumor activities.However,on account of its poor water solubility,low bioavailability,and toxicity,to date,there has been little progress with respect to the clinical development and trials of this compound[67].The effects of celastrol on non-alcoholic fatty liver disease has been evaluated in wild-type C57BL/6J and liver-specific SIRT1-deficient mice,and the molecular mechanisms have been further investigated.In wild-type mice fed a high-fat diet,treatment with celastrol was observed to reduce body weight,subcutaneous and visceral fat contents,and hepatic lipid droplet formation,and reductions in the concentrations of total cholesterol,triglycerides,free fatty acids,and alanine aminotransferase were detected in hepatocytes.Furthermore,celastrol treatment was observed to improve glucose tolerance and insulin sensitivity in high-fat diet-fed mice.In terms of the underlying mechanisms,it has been demonstrated that celastrol increases the expression of Sirt1 in the liver and primary hepatocytes,which has the effect of protecting the liver from high-fat diet-induced metabolic damage,mediated via the deacetylation of SREBP-1c.Moreover,celastrol was found to down-regulate the expression of SREBP-1c,enhance AMPKα phosphorylation,and up-regulate the expression of LKB1[68].
Others
Among other assessed natural product inhibitors of SREBPs,studies based on the use of a stable cell line expressing a luciferase reporter gene driven by an SRE-containing FAS promoter,have identified allyl isothiocyanate,one of the major isothiocyanates in cruciferous vegetables such asBrassica oleraceaL.var.gemmiferaZenker,as a novel SREBP inactivator,which inhibits SREBP activation of the 1007 FAS promoter(HH-7/FAS-Luc).In human hepatocellular carcinoma cells exposed to allyl isothiocyanate,SREBP-associated protein hydrolysis and the expression of target genes are down-regulated,along with a reduction in thede novosynthesis of fatty acids and cholesterol[69].Accordingly,the inhibition of SREBP activity can explain the weight loss effect attributable to this isothiocyanate and can thus be considered a promising strategy for improving metabolic disorders.
Syringic acid(4-hydroxy-3,5-dimethoxybenzoic acid),the main benzoic acid derivative identified inSyzygium aromaticum(L.)Merr.et Perry,has been established to reduce body weight and visceral fat mass;serum leptin,tumor necrosis factor,interferon,interleukin-6,and monocyte chemotaxis protein-1 levels;insulin resistance;hepatic lipid content;droppings;and early fibrosis and also increases the circulation of adiponectin.At the molecular level,it has been demonstrated that syringic acid down-regulates fat-producing(cell death-inducing DNA fragmentation factor-like effector A,PPAP-γ,SREBP-1c,SREBP-2,HMGCR,and FAS)and inflammatory(toll-like Receptor 4,myeloid differentiation primary response 88 ,nuclear factor-kappaB,tumor necrosis factor α,and TL6)genes in the liver and up-regulates the expression of fatty acid oxidation genes(PPAP-α,acyl-coenzyme A synthase,CPT1,and CPTt2).Compared with the high-fat diet group,syringic acid was also found to reduce the activity of liver adipogenic enzymes and increase the activity of fatty acid oxidases.These findings indicate that dietary syringic acid has weight loss,anti-inflammatory,and anti-steatosis effects,mediated via the regulation of lipid metabolism and inflammatory genes,and accordingly may serve as a novel natural therapeutic agent for the treatment of obesity or non-alcoholic hepatitis[70].
Curcumin,a major active component ofCurcuma longaL.,is a further natural product considered to have potential application as a lead compound for the prevention of obesity and insulin resistance[71].Researchers have established that curcumin can inhibit SREBP expression in vitro,which has the effect of reducing the biosynthesis of cholesterol and fatty acids.It is found to inhibit the activity of human SRE promoters in a dose-dependent manner and significantly down-regulate the mRNA expression of SREBPs target genes related to fatty acid and triglyceride synthesis.In vivo,curcumin has been demonstrated to ameliorate high-fat diet-induced body weight gain and fat accumulation in the liver or adipose tissues and improve serum lipid levels and insulin sensitivity in high-fat diet-induced obese mice.
In recent years,lipid metabolism reprogramming has emerged as a new hallmark of malignancies[72],and in this context,it has been established that cancer metabolism is associated with the activation of SREBPs.Currently,however,the application of SREBP inhibitors in the treatment of tumors is essentially at the stage of being a novel strategy.In this regard,fatostatin has been characterized as an SREBP inhibitor that can be applied to inhibit fat biosynthesis[73].Studies have shown that fatostatin inhibits SREBP-1,which mediates the transcriptional expression of corpus luteum,FAS,and SCD1 lipogenic genes,promoting the activation ofde novofat and cholesterol generation,and in turn inducing the proliferation of prostate cancer cells and thus the occurrence and development of tumors.By inhibiting SREBP-1,fatostatin reduces the expression of tumorrelated lipogenic genes,thereby further reducing the accumulation of intracellular fatty acids and thus inhibiting the growth of prostate cancer in vitro and in vivo[74].
Atherosclerosis is a chronic inflammatory disease involving immune and metabolic processes[75],the development of which is closely associated with disorders of lipid metabolism.Ligustrazine,a natural product derived fromLigusticum chuanxiongHort.,has been found to have anti-atherosclerotic effects associated with the improvement of dyslipidemia,by down-regulating the expression of progesterone-adiponectin receptor 3 and inhibiting the SCAP/SREBP-1c signaling pathway[76].A further natural compound with similar effects is betulinol,a component ofBetula platyphyllaSuk.,which has been found to enhance the expression of ABCA1 and ABCG1 by inhibiting the binding of transcriptional repressor SREBPs to the Ebox motif of the ABCA1 promoter.In apolipoprotein E mice fed a high-fat diet,the administration of betulinol via injection was observed to significantly reduced lesions in the facial aorta and aortic sinus[77].These observations,thus,indicate that inhibition of the SREBP pathway represents a promising strategy for the treatment of a range of metabolic diseases,including type 2 diabetes and atherosclerosis[78].
The benefits and drawbacks of natural SREBP inhibitors in the treatment of metabolic disease
As illustrated by the examples covered in this review,natural inhibitors of SREBPs have considerable potential with respect to the treatment of debilitating diseases and can provide novel solutions to multiple medical problems.SREBPs have been established to represent a causal link between abnormal fat and cholesterol generation and the occurrence and development of cancer[74],and accordingly,compounds that function as SREBP inhibitors are identified as potentially novel sources of anti-cancer therapy.In addition,researchers have found that by inhibiting cell mitosis,these inhibitors can also reduce the survival rate of cancer cells,which provides a new solution for the treatment of those cancers that are resistant to currently used anti-cancer drugs,such as glioblastomas that are characterized by rapid rates of lipid metabolism and proliferation[79].Consequently,the application of SREBP inhibitors in joint studies on metabolism and cancer would be anticipated to promote dual developments in both the fields.However,given that SREBPs play roles in multiple signaling pathways,the inhibitors of these SREBPs may have simultaneous and potentially differential effects on different processes.For example,among SREBPs involved in innate immune responses,SREBP-1a not only contributes to the activation of genes necessary for fat generation in macrophages but also those encoding NLRPLa,the core component of the inflammatory response[80].Consequently,when using SREBP inhibitors for therapeutic purposes,it is vital to carefully assess the potential influence on multiple pathways.Accordingly,prior to any therapeutic application of SREBP inhibitors,there needs to be comprehensive toxicity investigations and assessments of potential effects on non-target sites to ensure drug safety.
On the basis of the findings of studies conducted to date,natural compound inhibitors of SREBPs have shown significant effects with respect to improving metabolic disease in animal models,thereby providing potentially novel approaches for the treatment of cancer.and have been proved to have considerable value regarding research on innate immunity.However,a larger number of studies will be necessary to establish the safety and efficacy of SREBP inhibitorbased therapy in clinical practice(Table 2).
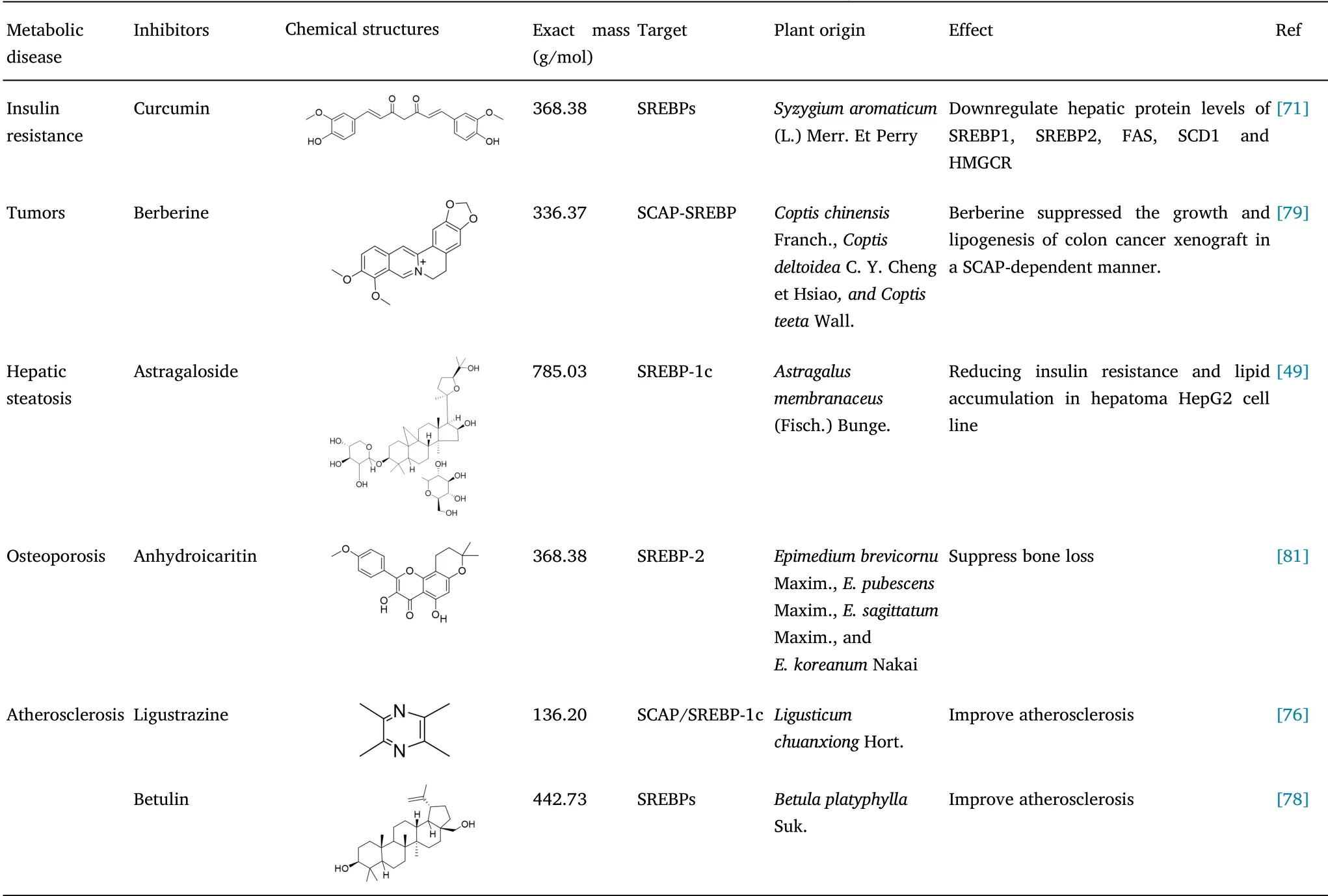
Table 2 SREBPs natural product inhibitors for improving metabolic disease
Conclusion
Traditional Chinese medicine plays a unique role in the treatment of diabetes,obesity,and other metabolic diseases,reducing side effects and improving complications.SREBPs inhibitors can improve metabolic diseases.With the deepening of research,a great number of natural small molecules have been found to be SREBPs inhibitors.This review introduces the action pathway and pharmacological action of SREBPs inhibitors according to the structural classification of natural products.It also introduces the treatment of SREBPs inhibitors on metabolic diseases.
In future research,the specific action pathway and molecular mechanism of SREBPs inhibitors should be clarified.It can be concluded that the natural product inhibitors of SREBPs have high research value and can be developed into drugs and nutritional products to treat a variety of metabolic diseases.
 Traditional Medicine Research2022年3期
Traditional Medicine Research2022年3期
- Traditional Medicine Research的其它文章
- Traditional Chinese medicine:an important broad-spectrum anti-coronavirus treatment strategy on COVID-19 background?
- Anti-asthmatic mechanism of the Huashanshen dripping pill via suppressing contraction of the airway smooth muscle
- Application of network pharmacology in the prevention and treatment of COVID-19 by traditional Chinese medicine
- Pharmacological efficacy of the traditional Chinese medicinal formula Kun-Tai-1A in the treatment of letrozole-induced polycystic ovary syndrome
- Integrated UHPLC-MS and network pharmacology to explore the active constituents and pharmacological mechanisms of Shenzao dripping pills against coronary heart disease
- Effects of Qingwen Baidu decoction on coagulation and multiple organ injury in rat models of sepsis
