Traditional Chinese medicine:an important broad-spectrum anti-coronavirus treatment strategy on COVID-19 background?
Run-Dong Chai,Ya-Dong Fan,Qi-Yao Li,Huan-Tian Cui,Hai-Zhao Liu,Ying Wang,Bo-Yang Gong,Lu Han,Shuai Zhang,Yu-Hong Bian*,Jun Kang,Yu Wang*
1Tianjin University of Traditional Chinese Medicine,Tianjin 300193,China.2School of life Sciences,Shandong University,Qingdao 266071,China.3School of life Sciences,Tianjin University,Tianjin 300072,China.
Abstract Coronaviruses exist widely in nature,can cause cross-species transmission,and pose serious threats to human and animal health.Over the past 20 years,coronaviruses have led to three major epidemics that have caused global panic,including severe acute respiratory syndrome,Middle East respiratory syndrome,and coronavirus disease-19.At present,coronavirus disease-19 not only spreads rapidly,but also mutates easily to escape host immune response,becoming more pathogenic.At present,there are no effective specific therapeutic drugs or vaccines.Drugs targeting severe acute respiratory syndrome coronavirus 2 and the host cell defense system that have been developed based on the structure and replication cycle of coronaviruses have a certain broad-spectrum antiviral effect;however,their efficacy still needs to be demonstrated in further clinical trials.Traditional Chinese medicine has an indispensable role in the ongoing response to coronavirus disease-19.Anti-virus treatment with traditional Chinese medicine has advantages such as broad-spectrum application,low toxicity and side effects,low susceptibility to drug resistance,and overall comprehensive regulation.Therefore,researches on effective components and mechanisms of action of the anti-viral effects of traditional Chinese medicine have increasingly gained attention.The present paper examines coronaviruses,specifically summarizing the genomes,replication mechanisms,and mutant strains.Afterward,the therapeutic effects and mechanisms of action of modern broad-spectrum anti-coronavirus drugs and traditional Chinese medicine are summarized.By considering the virus and the targets in the host comprehensively,in addition to the beneficial multi-target and multi-path antiviral effects of traditional Chinese medicines,this paper could guide the development of treatment strategies for broad-spectrum anti-coronavirus traditional Chinese medicines,and could facilitate the modernization and globalization of traditional Chinese medicine.
Keywords:COVID-19;SARS-CoV-2;traditional Chinese medicine;broad-spectrum antivirals
Background
The coronavirus disease-19(COVID-19)caused by severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)was epidemic globally in 2019,with considerable threats and losses to public health and economy.According to the World Health Organization(WHO),as of January 9,2022,380 million confirmed cases of COVID-19 have been reported globally,with the cumulative death toll of approximately 5.7 million(WHO,https://covid19.who.int/).Therefore,the screening and development of therapeutic drugs,includingvaccines,chemicaldrugs,biologicalproducts(antibodies/polypeptides/stem cells),plasma therapy,traditional Chinese medicines(TCMs)have been rapidly undertaken in China and in other countries.However,no report has examined the specific drugs recognized for COVID-19 treatment[1].
SARS-CoV-2 in an RNA virus,which mutate faster than DNA viruses,and a few strains exhibit enhanced transmission capacity and pathogenicity.While monitoring the protective efficacy of vaccines against mutant strains,the development of vaccines against mutant strains should also be accelerated.However,the development of novel drugs requires on average more than about US$ 2 billion,and lasts for 8-12 years;consequently,developing different drugs for different virus strains can greatly increase the cost of drug development for such viruses[2].Broad-spectrum antivirals(BSAs)can act on multiple viruses or multiple genotypes of the same virus;therefore,they have significant advantages over specific antiviral drugs.Therefore,evaluating the therapeutic targets and effects of approved and developed BSA drugs is important with regard to time and cost of preparing broad-spectrum antibiotics.
Since the outbreak of the COVID-19 epidemic in Wuhan,China,the clinical efficacy of TCM in COVID-19 treatment has been remarkable,and has attracted global attention.Therefore,China’s National Health Commission considers TCM as one of the important strategies of COVID-19 treatment[3].The symptoms observed in COVID-19 patients are similar to those of a “plague”(a severe infectious disease)recorded in theYellow Emperor’s Canon of Inner Classic(221 B.C.E.-220 C.E.),which was highly contagious and caused an epidemic[4].Lonicera japonicais a plant in family Caprifoliaceae.As early as the Eastern Han Dynasty(202 B.C.E.-8 C.E.),theDivine Farmer’s Materia Medica Classic(the date of completion is unclear)stated[5]“It mainly treats body swelling due to coldness or heat.” Wang Bingheng of the late Qing Dynasty(1808 C.E.)inCasual Jottings from the Hall of Repeated Felicitations,stated that[6]“L.japonicacan resolve the dirty and foul pathogens of epidemic febrile diseases”.Consequently,L.japonicaplayed a major role in the prevention and treatment of plagues in ancient times.According to the findings of modern medical research,the polysaccharide components inL.japonicahave remarkable antiviral effects,in addition to inhibitory effects against herpes simplex virus(HSV),coxsackievirus B5,coxsackievirus B3,and enterovirus 71[7].In vitro experiments have shown that chlorogenic acid inL.japonicacan inhibit the replication of influenza viruses.Furthermore,L.japonicacould alleviate inflammatory responses by inhibiting the production of inflammatory mediators,such as pro-inflammatory cytokines,chemokines,interleukin(IL)-1β,tumour necrosis factor(TNF)-α,and IL-6[8].Scutellaria baicalensiswas first recorded in theDivine Farmer’s Materia Medica Classic,which was written by Shen Nong,in the Han Dynasty.In 1798 C.E.,Wu Tang wroteSystematic Differentiation of Warm Pathogen Diseasesin the Qing Dynasty.It has recorded the Huang Qin Hua Shi decoction withS.baicalensisas the monarch drug,which is used to treat damp-warm syndrome or early-stage summer-heat and dampness,and has reportedly saved the lives of countless people.Modern pharmacological research has shown that baicalin and baicalein,the major chemical components ofS.baicalensis,can inhibit many viruses[9].Additionally,baicalin and baicalein could inhibit 3CL protease and coronavirus S protein,thus reducing the amplification of SARSCoV-2[10].Recently,the “three preparations and three formulas of traditional Chinese medicine” used to treat the COVID-19 epidemic have also includedS.baicalensis,L.japonica,and other herbs.Such reports demonstrate that TCMs have played an important role in the treatment of epidemic diseases in different eras.
Therefore,starting from SARS-CoV-2,this paper explored the mechanisms of action and targets of coronavirus infection and summarized the specific and broad-spectrum antiviral compounds in TCM.This paper could offer novel insights and facilitate the formulation of strategies for the prevention,control and treatment of the epidemic.
SARS-CoV-2 mutations have led to a more severe epidemic
The main features of SARS-CoV-2 virus are concealed transmission,multi-point dissemination,and concentrated outbreak[11].At present,although the number of severe cases has decreased,the number of mild and asymptomatic infections has increased,which suggests that the mutant viruses may achieve immune escape,leading to missed diagnoses,misdiagnoses,and wider spread,further exacerbating to challenges associated with the prevention and control of COVID-19.
SARS-CoV-2 structure
Coronaviruses belong to family Coronaviridae,and can be divided into α(α-CoV),β(β-CoV),γ(γ-CoV),and δ(δ-CoV)coronaviruses.α and β coronaviruses can infect mammals,and viruses found in humans are genetically similar to β-CoV.β-CoV is further divided into different lineages(A,B,C,and D):SARS-CoV and SARS-CoV-2 are classified into lineage B,which has about 200 published virus sequences[12].The whole genome of SARS-CoV-2 is 80% identical to that of SARS-CoV.The similarity of spike protein sequences between SARS-CoV-2 and SARS-CoV is approximately 76-78%.In addition,the similarity in receptor binding domain(RBD)is 73-76%,whereas that of receptor binding motif is 50-53%.The spike protein sequence similarity between SARS-CoV-2 and SARS-CoV explains their binding to the same receptor,angiotensin converting enzyme 2(ACE2),in host cells[11].
The genome of SARS-CoV-2 is about 30 kb(Figure 1);it contains 14 open reading frames(ORFs)and encodes 29 viral proteins[13].The genome structure is arranged in the order of 5’-3’,with a methylation cap structure at the 5’ end and a polyadenylation tail structure at the 3’ end.The genome contains two overlapping ORFs,ORF1a and ORF1b.ORF1a/b first translates polyprotein 1a and polyprotein 1b,which are digested by two viral proteases into 16 nonstructural proteins.They are essential for virus replication and transcription.The four ORFs at the 3’ end of the genome encode structural proteins,including nucleocapsid protein(N),spike glycoprotein(S),membrane protein(M),and envelope protein(E),which are responsible for the assembly of virions and participate in the suppression of host immune response(Figure 2).A series of helper genes encoding helper proteins(ORF3a,ORF3b,ORF6,ORF7a,ORF7b,ORF8b,ORF9b,and ORF14)are located among these structural genes.In addition to structural proteins ORF3a and ORF7a,helper proteins are involved in regulating the process of host infection by SARS-CoV-2[13].
Replication cycle of SARS-CoV-2
The replication cycle of SARS-CoV-2 is mainly divided into six processes:adsorption,penetration,uncoating,replication,assembly,and budding.S protein plays a key role in virus-cell receptor interactions,mediating the process of virus adsorption and invasion to host cells.S protein is a homotrimer,which distributes structures resembling spikes all over the surface of SARS-CoV-2 and contains about 1,200 residues.It can be cut into two functional subunits,S1 and S2,by furin,and the S subunits contain RBD and the N-terminal domain(NTD).RBD recognizes ACE2 receptor on the host cell membrane by altering the conformational state.S2 subunit contains fusion peptide,two heptapeptide repeats(HR1 and HR2),transmembrane domain,and intracellular domain.When RBD binds to the host cell,the S2 subunit promotes the conformational rearrangement of S protein,promotes the fusion of SARS-CoV-2 with the host cell membrane,and releases its own genomic RNA into the cell[14].At such a stage,SARS-CoV-2 has completely entered the host cell and is ready for replication.
Before the initiation of replication,the nonstructural proteins generated by the division of PLpro and 3CLpro gather on the doublemembrane vesicles of the double-membrane structure to form a replication-transcription complex(RTC)to complete replication.Under the action of RNA-dependent RNA polymerase(RdRp),helicase,and RTC,genomic RNA as a template is transcribed to form negative-strand RNA[15],and negative-strand RNA is used as a template to synthesize new genomic RNA and subgenome RNA:among them,genomic RNA enters a new cycle,and subgenome RNA as the mRNA is recognized by host ribosomes and translated to structural proteins.Among the proteins above,N protein first wraps the newly synthesized genomic RNA to form a spiral nucleocapsid[16],and then the nucleocapsid binds to M,E,and S proteins,and transports the genomic RNA to Golgi apparatus.Through glycosylation and other modifications,a mature virus is formed,a new virus is released by budding on the surface of host cells through exocytosis,and the new virus continues to invade and replicate[17](Figure 3).
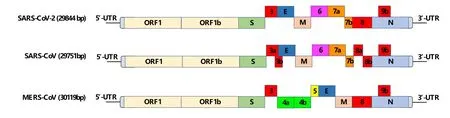
Figure 1 Genomes composition of coronavirus.MERS-CoV,Middle East respiratory syndrome coronavirus;ORF,open reading frames.
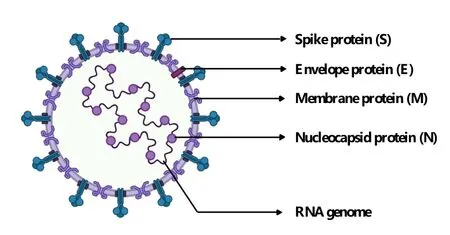
Figure 2 Structure of SARS-CoV-2
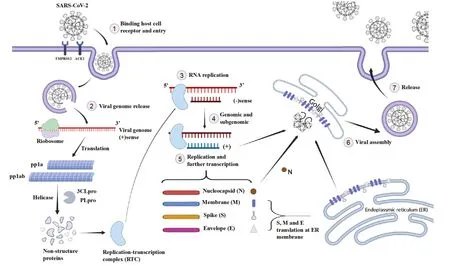
Figure 3 Overview of replication cycle of SARS-CoV-2.3CLpro,3-chymotrypsin like protease;ER,endoplassmic reticulum;RTC,replicationtranscription complex.
Identification,clinical characteristics,and harm caused by SARS-CoV-2 mutants
The WHO first put forward the definition of variant of concern(VOC)in February 25,2021[18].SARS-CoV-2 VOCs refer to mutant strains that alter or potentially alter the transmissibility and pathogenicity of SARS-CoV-2 and its immune escape,and exhibit decreased effectiveness of diagnostic reagents or treatment methods compared to that in the early reference strains,resulting in increased global public health risks.Following evaluation,a VOC should exhibit at least one of the following characteristics:(1)increased viral infectivity;(2)increased pathogenicity or changes in clinical symptoms;and(3)decreased effectiveness of public health and social measures or diagnosis,vaccines,and treatment methods.At present,the specified VOCs include the Alpha,Beta,Gamma,Delta,and Omicron variants.
The Alpha variant was first discovered in the UK in early December 2020,and mainly including nine genotypes(B.1.1.7,Q.1,Q.2,Q.3,Q.4,Q.5,Q.6,Q.7,and Q.8)[19].Alpha(B.1.1.7)has 17 variants,including nine variants on the S protein(Figure 4)[20,21].The overall death risk associated with Alpha strain increased by 61%,and the high viral load of patients may be related to the severity of the associate disease[22].By the end of August 2021,the Alpha strain had been replaced by the Delta strain[19].
The Beta variant was first discovered in Cape Town,South Africa,in December 2020,and mainly includes three genotypes(B.1.351,B.1.351.2,and B.1.351.3).The Beta variant has eight mutation or deletion sites on the S protein(Figure 4),which can change the conformation of RBD,enhance affinity to the ACE2 receptor,and increase infectivity[23,24].According to research findings,the Beta strain could increase the mortality of hospitalized patients,decrease antibody neutralizing activity against the Beta strain compared to that in early epidemic strains,and increase risk of reinfection.In addition,the mutation of E484K and K417N led to the complete loss of neutralizing activity of some antibodies against B.1.351,suggesting that the variant can escape the immune system.AstraZeneca AZD1222 vaccine,recombinant protein vaccine NVXCoV2373,and Pfizer BNT162b2 vaccine are less effective in preventing infections or diseases caused by the Beta strain,whereas adenovirus vector vaccine(Ad26.COV2.S)and Pfizer BNT162b2 vaccine are not significantly less effective in preventing severe disease[25].
The Gamma variant was first discovered in December 2020,in Amazon,Brazil,with three genotypes(P.1,P.1.1 and P.1.2).It has 11 mutations on S protein,including D614G,and L18F,T20N,P26S,D138Y,and R190S in NTD,in addition to K147T,E484K,and N501Y in RBD,and H655Y near furin cleavage site(Figure 4)[26].The RBD mutation of the P.1 variant is similar to that of B.1.351,with E484K and N501Y mutations that can cause immune escape.Compared with those of the early prevalent strains,the transmission ability of the Gamma strain is enhanced(about 40%),which can increase hospitalization rates.The antibody produced by Pfizer BNT162b2 or mRNA-1273 vaccine neutralizes the activity of the Gamma strain,and increases risk of reinfection.
The Delta variant was first discovered in India in October 2020,and mainly includes three genotypes(B.1.617.1,B.1.617.2,and B.1.617.3).Eight mutation sites are located on S protein(Figure 4)[27].Through cell structure analysis,mutations at sites L452R and E484Q in RBD have been observed to be able to destroy the binding reaction between S protein antibody and spike protein RBD,decreasing the binding ability of virus and monoclonal antibodies,which further affects the neutralizing ability of antibodies[28].Compared to those of the early epidemic strains,the transmission ability of the Delta strain is enhanced(about 100%);in addition,the viral load,hospitalization rate,and risk of reinfection in patients without vaccination infected with the Delta strain are relatively high,and the antibodies produced by infection with early epidemic strains have relatively reduced neutralizing activity against the Delta strain[29].The protection conferred by Pfizer BNT162b2 vaccine against Delta strain infection decreases significantly six months after vaccination;in addition,the protection conferred by Pfizer BNT162b2 and AstraZeneca AZD1222 vaccines against clinical symptoms and infections decrease after six months;however,protection against severe disease does not decrease significantly after vaccination[30-32].
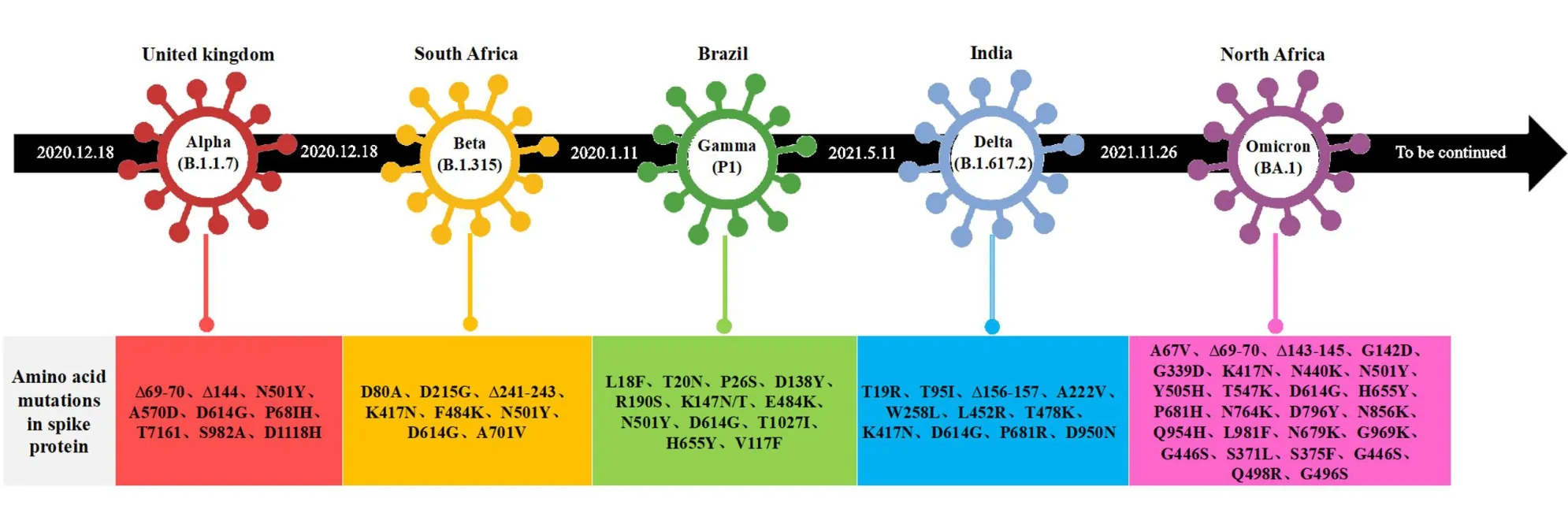
Figure 4 Timeline and amino acid changes in SRAS-CoV-2 variants of concern
The Omicron variant was first discovered in South Africa on November 9,2021,and it includes three genotypes(BA.1,BA.2,and BA.3).The mutation sites include T91 on E protein,P13L,E31del,R32del,S33del,R203K,and G204R on N protein,D3G,Q19E,and A63T in matrix,N211del/L212I,Y145del,Y144del,Y143del,G142D,T95I,V70del,H69del,and A67V in NTD,Y505H,N501Y,Q498R,G496S,Q493R,E484A,T478K,S477N,G446S,N440K,K417N,S375F,S373P,S371L,and G339D in RTD,D796Y in fusion peptide,and L981F,N969K,and Q954H in HR1[33].Considering the numerous mutation sites,the Omicron variant could exhibit immune escape and increased transmission[34].The results of a preliminary experimental study conducted by Pfizer showed that the protective efficacy of two doses of Pfizer vaccine declined,and the third dose of Pfizer vaccine could play a protective role against the Omicron variant.
Broad-spectrum antiviral drug development strategies and research progress
Currently,two major strategies are adopted for the development of broad-spectrum antiviral drugs,including interference with the virus replication cycle and targeting of the host cell defense system[35].BSA plays a therapeutic role by intervening the key links in the process of virus infection.This paper summarized the BSA drugs that have been marketed and are in the clinical research stages from both virus and host perspectives as shown in Table 1.
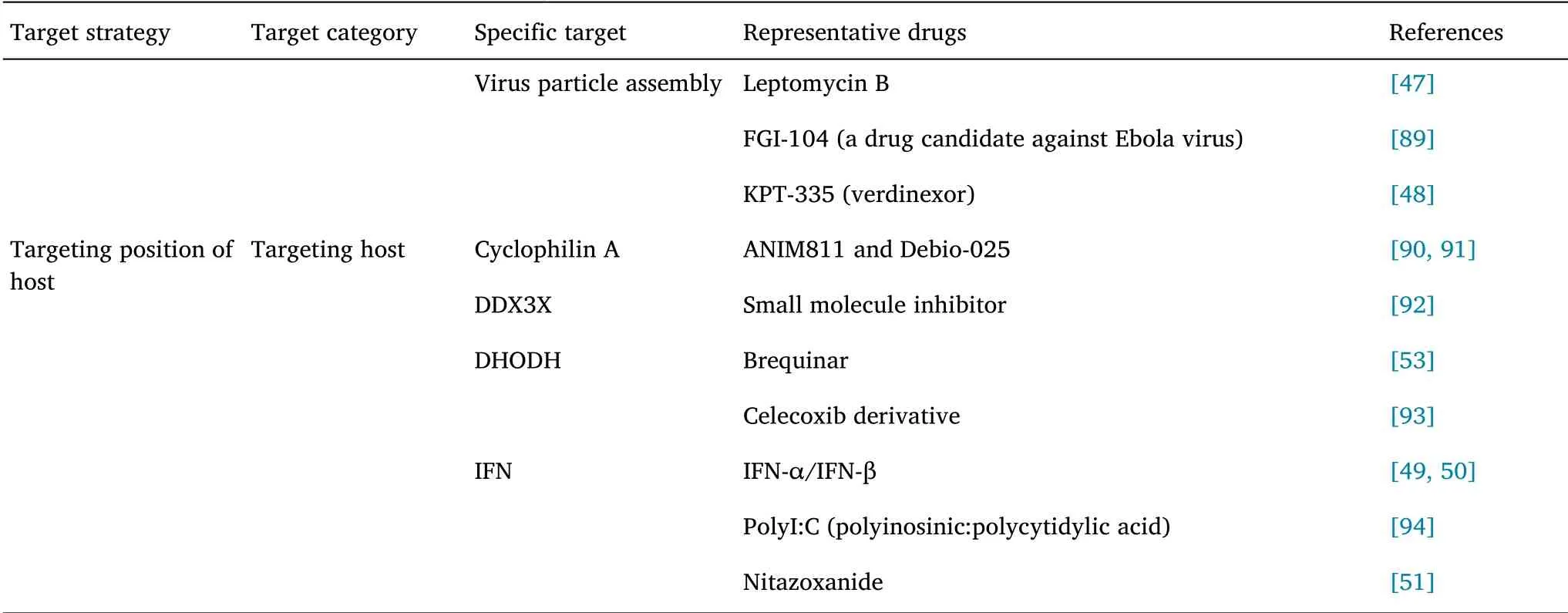
Table 1 Summary of broad-spectrum anti-coronavirus inhibitors(Continued)
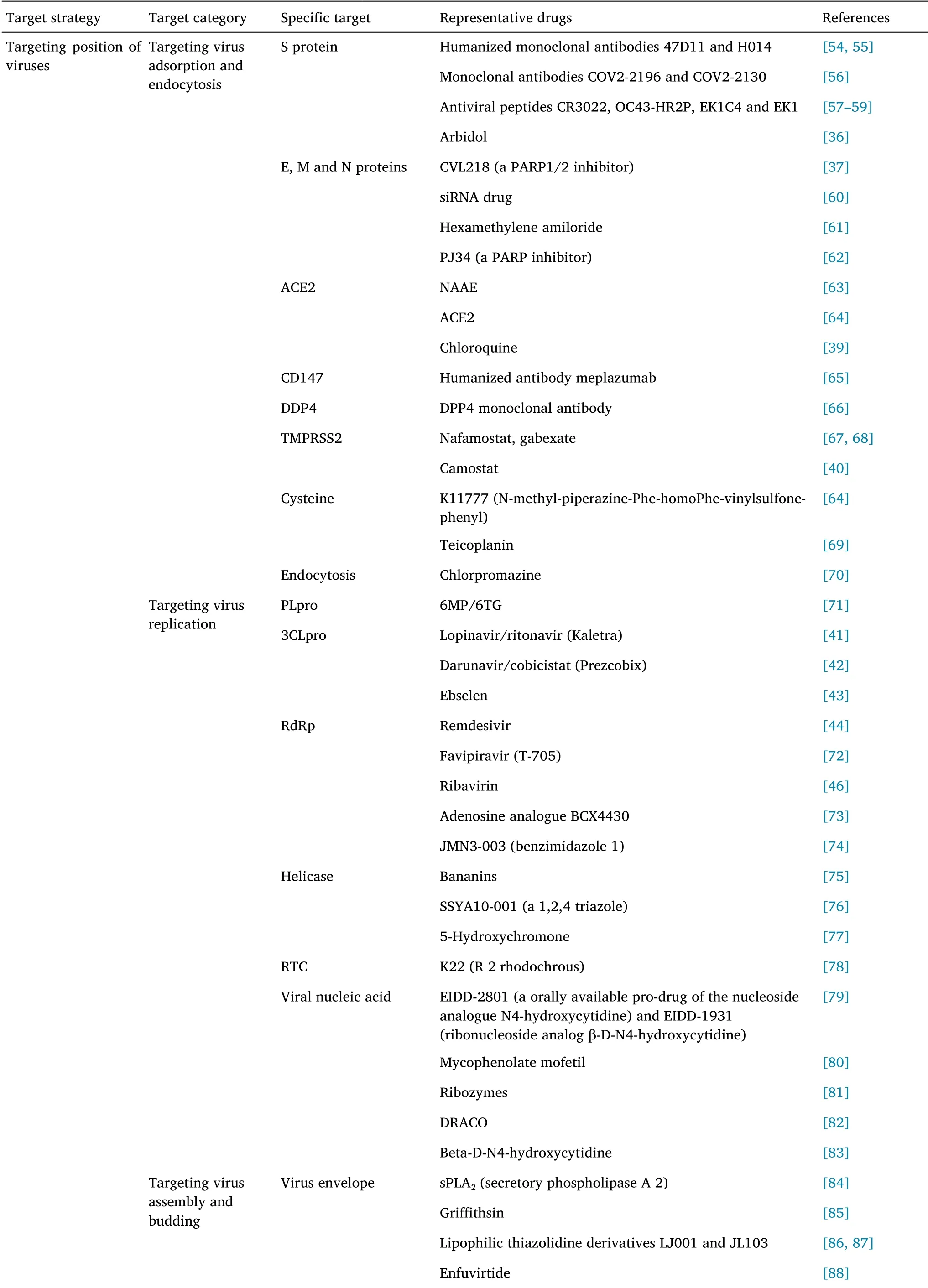
Table 1 Summary of broad-spectrum anti-coronavirus inhibitors
Inhibitors targeting viral adsorption and endocytosis
Targeting S protein is one of the major strategies in broad-spectrum antiviral drug development.Arbidol is one of the most representative broad-spectrum non-nucleoside antiviral drugs.In vitro experiments have demonstrated that arbidol can inhibit SARS-CoV,Middle East respiratory syndrome coronavirus(MERS-CoV),and Ebola virus[36]and has been approved for marketing in Russia and China for COVID-19 prevention and treatment.According to Ge et al.,CVL218,a poly(adenosine-diphosphate-ribose)polymerase-1 inhibitor currently in phase I clinical trial,could inhibit the replication of SARS-CoV-2,and molecular docking simulation studies have suggested that its mechanism of action is prevention of the binding of N protein to viral RNA by binding to the NTD of SARS-CoV-2 N protein[37].
In addition,targeting receptor proteins involved in virus invasion could inhibit coronavirus infection.Chloroquine,a marketed antimalarial agent,affects the activity of glycosyltransferase or glycosyl-modifying enzymes in host cells to alter the pH values of cells,interfere with glycosylation of ACE2,and block the binding of virus to ACE2,to inhibit virus invasion and endocytosis.In addition,in vitro research has demonstrated that chloroquine could restrict the replication of numerous viruses,including human immunodeficiency virus(HIV),SARS-CoV,MERS-CoV,Dengue virus,Ebola virus,and Zika virus[38],and has displayed certain curative effects in clinical settings.Chloroquine is used in the treatment of COVID-19,with relatively mild toxic and side effects.However,acute lethality may occur at high doses[39].Camostat,a drug used for clinical treatment of chronic pancreatitis in Japan,can inhibit the activity of TMPRSS2.In vitro experiments have showed that it could inhibit SARS-CoV,MERS-CoV,and SARS-CoV-2,and could improve the survival rate of mouse models of SARS-CoV infection[40].
Inhibitors targeting virus replication
Proteases(PLpro,3CLpro,RdRp,and helicase)play vital roles in the life cycles of viruses,and therefore are also attractive targets in research on BSA drugs.Lopinavir/litonavir is a first-line treatment for HIV,which can play an antiviral role by inhibiting 3CLpro.Lopinavir is a sensitive substrate of cytochrome CYP3A4 and P-glycoprotein,with a relatively high protein binding rate in plasma.The combination of lopinavir and litonavir could significantly improve the prognosis of SARS patients[41].Darunavir/cobicistat is also a HIV treatment drug,and the combination can also enhance the efficacy[42].Wang et al.resolved the three-dimensional structure of SARS-CoV-2 3CLpro,and found that Ebselen had significant inhibitory effects and high safety,and therefore has been used in clinical trials for treating hearing impairment and other diseases[43].Remdesivir can act on a series of RNA viruses such as Filoviridae,Paramyxoviridae,and Coronaviridae by inhibiting their RdRp[44].Further research has revealed that remdesivir at low micromolar concentrations could effectively block SARS-CoV-2 infection[45].In addition,favipiravir exhibits anti-influenza properties based on its influence RdRp,with no effect on human DNA and RNA.At present,various clinical trials for favipiravir in COVID-19 treatment are being performed.Ribavirin,a guanine analogue,which acts by inhibiting virus RdRp activity,is used to treat SARS-CoV.However,the earlier clinical data on coronavirus therapy reported serious toxic and side effects,and combined therapy could be a better strategy[46].
Inhibitors targeting virus assembly and budding
Viral ribonucleoprotein formed after virus transcription and replication is assembled by the protein transport system of host cells,and discharged from cells by budding.Therefore,targeting such protein-mediated signaling pathways can inhibit the spread of virus in the body.Due to the similarity of mechanisms of transmission of various viruses in the body,such inhibitors also have broad-spectrum antiviral activity;however,current associated research has not yet entered the clinical stage.Leptomycin B is a highly specific inhibitor of export pathways.Leptomycin B can inhibit the export of viral ribonucleoprotein from nucleus to cytoplasm,thus inhibiting virion assembly[47].Leptomycin B has also been shown to inhibit the replication of DNA viruses,such as HSV type 1;however,toxicity and side effects could be observed in animals[48].In 2014,Perwitasari et al.have reported that KPT-335,a small molecule inhibitor,can also achieve antiviral activity by antagonizing the protein transport system.In vitro research has demonstrated good antiviral effects against HIN1,H5N1,and HN9.Furthermore,in vivo research has shown that preventive and therapeutic administration of KPT-335 could protect mice from harm by influenza A virus,and the production of virions and pro-inflammatory cytokines in mouse lungs was reduced significantly following drug administration[48].
Immunomodulatory drugs targeting host cells
Virus replication is highly dependent on enzymes and substrates provided by host cells.When a virus invades the body,it induces host cell defense response against virus replication and reproduction.Therefore,improving a host’s immunity is essential for eliminating viruses.Interferon(IFN)is an important immunomodulator.Currently,in clinical treatment,IFN or IFN inducer supplementation is used to enhance immunity and control virus infections[49,50].IFN-α or IFN-β combined with other antiviral drugs,such as lopinavir,have been used in clinical treatment with good antiviral effect,especially in the early infection stages of MERS-CoV patients.Nitazoxanide is a thiazole compound,which has been approved for marketing by Food and Drug Administration(FDA).Nitazoxanide is rapidly deacetylated in blood after oral intake and absorption,forming the active metabolite tizoxanide and exhibiting inhibitory effects against various viruses,such as Ebola virus and vesicular stomatitis virus,and has entered phase III clinical trials[51].RNA helicase X-linked DEAD-box polypeptide 3 can enhance the antiviral capacity of the innate immune system by interacting with specific proteins of the type I IFN bypass pathway.Dihydroorotate dehydrogenase(DHODH)is a rate-limiting enzyme located on the inner membrane of mitochondria.It can convert dihydroorotate into orotic acid,and the process influences the rate of nucleic acid pyrimidine synthesis by organisms.Subsequently,orotic acid is converted into uridine monophosphate,which is one of the precursors of the synthesis of all other pyrimidine nucleotides[52].Inhibition of DHODH activity can restrict the replication of several viruses,including Ebola virus,vesicular stomatitis virus,Zika virus.DHODH inhibitors can also trigger the expression of IFN-stimulating genes,and such activity could enhance the antiviral effects of such compounds[53].
TCM provides novel perspectives that can facilitate broad-spectrum antiviral development
COVID-19 has significant infectivity and fall under the “plague” and“epidemic disease” categories in TCM theories.For thousands of years,TCM has accumulated rich experience in prevention and treatment of “plague”.According to Tong Xiaolin and Zhang Boli,COVID-19 is caused by cold and damp pathogens,and falls under the category of “cold and damp epidemic”[95].TheDiagnosis and Treatment Protocol for COVID-19(trial version 8)divides the clinical treatment period of COVID-19 into four stages,including initial,middle,severe,and recovery stages[96].
TCM efficacy against COVID-19 is remarkable
According to the WHO,during the SARs outbrea k,compared to conventional western medicine treatment,TCM treatments could reduce fever and hospitalization days of patients,with low high cost and no challenges such as transfer,death,sequelae,and medical staff infections[97,98].Among SARS patients treated with glucocorticoids,the combination of TCM and western medicine significantly reduced the mortality rate,shortened fever duration,reduced abnormalities in chest radiographs,and alleviated secondary fungal infection after glucocorticoid use[99].A meta-analysis of clinical efficacy among 3,444 patients in 30 studies showed that the average antipyretic time of the TCM treatment group was significantly shortened[100].Among the confirmed cases of COVID-19 in China,the coverage rate of TCM was 91.5%,and the total effective rate of TCM was >90%[101].For patients with mild or moderate illness,early intervention using TCM has been demonstrated to be effective in preventing the disease from advancing to a severe or critical state.In severe cases,TCM treatment stabilized patients and prolonged the treatment window.Increasing evidence has revealed that early intervention with TCM for COVID-19 improves cure rate,shortens of disease course,delays disease progression,and reduces mortality rate[102,103].
In the fight against the COVID-19 epidemic,through clinical screening,the “three preparations and three formulas of TCM”emerged,including:Jinhua Qinggan granules(L.japonica,Gypsum fibrosum,Ephedra sinica,Semen Armeniacae amarum,etc.,approved by China Food and Drug Administration(CFDA),No.Z20160001),Lianhua Qingwen capsules(Forsythia suspensa,L.japonica,E.sinica preparata,Semen Armeniacae amarum preparata,G.fibrosum,etc.,approved by CFDA,No.Z20040063),Xuebijing injection(Carthamus tinctorius,Radix paeoniae rubra,Ligusticum wallichii,etc.,approved by CFDA,No.Z20040033),Qingfei Paidu decoction(E.sinica,Glycyrrhiza uralensis preparata,Semen Armeniacae amarum,G.fibrosum,Ramulus Cinnamomi,etc.,approved by CFDA,No.C20210001),Huashi Baidu formula(E.sinica,Agastache rugosus,G.fibrosum,Semen Armeniacae amarum,Rhizoma Pinelliae Preparata,etc.,approved by CFDA,No.C20210002),Xuanfei Baidu formula(E.sinica,Semen Armeniacae amarum,G.fibrosum,Semen Coicis,Atractylis lancea,etc.,approved by CFDA,No.C20210003)and other effective formulas have been integrated in theDiagnosis and Treatment Protocol for COVID-19(trial version 8).They can reduce the incidence rate,severe illness rate,and death rate associated with COVID-19,promote the negative conversion of nucleic acid,improve cure rate,and shorten the recovery period(see Supplementary Table S1 for all drug compositions)[104].On April 14,2020,the National Medical Products Administration approved the new indication of COVID-19 for the “three preparations of TCM”.On March 2,2021,three TCM preparations,Qingfei Paidu granules,Huashi Baidu granules,and Xuanfei Baidu granules,which originated from ancient classic prescriptions,were approved for marketing.In addition to the above preparations and formulas,TCM injections,such as Xiyanping injection(approved by CFDA,No.Z20026249),Reduning injection(approved by CFDA,No.Z20050217),Tanreqing injection(approved by CFDA,No.Z20030054),Shenfu injection(approved by CFDA,No.Z51020664),Shengmai injection(approved by CFDA,No.Z51021882),and Shenmai injection(approved by CFDA,No.Z13020886),have several advantages,including high bioavailability,relatively clear components,and rapid absorption,and are suitable for use in complementary treatment of severe or critical COVID-19 cases[105-107].The results of 40 representative clinical trials and basic experiments have demonstrated that the corresponding Chinese patented medicines or TCM formulas have remarkable therapeutic effects and potential at different treatment stages,based on aspects such as clinical manifestation,lung characteristics,and laboratory results[102].Nevertheless,more high-quality randomized controlled trials are required to verify the effectiveness of TCM in treating COVID-19,and to investigate the toxicity and side effects of such drugs.The effects and mechanisms of TCM against COVID-19 include but are not limited to(1)inhibiting the invasion and replication of SARS-CoV-2 by targeting S protein,ACE2,TMPRSS2,Plpro,3Clpro,RdRp,and Spike-ACE2 interaction;(2)regulating immune and inflammatory responses by targeting inflammatory factors,such as IL-1,TNF-α,and IL-8 secreted by monocytes,macrophages,dendritic cells,CD4+T cells,and CD8+T cells,as well as chemokines such as C-C motif chemokine ligand 5,C-C motif chemokine ligand 2 and IFN-γ induced protein 10;(3)preventing and treating acute respiratory distress and multiple organ dysfunction syndrome by targeting IL-6,C-reactive protein,d-dimer,and procalcitonin to inhibit interactions among viral toxicity,endothelial damage,cytokine storm,hyperimmunity,and microthrombi[108].
TCM can provide important ideas for broad-spectrum anti-SARSCoV-2
According to the WHO,about 80% of the global population rely on medicinal plants or herbs for their medicinal needs[109].To date,dozens of Chinese herbal medicines and hundreds of natural TCM components have been reported to have antiviral activities[110].Anti-virus treatment with TCM has various advantages including broad spectrum application,low toxicity and side effects,low susceptibility to drug resistance,overall comprehensive regulation,etc.Consequently,research on the active components and mechanisms of action of the anti-viral effects of TCM have increasingly attracted the attention of researchers.Against a background of surging demand for COVID-19 treatment,researchers have summarized the potential effects and mechanisms of more than 200 natural products of TCMs,including flavonoids,alkaloids,terpenoids,polyphenols,and quinones,and their anti-inflammatory,anti-viral,anti-oxidative,anti-apoptotic,anti-pulmonary fibrosis properties[111]TCM constitute a wide variety of natural medicinal plants and metabolites,which are important sources of medicinal BSA screening material.Such natural plant metabolites can block the fusion of S protein of coronavirus and the ACE2 of hosts,inhibit the activity of key enzymes involved in the CoV replication cycle,including Plpro and 3Clpro,and inhibit cell signaling pathways[112,113].Their diverse multidimensional chemical structures makes them advantageous in serious disease treatment,and some of them have been reported to be promising alternative drugs and lead compounds[112].In addition,the versatility of secondary metabolites can also address the drug resistance of clinical antibiotics and antiviral drugs.Table 2 summarizes the anti-coronavirus targets and mechanisms of some representative TCMs and their active ingredients.
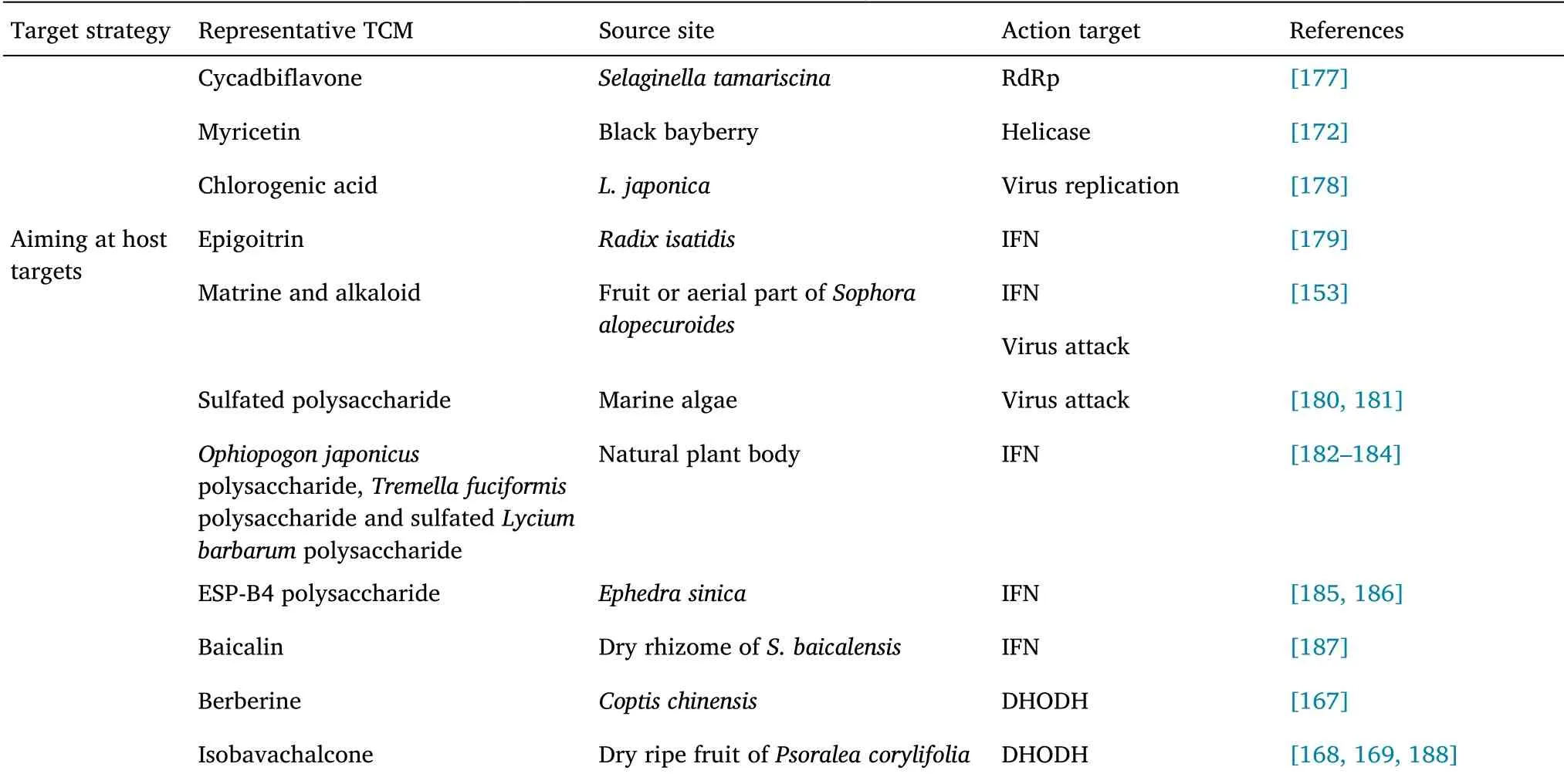
Table 2 Summary of TCM anti-coronavirus targets and mechanisms(Continued)
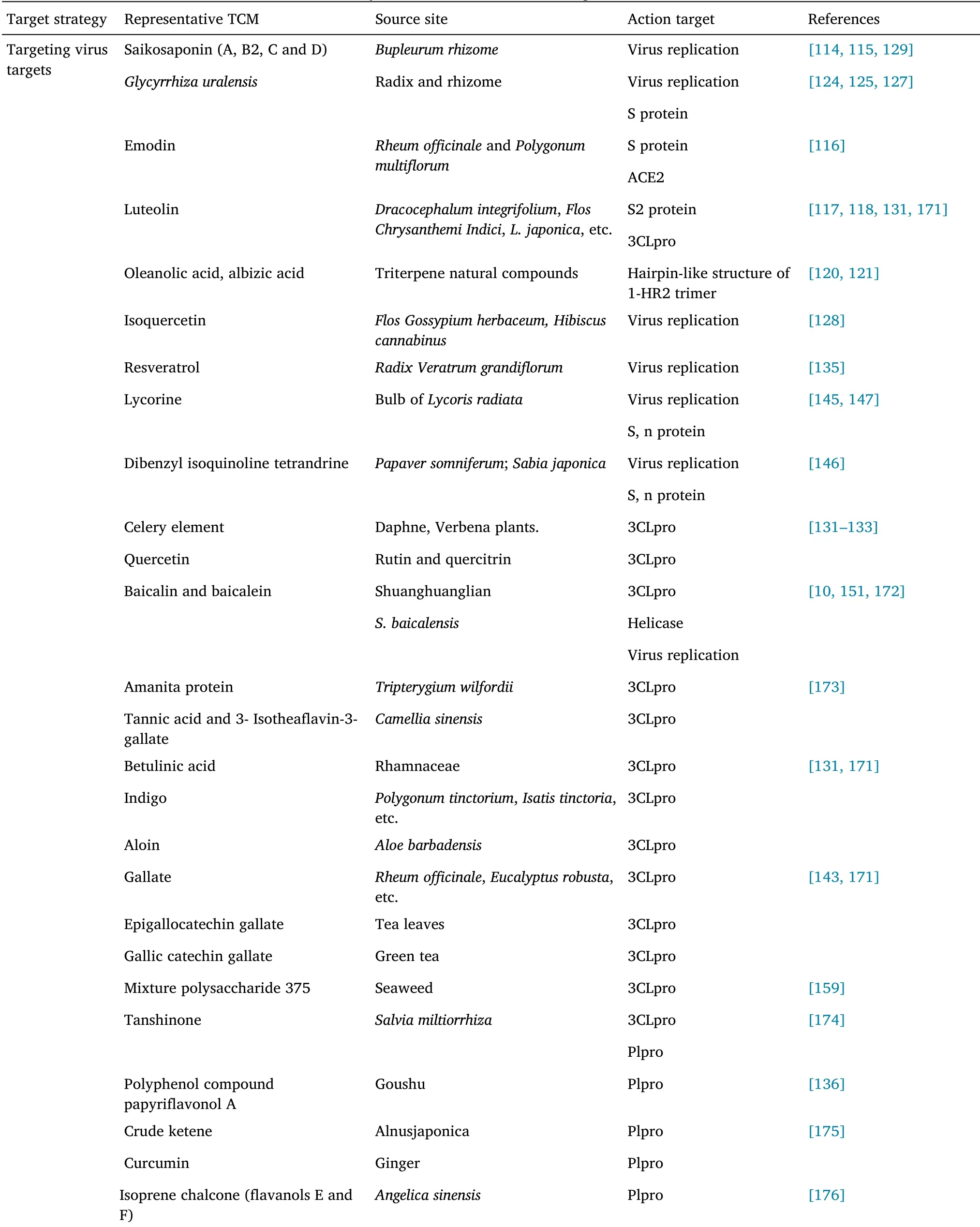
Table 2 Summary of TCM anti-coronavirus targets and mechanisms
Targeting virus adsorption and endocytosis.Saikosaponin B2 has significant inhibitory effects against SARS-CoV and human coronavirus(HCoV)-229E infections,and its mode of action may involve interference in the early stages of virus replication,such as virus absorption and penetration[114,115].Emodin,which has an anthraquinone structure and is derived fromRheum officinaleandFallopia,can block interaction between S protein and ACE2 significantly,in a dose-dependent manner and affect the adsorption and invasion of SARS-CoV into host cells.It also inhibits the infectivity of S protein pseudoretrovirus to Vero E6 cells.Therefore,emodin is considered a potential drug for SARS-CoV2 treatment[116].Luteolin binds to S2 protein on the surface of SARS-CoV(EC50:10.6 μM)and interferes with virus entry into host cells[117,118].A study screened the National Institutes of Health Clinical Collection in cell culture using a luciferase reporter-expressing recombinant murine coronavirus.Among the 727 compounds screened out,84 compounds had remarkable anti-coronavirus effects,51 compounds prevented the virus from entry,and the other 19 compounds inhibited virus replication.Among them,homoharringtonin and hexachlorophenol,which exhibited the strongest inhibitory activities,exhibit antiviral activity against numerous human and animal coronaviruses[119].In addition,triterpene natural compounds,such as oleanolic acid and echinocystic acid,can cover the spiral surface of HR2 through their hydrophobic pentacyclic skeleton,which hinders the formation the hairpin-like structure of HR1-HR2 trimer required for the fusion of virus and cell membrane,thus showing broadspectrum antiviral activity against influenza,Ebola,and HIV virus[120,121].
Glycyrrhiza uralensisFisch.is a common herb that harbors more than 20 types of trimers and about 300 types of flavonoids,which have great potential as antiviral or antimicrobial drugs.Glycyrrhizic acid(GL)and glycyrrhetinic acid(GA),the major active components ofGlycyrrhiza uralensis,can interfere with hepatitis virus,HIV,herpes virus,and influenza virus development[122,123].The effect of GA,an aglycone metabolite of GL,on SARS-CoV-2 infection was analyzed using a S protein-pseudotyped Lenti virus vector.GA could block protein-pseudotyped Lenti virus infection in a dose-dependent manner and directly target protein-pseudotyped Lenti virus virus instead of host cells.The results of surface plasmon resonance and molecular docking showed that GA interacted with recombinant S protein at the interface between S protein and ACE2,as well as the inner sites of RBD,and blocked the binding of S protein to host cells[124,125].In addition,in vitro research findings have indicated that GL has antiviral effects on SARS infection[126].GL can inhibit the adsorption and penetration of viruses in the early stages of the replication cycle,as well as inhibit the replication of clinically isolated coronaviruses(FFM-1 and FFM-2).The underlying mechanism may involve regulating and inducing the expression of nitrous oxide synthase and production of nitrous oxide in macrophages[127].Such findings indicate that adding GL after virus adsorption is more effective than adding GL during adsorption.
Targeting virus replication.Isoquercetin can significantly inhibit influenza A and B virus replication.In the presence of isoquercetin,continuous virus passage would not lead to the emergence of drugresistant virus,whereas adding isoquercetin to amantadine or oseltamivir treatment could inhibit the emergence of amantadine or oseltamivir-resistant virus.In a mouse model infected with influenza virus,mice infected with influenza A virus and injected with isoquercetin intraperitoneally had significantly reduced virus titer and improved lung pathology[128].Saikosaponin(A,B2,C and D)has a clear inhibitory effect on HIV,measles,influenza virus,herpes simplex,and chickenpox virus.Saikosaponin A weakened the replication of three influenza A virus strains(including the highly pathogenic H5N1 strain)in human alveolar epithelial A549 cells.In a H1N1 lethal model established in C57BL/6 mice,saikosaponin A reduced virus replication,down-regulated the expression of NF-κB,reduced the nuclear output of caspase-3-dependent virus ribonucleoprotein,and increased the production of proinflammatory cytokines significantly,with abnormal anti-influenza virus activity and changes in lung tissue pathology[129].
The antiviral activity of some flavonoids against CoV is considered to be directly caused via 3CLpro inhibition[130].Apigenin and quercetin exhibit anti-SARS-CoV activity by inhibiting 3CLpro,with IC50values of 38.4 ± 2.4 μM and 23.8 μM[131-133],respectively.In addition,herbacetin,rhoifolin,and pectolinarin could effectively block the SARS-CoV 3CLpro activity[133].Tryptophan-based fluorescence and induced-fit docking analyses have showed that S1,S2,and S3 sites are involved in its binding with 3CLpro.Studies conducted based on in-silico molecular docking and molecular dynamics supported by in-vitamin associations show that various plant polyphenols have the potential to inhibit 3CLpro activity,and the IC values of quercetin,ellagic acid,curcumin,epigallocatechin gallate,and resveratrol are in the 11.8-23.4 µM range[134].Resveratrol significantly inhibits MERS-CoV infection and reduces it replication in vitro[135].In addition,polyphenols isolated fromBroussonetia papyriferahave greater effects on Plpro than on 3LPRO[136].Papyriflavonol A is the most effective Plpro inhibitor in SARSCoV,with an IC50of 3.7 μM.Baicalin and baicalein,two components of Shuanghuanglian,have been characterized as non-covalent and non-peptidomimetic inhibitors of SARS-CoV-2 3CLpro activity,and have demonstrated effective antiviral activity in cell-based systems.The binding patterns between baicalein and SARS-CoV-2 3CLpro determined using X-ray protein crystallography is significantly different from those of the known 3CLpro inhibitor.Baicalein is effectively embedded in the core of the substrate binding pocket by interaction with two catalytic residues,key S1/S2 subsites and oxygen anion ring,and acts as a “barrier” in front of the catalytic binary,effectively preventing the substrate from entering the catalytic binary in the active site[10].
Amanita protein(IC50:2.6 μM)and pristane(IC50:5.5 μM),with a quinone formyl triterpene structure,and isolated fromTripterygium wilfordii,as well as tannic acid(IC50:3 μM)and 3-isotheaflavin-3-gallate(IC50:7 μM),isolated fromCamellia sinensis,have clear 3CLpro inhibitory activity[137,138].Tanshinone,with an abietylene diterpene structure,and isolated fromSalvia miltiorrhiza,has specific or selective inhibitory effects on SARS coronavirus 3CLpro and Plpro,and their activities are slightly affected by subtle changes in structure and targeting enzymes.However,the effect of tanshinone on Plpro activity was much greater than that on 3CLpro(the IC50ranged between 0.8 µM and 30 μM)[139].The IC50values of cryptotanshinone,tanshinone IIA,and dihydrotanshinone were 0.8,1.6,and 4.9 μM,respectively.Crude ketene fromAlnus japonicaand curcumin from ginger demonstrated effective inhibitory activity against Plpro,with IC50values of 4.1 μM and 5.7 μM[140],respectively.Structure-activity relationship analysis showed that the α,β-unsaturated carbonyl part was vital for the inhibitory activity[141].Isoprenyl chalcones(such as flavanol E and flavanol F)isolated fromAngelica sinensishave strong non-competitive inhibitory effects against Plpro,with IC50ranging from 1.2 μM to 5.6 μM[142].Furthermore,betulinic acid,indigo,aloenin,luteolin,quinmethyl triterpene,quercetin,or gallate have certain inhibitory effects against SARS-CoV 3CLpro activity,with IC50values ranging from 3 µM to 300µM[133].Quercetin,epigallocatechin gallate,and gallocatechin gallate(exhibit good inhibition by binding to 3CLpro active sites and the 3-OH gallate group(necessary for virus inhibition activity),with IC50values of 73,73,and 47 μM,respectively[133].Notably,quercetin could alter expressions of 98(30%)out of 332 human genes encoding SARS-CoV-2 protein targets,which might interfere with the functions of 23(85%)out of 27(85%)genes of SARS-CoV-2[143].
Alkaloids are expected to have inhibitory effects against SARS-CoV-2.Different types of alkaloids achieve anti-SARS activity by inhibiting protease activity and RNA and protein synthesis[144].Some alkaloids,such as tetrandrine,cephalosporin,and lycorine,act on SARS coronavirus as nucleic acid intercalators,by degrading nucleic acids and inhibiting S and N protein synthesis[145].Bisbenzylisoquinoline alkaloids,including tetrandrine,fangchinoline and cephalosporin,inhibited virus-induced cell death significantly at the early stages of virus infection,with IC50values of 14.51 μM,12.4 μM,and 10.54 μM,respectively,which inhibited the expression of S and N proteins,and had potential antiviral activity against HCoVOC43 infection and inhibited virus replication[146].In vitro experiments have showed that lycorine has potential inhibitory activity against coronavirus replication,with HCoV-OC43,MERS-CoV,and HCoV-NL63 having EC50values of 0.15 μM,1.63 μM,and 0.47 μM,respectively.Lyricine could reduce the viral load in the central nervous system of BALB/c mice,and prevent the lethality induced by HCoV-OC43[147].Furthermore,tylophorine and its analogues have phenanthroline alkaloid(IC50:58 nM)and 7-methoxycryptopleurine(IC50:20 nM)structures,which have strong inhibitory effects against coronavirus replication[148].
Biflavonoid skeleton is a potential RdRp inhibitor;specifically,amentoflavone and robustaflavone are the most promising inhibitors.An in vitro study has shown that sotetsuflavone is the greatest inhibitor of dengue virus RdRp activity,with an IC50value of 0.16 μM[149].Myricetin and baicalein could bind to the key residues of the adenosine triphosphate/adenosine diphosphate binding capsule of SARS-CoV helicase protein nsP13 in vitro,and interfere with the adenosine triphosphatases activity of SARS-CoV helicase protein,so that they can be used as chemical inhibitors of SARS-CoV[150].In addition,baicalein could inhibit the synthesis of H1N1 virus mRNA in a dose-dependent manner,and has stronger antiviral effects than ribavirin[151].Furthermore,chlorogenic acid could inhibit the transcription and protein translation of viral mRNA,reduce virus generation,protect Madin-Darby canine kidney cells from virus infection in a dose-dependent manner,protect mice from death caused by H1N1 and H3N2 by 60% and 50%,effectively reduce virus titer,and alleviate pulmonary inflammation[152].
Targeted host cell immunomodulation.Matrine alkaloids could directly inactivate viruses,block virus adsorption and entry into cells,and inhibit virus replication in cells,thus producing antiviral effects[153].By up-regulating the phosphatidylinositol-3 kinase/protein kinase B signaling pathway and down-regulating the toll-like receptor(TLR)4/myeloid differentiation primary response 88 signaling pathway of virus-infected cells,they can promote the expression of IFN and inhibit the expression of inflammatory factors,and prevent damage to cells by virus.Matrine and sodium chloride injection had significant therapeutic effects in a HCoV-229E mouse model,and the underlying mechanism is associated with inhibition of virus replication,regulation of immune function,and inhibition of the release of inflammatory factors.Oxymatrine can significantly reduce the activity of gene promoters of immune recognition signal molecules,including TLR3,TLR4,TLR7,myeloid differentiation primary response 88,and TNF receptor associated factor 6,inhibit the Akt pathway induced by influenza virus,and reduce the expression of inflammatory cytokines and matrix proteins.Oxymatrine can also promote the production of IFN-γ in CD4+T cells in a dose-dependent manner,thus inhibiting hepatitis B virus replication.Baicalin exerts its anti-influenza activity by regulating the expression nonstructural protein 1 of influenza A virus protein,which leads to up-regulation of IFN-induced antiviral signal transduction and a decrease in PI3K/Akt signal transduction in cells[154].Epigoitrin found inRadix Isatidisinhibits H1N1 virus replication by promoting the generation of IFN-β in the virus susceptibility model induced by corticosterone,promotes the expression of mitochondrial antiviral signal,and plays an antiviral role.
TCM polysaccharides are important bioactive macromolecules in TCMs,and they exhibit numerous pharmacological activities,including antiviral,antibacterial,anti-inflammatory,antioxidant,immunomodulating,and intestinal flora regulation activities.TCM polysaccharides have broad sources,low toxicity,and good biocompatibility,and can exert antiviral activity at different stages of virus infection[155].In addition,TCM polysaccharides have multicomponent,multi-pathway,and multi-target characteristics,and drug resistance does not easily emerge.Considering the entire virus life cycle,TCM polysaccharides can directly kill the virus,prevent its adhesion and further invasion,and inhibit its replication,transcription and translation in cells.In addition,TCM polysaccharides can play antiviral roles by regulating immunity,antiinflammation,anti-oxidation,and intestinal flora.Interaction betweenS.baicalensispolysaccharide and Newcastle disease virus led to a decrease in the titer of the virus,which indicated that this polysaccharide could directly kill the virus.In addition,Ophiopogon japonicuspolysaccharide[156],Tremella fuciformispolysaccharide and sulfatedLycium barbarumpolysaccharide[157]can directly kill the virus[158]in vitro.Four types of acidic polysaccharides have been isolated fromPogostemon cablin.Through a comparison of three dosing methods,four types of polysaccharides could effectively inhibit the replication of porcine epidemic diarrhea virus in vitro.The mixed polysaccharide 375 from a seaweed,Ecklonia kurome,completely inhibited 3CLpro activity(IC50:0.48 μM).In addition,homogeneous polysaccharide 37502 from 375 could bind well to 3CLpro molecules(kD:4.23 × 106),and could effectively interfere with the binding of S protein to ACE2 receptor(EC50:2.01 μM)[159].Sulfated polysaccharides from marine algae can inhibit the adhesion and invasion of HSV,dengue virus,and other viruses,in vitro[160].In addition,Poria Cocospolysaccharide[161],Rehmannia glutinosapolysaccharide[162],Lycium barbarumpolysaccharide[163],Astragalus membranaceuspolysaccharide[164],Glycyrrhiza uralensispolysaccharide[165]can also be used as adjuvants during vaccine injection and can increase vaccine immunogenicity,enhance humoral and cellular immunity,and facilitate the achievement of long-term protection effects.Notably,the solubility and bioavailability of some TCM components,such as polysaccharides,resveratrol,quercetin,baicalin,curcumin,emodin,and tanshinone IIA are limited,which leads to poor blood absorption following oral administration.Such compounds may exert their therapeutic effects through interaction with intestinal microbes[127].ESP-B4 is a homogeneous polysaccharide isolated fromE.sinica,with a uronic acid content of 77.5% and relative molecular weight of 23 kD.Lin et al.found that ESP-B4 could ameliorate acute lung injury caused by H1N1 virus in mice,and significantly increase the abundance ofLactobacillalesandBifidobacteriumamong intestinal flora of mice,which indicates that ESP-B4 might indirectly play an antiviral role by regulating intestinal flora[166].
Molecular docking tests have showed that six compounds,includingemodin-1-0-β-D-glucopyranoside,quercetin-3-Oarabinoside,and2,3,5,4’-tetrahydroxystilbene-2-O-β-Dglucopyranoside,fromPolygonum cuspidatum,have strong inhibitory effects against DHODH activity.Berberine,a natural alkaloid inRhizoma coptidis,is a novel and powerful inhibitor of PfDHODH activity,with an IC50value of 1.83 ± 0.08 μM.In addition,enzyme kinetic analysis has showed that berberine is a noncompetitive inhibitor of PfDHODH.Heat displacement and molecular docking simulation analyses have showed that berberine could bind to PfDHODH.In addition,the inhibitory activity of coptisine against human DHODH is weak,which indicates that coptisine is a selective inhibitor of PfDHODH[167].Enzymology analysis,heat displacement measurement,pull-down,nuclear magnetic resonance,and isothermal titration calorimetry experiments have showed that isobavachalcone could inhibit human DHODH[168].(4S,6R)-4-hydroxy-6-isopropyl-3-methylcyclohex-2-enone and(4S,5R,9S,10R)-8(17),12,14-labdatrien-18-oic acid isolated from aerial parts ofChloranthus elatiorhas good anti-hDHODH activity,with IC50values of 18.7 µM and 30.7 µM,respectively[169].Celestrol,extracted from“Tripterygium wilfordii”,could reduce the levels of uridine and DHODH protein in tumor tissues[170],with no systemic toxicity at the pharmacological dose(2 mg/kg,i.p.,21 days).
Conclusion and future prospects
With increasing viral mutations,compared to chemical drugs that have distinct active ingredients,TCMs have multi-level,-target,and-pathway characteristics,which may confer advantages in terms of dealing with viral mutations through the exertion of broad effects.Consequently,TCM has great application prospects in the treatment and control of viral infections that can cause an outbreak in the future.Most natural products listed in this paper have good biologicalactivityinvitro;however,moreanimal experiments/clinical studies are required to investigate their bioavailability and biological activity in vivo,and to confirm their effectiveness in resisting coronavirus infection and enhancing virus immunity.In addition,standardization of preparation,purification,identification,and analysis of Chinese herbal medicines is necessary for the establishment of stable and safe lead molecules.In addition,the investigation of acute and long-term toxicity and pharmacokinetic characteristics of the active compounds,as well as the necessary structural modifications,dosages,and intellectual property rights based on bioavailability,must also be considered when developing candidate drugs.The combination of drug development and clinical treatment should be performed not only through screening,molecular experiments,animal experiments and small-scale clinical trials,but also through clinical observation to confirm curative effects,as well as through continuous monitoring,to enable the exploitation of the benefits of both empirical research and clinical practice.Furthermore,different research techniques and disciplines should be integrated fully in drug screening and evaluation,including technologies such as supercomputing,multiomics,artificial intelligence and other disciplines.
 Traditional Medicine Research2022年3期
Traditional Medicine Research2022年3期
- Traditional Medicine Research的其它文章
- Anti-asthmatic mechanism of the Huashanshen dripping pill via suppressing contraction of the airway smooth muscle
- Application of network pharmacology in the prevention and treatment of COVID-19 by traditional Chinese medicine
- Pharmacological efficacy of the traditional Chinese medicinal formula Kun-Tai-1A in the treatment of letrozole-induced polycystic ovary syndrome
- Recent advances in research on natural product inhibitors of SREBPs
- Integrated UHPLC-MS and network pharmacology to explore the active constituents and pharmacological mechanisms of Shenzao dripping pills against coronary heart disease
- Effects of Qingwen Baidu decoction on coagulation and multiple organ injury in rat models of sepsis
