Anti-asthmatic mechanism of the Huashanshen dripping pill via suppressing contraction of the airway smooth muscle
Yu Liu,Ni-Na Cui,Xuan-Shuo Liu,Peng-Cheng Lin,Lorenzo Pecoraro,Giuseppe Venturella,Shu-Li Man*,Wen-Yuan Gao,*
1The Innovation Platform for the Development and Construction of Special Project of Key Laboratory for Tibet Plateau Phytochemistry of Qinghai Province(2017-ZJ-Y19),College of pharmacy,Qinghai Nationalities University,Xining 810007,China.2State Key Laboratory of Food Nutrition and Safety,Key Laboratory of Industrial Microbiology,Ministry of Education,Tianjin Key Laboratory of Industry Microbiology,National and Local United Engineering Lab of Metabolic Control Fermentation Technology,China International Science and Technology Cooperation Base of Food Nutrition/Safety and Medicinal Chemistry,College of Biotechnology,Tianjin University of Science&Technology,Tianjin 300457,China.3Tianjin Key Laboratory for Modern Drug Delivery and High Efficiency,School of Pharmaceutical Science and Technology,Tianjin University,Tianjin 300072,China.4Department of Agricultural,Food and Forest Sciences,University of Palermo,Palermo,Italy.
Abstract Background:The Huashanshen(HSS)dripping pill has been widely used in asthma for a long time in China.However,the relaxant mechanism of HSS is not well understood.Methods:In this report,high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry was used to identify the constituents in rat plasma after oral administration of HSS.Ovalbumin-sensitized allergic asthma and isolated trachea were studied for the anti-asthmatic mechanism of HSS.Results:D-anisodamine,L-anisodamine,scopolamine and atropine were detected in the rat plasma containing HSS.It was clear that the HSS inhibited the release of inflammatory mediators,regulated the balance of T-helper 1 and T-helper 2 to reduce the airway inflammation,and relaxed the tracheal smooth muscle by controlling the KCa channel,Ca2+ influx and release to reduce the airway hyperresponsiveness.Conclusion:Atropine,anisodamine and scopolamine might be active compounds of HSS which inhibited the release of inflammatory mediators,regulated the balance of Th1/Th2,and relaxed the tracheal smooth muscle to reduce airway hyperresponsiveness.
Keywords:Huashanshen dripping pill;HPLC-QTOF-MS;allergic asthma;airway remodeling;inflammation;airway smooth muscle
Background
Asthma is a chronic inflammation that results in airway obstruction and hyper-responsiveness of the airway smooth muscle(ASM)in response to direct or indirect stimuli,resulting in bronchoconstriction[1,2].Allergic asthma is the most prevalent form of asthma,in which individuals have an imbalance of T-helper(Th)1/Th2 cells and antigen-specific immunoglobulin E(IgE)-mediated response to common aeroallergens[3,4].When an allergen presents antigen-presenting cells to Th0 cells,this results in Th1 and Th2 cell differentiation[1,5].The Th2 cells produce interleukins(IL),as cytokines,which induce airway inflammation.For example,IL-4 induce B-cells to produce IgE.Meanwhile,IL-5 is implicated in the maturation of eosinophils,and their survival and migration into the airways[5-7].
ASM is the main cell that regulates airway resistance and hyper-reactivity,hallmark features of asthma and exacerbations of the disease[8].In addition to the fact that the inflammatory response generated by allergic asthma modulates the contractility of ASM,a variety of regulatory mechanisms have been suggested for the relaxant effects of medicinal plants on tracheal smooth muscles,such as potassium channel opening,calcium channel-blocking,ß2-adrenergic receptor stimulation,nitric oxide(NO)production,cyclooxygenase(COX)pathways,and phosphodiesterase(PDE)activity[9,10].
Huashanshen(HSS)was first recorded inA Supplement to Compendium of Materia Medica,compiled by Zhao Xuemin(1719-1805 C.E.).It has been widely available for the treatment of asthma for a long time in China.Taking full advantage of its antiasthmatic,expectorant and antitussive effects,the HSS dropping pill is made from Huashanshen and is composed ofPhysochlainae Radixextract(Supplementary Figure S1).This process is achieved by refining it into a paste,and then making it into the dropping pill.The HSS dropping pill is a patented medicine officially listed in theChinese Pharmacopoeia(approved number:Z10900001)[11].HSS dropping pill contained 11 alkaloids such as atropine,anisodamine and scopolamine[12-14].Anisodamine was reported to reduce eosinophilic airway inflammation and airway hyper-reactivity[15].
However,the anti-asthma mechanism of the HSS dropping pill has been rarely studied.Meanwhile,it was not known which compound was responsible for the anti-asthma effect.This study aimed to examine the main components in rat plasma after oral administration of the HSS dropping pill,so as to explore the possible mechanism of its treatment.Then,the anti-asthmatic mechanism of the HSS dropping pill was studied through an isolated tracheal model.
Materials and methods
Materials
HSS dropping pills were obtained from Tianjin Zhongxin Pharmaceutical Group Co.,Ltd.(Tianjin,China).Formic acid,methanol and acetonitrile were obtained from Merck Company(Darmstadt,Germany).Atropine,D/L-anisodamine and scopolamine standards were obtained from the Shanghai Standard Technology Co.,Ltd.(Shanghai,China).Ovalbumin(OVA,grade II)and aluminum hydroxide were obtained from Sigma-Aldrich(St.Louis,MO,USA).The study was conducted in accordance with theBasic &Clinical Pharmacology &Toxicology Policyfor experimental and clinical studies[16].
Preparation of plasma samples
All animals were purchased from the Experimental Animal Center,Chinese Academy of Medical Sciences,Peking(license No.SCXK(Jing)2016-0006,Beijing,China).Rats were administered(200 mg/kg,p.o.)HSS three times a day for three consecutive days.One hour after the last administration,blood samples were collected into heparinized tubes by retroorbital sinus puncture.The method of preparing plasma samples was the same as in our previous research[14].All experimental protocols were approved by the Animal Ethics Committees of the Faculty of Medicine,Tianjin University,Tianjin,China(approved date:20200331).
High performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry(HPLC-QTOF-MS)analysis
The instruments consisted of an Agilent G6500 HPLC-QTOF-MS(Agilent,Santa Clara,CA,USA)equipped with electrospray ionization.Chromatographic separation was carried out on a Kinetex XB-C18 column(50 mm×2.1 mm,1.7µm;Phenomenex,Torrance,CA,USA).The flow rate was 0.25 mL/min,and the temperature of the column was set at 30 °C.Mobile phase A was acetonitrile and mobile phase B was 0.1% formic acid/H2O.The gradient condition was 2-7% A for 0-2 min,7% A for 2-4 min,7-30% A from 4-10 min,30-50% A for 10-12 min,50-95% for 12-16 min,and 95% A for 16-20 min.The 2-µL sample was injected into the system for analysis.
To ensure the accuracy and reproducibility of the experiment,the mass spectrometer was calibrated in the range of 50-1,500 Da before the experiment.The operation was carried out by the Hystar Software Company(Bruker Daltonik GmbH,Bremen,Germany).The corresponding retention times and fragment ions of the samples were determined,and compared with those of the atropine,D/L-anisodamine and scopolamine standards.The mass analysis was in positive ionization mode,and the instrument parameters were set as follows:drying gas temperature 320 °C,drying gas flow 8.0 L/min,sheath gas temperature 350 °C,nebulizer pressure 35 psi,sheath gas flow 11 L/min,capillary voltage 3,500 V and nozzle voltage 1,000 V.
The effects of HSS on alleviating the airway inflammation
Induction of allergic asthma with OVA.Male BALB/c mice(20 ± 2 g),6 weeks old,were divided into four groups(Figure 1):control group(saline),model group(saline),HSS dropping pills group(108 mg/kg,20 mg/pill × 6 pills/60 kg × 9 × 6,where 9 was the conversion coefficient and 6 was the expansion multiple)and dexamethasone(DM)group(2 mg/kg)[14,17].On the first and eighth days,every mouse of the model,HSS,and DM mice groups was administered 100 µg OVA and 2 mg aluminum hydroxide(i.p.).Seven days after the final sensitization,mice were stimulated with 5% OVA solutions(atomized for 20 minutes)for five days.The challenges were then continued for 14 days at once every two days.The treatment groups received oral administration at 1 h after the OVA challenge from the 15th to 34th days.
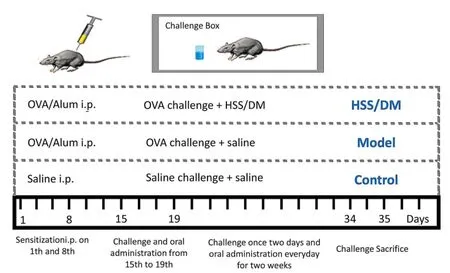
Figure 1 Treatment protocols of allergic asthma induced by OVA.Alum i.p.,aluminum hydroxide;Saline i.p.,physiological saline;OVA,ovalbumin;DM,dexamethasone;HSS,huashanshen.
Behavior of allergic mice.Before the sacrifice,the behaviors of the mice were obsesrved in the 500 mm× 500 mm × 400 mm activity box(DB018,Beijing Zhishuduobao Biotechnology Co.,Ltd.,Beijing,China)for 5 min.All trajectories were recorded by a camera and analyzed.The movement ability change of the mice was monitored by analyzing the trajectory and average velocity of the allergic mice.The whole set of open field instruments was provided by the Beijing Zhishuduobao Biotechnology Co.,Ltd.
Collection of bronchoalveolar lavage fluid(BALF).All of the right lung was tied for histological analyses.BALF was flushed back and forth in the left lung(0.5 mL normal saline).BALF was centrifuged at 2,000 rpm for 8 min,and then stored at-20 °C.
Histological analysis.After BALF,the lobes of the right lung were fixed in 10% formalin processed for embedding in paraffin.They were then cut into 5-µm thick sections.Next,they were stained with hematoxylin and eosin.Six histological sections per lung were randomly analyzed in each group.The histopathological examination was performed by pathologists who did not know the treatment of each group.
Cytokine measurements.The enzyme-linked immunosorbent assay kits((Nanjing SenBeiJia Biological Technology Co.,Ltd.)were used for the detection of mouse IgE,IL-4,IL-5,interferon-γ(INF-γ)and endothelin 1(ET-1)in BALF.The known antigen or antibody was adsorbed on the surface of the solid carrier(polystyrene micro reaction plate),and the enzyme-labeled antigen antibody reaction was carried out on the solid surface.The free components in the liquid phase were washed out by washing method.The specific experimental steps can be found in the published literature[14].The brief steps are as follows:add the prepared sample,react at 37 °C for 30 min,wash for five times,add enzyme labeling reagent,react at 37 °C for 30 min,wash again for five times,add color developing solution for 10 min,add termination solution,and determine its optical density value.
Protein measurements.In accordance with the manufacturer’s manual(Nanjing SenBeiJia Biological Technology Co.,Ltd.),the protein levels were measured by a protein kit(Nanjing SenBeiJia Biological Technology Co.,Ltd.)in BALF.The specific experimental steps were performed in accordance with the previously published literature[18].The brief steps are as follows:under alkaline conditions,divalent copper ions can be reduced to monovalent copper ions by proteins.Monovalent copper ions can interact with bicinchoninic acid.Two molecules of bicinchoninic acid chelate a copper ion to form a purple complex.The complex is water-soluble and shows strong absorbance at 562 nm.Within a certain concentration range,the absorbance has a good linear relationship with the protein content.So,the concentration of the protein to be tested can be calculated from the standard curve using the absorbance of the protein to be tested at 562 nm.
The effect of HSS on relaxing the ASM
Isolated trachea tissue preparation.After the Wistar rats were sacrificed and died,the trachea was divided into three or four cartilage rings.The tracheal ring was fixed between two hooks placed in a 20 mL organ bath containing Krebs solution(KCl 4.55 mM,KH2PO41.1 mM,NaCl 118 mM,NaHCO325 mM,MgSO45.7 mM,CaCl22.52 mM and glucose 11 mM)at 37 °C[19],and continuously aerated with a 5% CO2-95% O2mixture.While cleaning the tissue with Krebs solution every 15 minutes,1 g of isometric tension was applied for at least one hour.The tracheal ring contractile response was measured with an isometric transducer(MD3000,Beijing Zhishuduobao Biotechnology Co.,Ltd.,Beijing,China).
Effects of HSS on the acetylcholine(Ach)-induced tracheal contraction.For accuracy,Huashanshen extract was detected instead of HSS.The relaxant effects of HSS(Huashanshen extract,1-16 ng/mL)were evaluated in cumulative concentrations(concentration-response curves,CRC)under pre-contracted by 10 µM Ach.Furthermore,the CRC of HSS indicated their ability to decrease the maximal tracheal contraction induced by Ach in the absence and presence of HSS.The maximal tension induced by Ach was considered as 100%.
Effects of HSS on the basal tone of the tracheal rings.To determine the underlying mechanism of action of HSS,tracheal rings were pre-incubated with inhibitors,such as propranolol(nonselective β-adrenergic antagonist,10 µM),theophylline(PDE unspecific inhibitor,10 µM),indomethacin(COX inhibitor,10 µM),and n(omega)-nitro-L-arginine methyl ester(L-NAME,a nitric oxide synthase(NOS))inhibitor,10 µM)for 15 minutes prior to the Ach-induced contraction.The relaxation CRC of HSS(Huashanshen extract,1-16 ng/mL)was obtained.
Role of the potassium channel in the HSS-induced tracheal relaxation.To detect the involvement of the K+channel in the HSS-induced relaxant effect,tracheal rings were pre-incubated with tetraethylammonium(TEA,Ca2+-activated potassium KCa channels inhibitor,1 mM),glibenclamide(an adenosine triphosphate(ATP)-sensitive potassium KATPchannel specific inhibitor,10 µM)and 4-aminopyridine(4-AP,voltage-gated potassium KVchannel inhibitor,100 µM)for 15 minutes before ACh was added.The non-incubated tracheal rings with inhibitors were considered as the control.
Role of calcium in the HSS-induced tracheal relaxation.To assess the inhibition of extracellular Ca2+in the HSS-caused relaxation,the tracheal rings were stabilized in a Ca2+-free solution for 20 min after sensitization in the normal Krebs solution.The tissues were washed with Ca2+-free and high-K+(60 mM)Krebs solutions,and were stabilized for 15 min.A CRC for the CaCl2-induced(0.03-30 mM)contraction was then obtained in the absence of HSS(control group).The tracheal rings were washed with Ca2+-free Krebs solution containing high-K+(60 mM)and stabilized for 20 min when the maximal contraction was reached.Subsequently,the tissues were incubated with HSS(Huashanshen extract,16 ng/mL)for 15 min,and the second CRC for the CaCl2was then obtained.The contractile effect of HSS induced by CaCl2was compared with the non-incubation with HSS.The incubation with nifedipine(calcium channel blocker,4 ng/mL)was used as a positive control.
To determine the relationship in the relaxing effect induced by HSS and release of intracellular Ca2+,the tissues were stabilized in the Krebs solution without Ca2+for 20 min.The tracheal rings were then pre-incubated with HSS(Huashanshen extract 16 ng/mL)for 15 min before Ach was added to improve the release of intracellular Ca2+.The saline and nifedipine(4 ng/mL)were considered as a control and positive control,respectively.
Role of the potassium channel and calcium in alkaloids-induced tracheal relaxation.To determine whether the alkaloids in plasma act on the K+channel,atropine(atropine sulfate,4-64 ng/mL),anisodamine(racanisodamine,4-64 ng/mL)and scopolamine(scopolamine hydrobromide,4-64 ng/mL)were examined with tracheal rings.The method used here was the same as that described in histological analysis.
Meanwhile,atropine(atropine sulfate,16 ng/mL),anisodamine(racanisodamine,16 ng/mL)and scopolamine(scopolamine hydrobromide,16 ng/mL)were detected in the calcium influx or release in the ASM.The method used here was the same as that described in cytokine measurements.
Data analysis
Data were analyzed by statistical software IBM SPSS 21.0,and expressed as the mean ± standard error of the mean.Statistical significance was determined by the one-way analysis of variance,followed by Tukey’s multiple comparison test.
Results
Identification of HSS absorption in rat plasma
HPLC-QTOF/MS was used to analyze the components in the rat plasma.In our previous work,D-anisodamine in rat plasma was analyzed by the Dionex UltiMate 3000 UHPLC System(Thermo Fisher Scientific Co.,Ltd.,Waltham,MA,USA)with QTOF-MS(Bruker Daltonik GmbH Co.,Ltd.,Berlin,Germany)and Kinetex XB-C18 column(50 mm × 2.1 mm,1.3 µm;Phenomenex Co.,Ltd.,Torrance,CA,USA).However,an Agilent G6500 HPLC-QTOF-MS and Kinetex XB-C18 column(50 mm × 2.1 mm,1.7 µm;Phenomenex Co.,Ltd.,Torrance,CA,USA)were used to analyze the tropane alkaloids absorbed in the present study.Atropine,anisodamine and scopolamine were clearly detected as the muscarinic antagonists[20]in the plasma containing HSS(Figure 2).In accordance with the components analysis of HSS in the previous report[14],D-anisodamine,scopolamine,L-anisodamine and atropine were identified by comparing their retention times and mass spectra with the characteristics of the standard(Table 1 and Supplementary Figure S2).D-Anisodamine produced a precursor ion[M + H]+at m/z 306.1697.The fragment ions at m/z 140.1072[M + H]+and m/z 122.0961[M +H]+were produced by the loss of tropic acid(C9H10O3)and a neutral fragment(C9H10O3+ H2O)from the parent ion,respectively.The characteristic ions of L-anisodamine were the same as those of D-anisodamine,while the distinct retention times values were an indicator of their differences.Scopolamine produced a precursor ion[M + H]+at m/z 304.1537.The fragment ion at m/z 156.1014[M + H]+was produced by the loss of a neutral fragment(C9H8O2)from the parent ion.Atropine produced a precursor ion[M+ H]+at m/z 290.1749.The fragment ion at m/z 124.1119[M +H]+was produced by the loss of tropic acid(C9H10O3)from the parent ion.The results demonstrated that anisodamine,scopolamine and atropine were the active components in HSS.
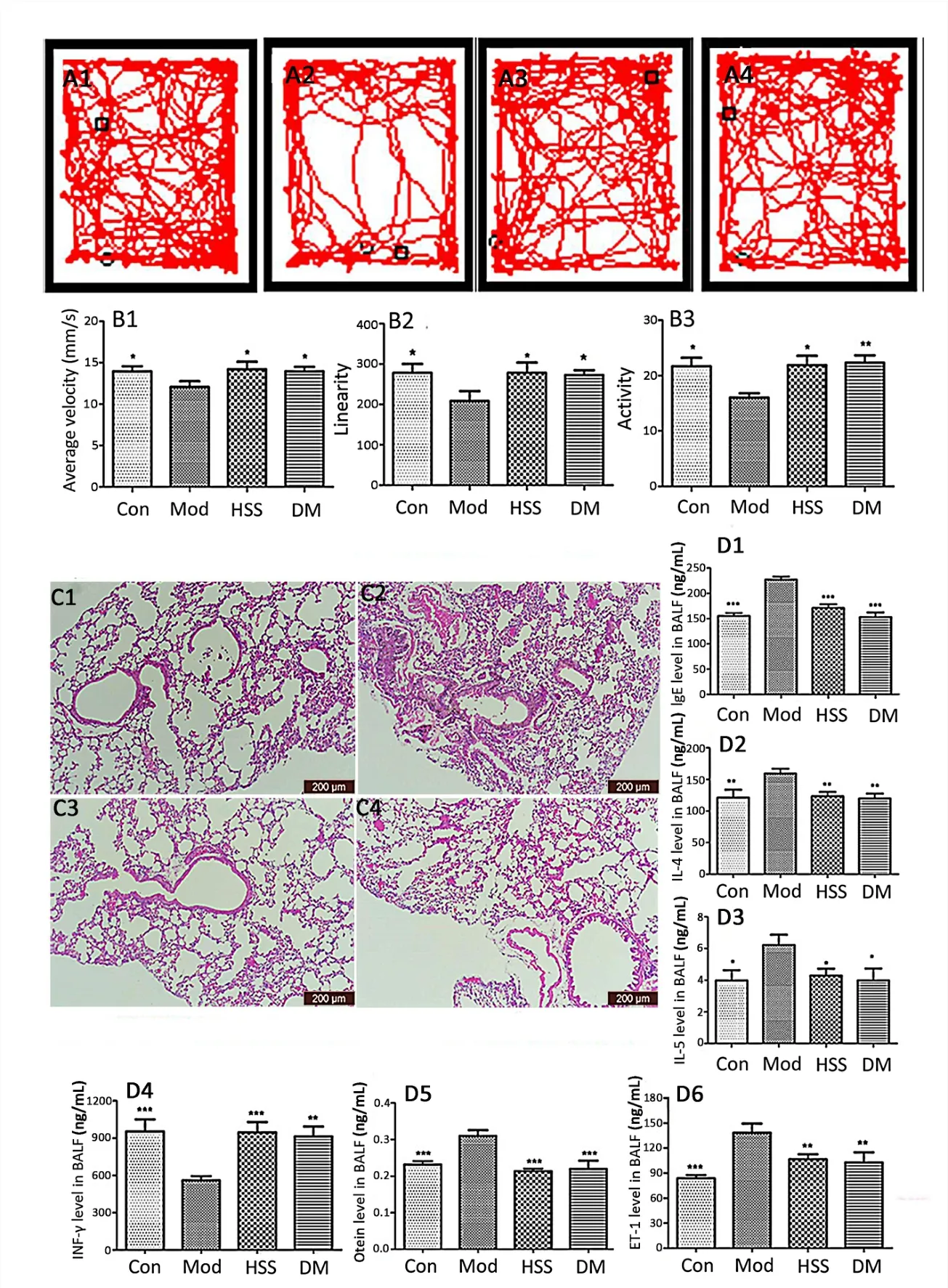
Figure 2 HSS-modified mice behaviors,airway remodeling and inflammatory cell infiltration in the lung tissue of allergic asthma models.(A1-A4)Crawl routes of mice.A1,control group;A2,model group;A3,HSS group;A4,DM group.The square and circle symbols mean the starting point and end point,respectively,for the mice.(B1-B3),average velocity,linearity and,activity of allergic mice respectively.(C1-C4)Histological analysis of allergic mice(x 100).C1,control group;C2,model group;C3,HSS group;C4,DM group.(D1-D6),IgE level,IL-4 level,IL-5 level,INF-γ level,ET-1 level and protein level in BALF respectively.* p <0.05,** p <0.01,*** p <0.001,compared with model groups.HSS,huashanshen;DM,dexamethasone;IgE,immunoglobulin E;IL,interleukins;BALF,bronchoalveolar lavage fluid;INF-γ,interferon-γ;ET-1,endothelin 1.
HSS alleviated the airway inflammation of allergic asthma
The behavior of the allergic mice was observed before they were sacrificed.As shown in Figure 2A1-4 and B1-3,compared with the control mice,the average velocity,linearity and activity of the OVA-induced mice significantly decreased(13.96 ± 0.45 vs 12.07 ±0.68,278.32±3.65 vs 208.20±3.20,21.68±1.76 vs 16.05±0.82,p<0.05).However,the treatment groups of HSS(14.20 ± 1.25 vs 12.07 ± 0.68,278.18 ± 4.20 vs 208.20 ± 3.20,21.89 ± 0.19 vs 16.05 ± 0.82,p<0.05)and DM(13.96 ± 1.28 vs 12.07 ± 0.68,272.39±6.25 vs 208.20±3.20,22.31±1.31 vs 16.05±0.82,p<0.05 orp<0.01)dramatically increased the activity,linearity and average velocity compared with that of the model mice.
To examine whether HSS could inhibit the inflammation of allergic asthma,the lung tissues were observed.Compared with the control group,inflammatory cell infiltration,tracheal contraction,airway epithelial hyperplasia and basement membrane thickening were found in the lung tissue of the model group(Figure 2C1 and C2).The histological changes of the lungs in the HSS and DM groups were markedly inhibited(Figure 2C3 and C4)compared with that of the model mice(Figure 2C2).
Compared with the saline-sensitized group,the IgE,IL-4,IL-5,ET-1 and otein levels in the BALF of the model mice(155.13 ± 13.96 vs 227.07 ± 14.72 ng/mL,p<0.001;121.17 ± 30.63 vs 159.32 ±18.72 ng/mL,p<0.01;3.98±1.59 vs 6.23±1.58 ng/mL,p<0.05;83.57 ± 9.38 vs 138.25 ± 27.03 ng/mL,p<0.001;0.23 ± 0.02 vs 0.31 ± 0.04 ng/mL,p<0.001)were dramatically increased,while the INF-γ level(954.87 ± 238.37 vs 559.36 ± 84.35 ng/mL,p<0.001)in BALF decreased in the model group(Figure 2D1-D6 and Supplementary Table S1).The HSS-treated(170.87 ±17.76 vs 227.07± 14.72 ng/mL,p<0.001;123.38 ± 16.50,vs 159.32 ± 18.72 ng/mL,p<0.01;4.28 ± 1.09 vs 6.23 ± 1.58 ng/mLp<0.05;106.51 ± 14.49 vs 138.25 ± 27.03 ng/mL,p<0.01)and DM groups(152.90 ± 22.18 vs 227.07 ± 14.72 ng/mL,p<0.001;119.74 ±18.63,p<0.01;3.98 ± 1.85,p<0.05;102.56 ± 30.24,p<0.01)dramatically reduced the content of IgE,IL-4,IL-5 and ET-1,and increased the INF-γ level(946.54±205.11 vs 559.36±84.35 ng/mL,p<0.001;913.21 ± 197.52 vs 559.36 ± 84.35 ng/mL,p<0.01)in BALF compared with that of the model mice.
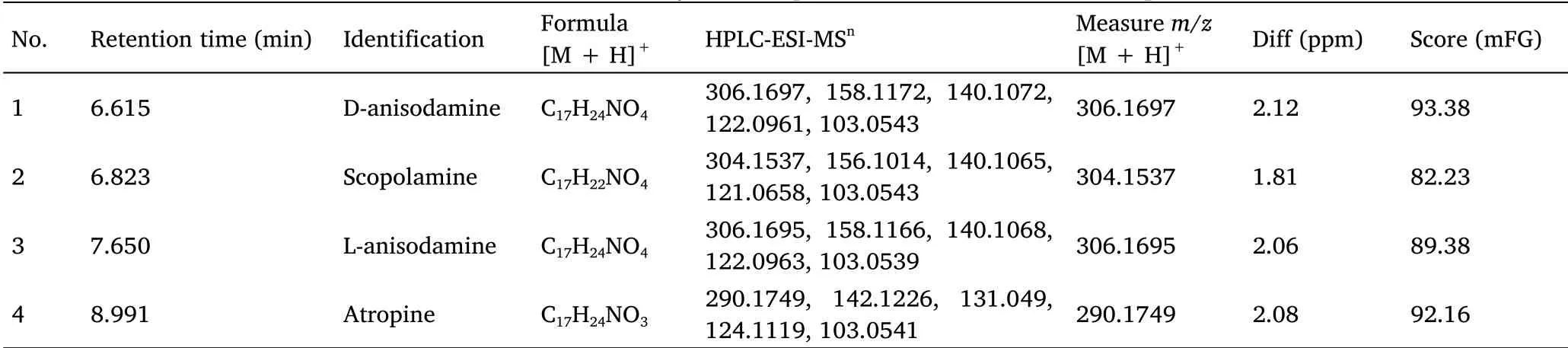
Table 1 HPLC-QTOF-MS analyses of tropane alkaloids absorbed in rat plasma
HSS relaxed the Ach-induced contraction of the ASM
The dose-effects of HSS were measured by tracheal rings to further evaluate the diastolic degree of the tracheal smooth muscle.The result showed significant concentration-dependent relaxant effects of HSS in the tracheal rings contracted by Ach(Figure 3A and B).Incubation with HSS(Huashanshen extract at 16 ng/mL)dramatically induced the maximum relaxation at 102.27 ± 12.04%.To assess the involvement of factors in the HSS relaxing trachea activity,propranolol,theophylline,indomethacin and L-NAME were detected in the control and model groups separately.The percentages of relaxation for propranolol(90.12 ± 10.23% vs 98.22 ± 11.03%),theophylline(92.33 ± 6.88% vs 98.77 ± 10.89%),indomethacin(93.66 ± 13.01% vs 89.45 ± 5.99%)and L-NAME(89.25 ± 10.57%vs 92.11 ± 13.54%)at the maximum dose between the two groups without significant differences showed that the tracheal relaxant effects of HSS were unaffected by the β-adrenergic receptor,PDE,COX and NOS(Figure 3C-F).
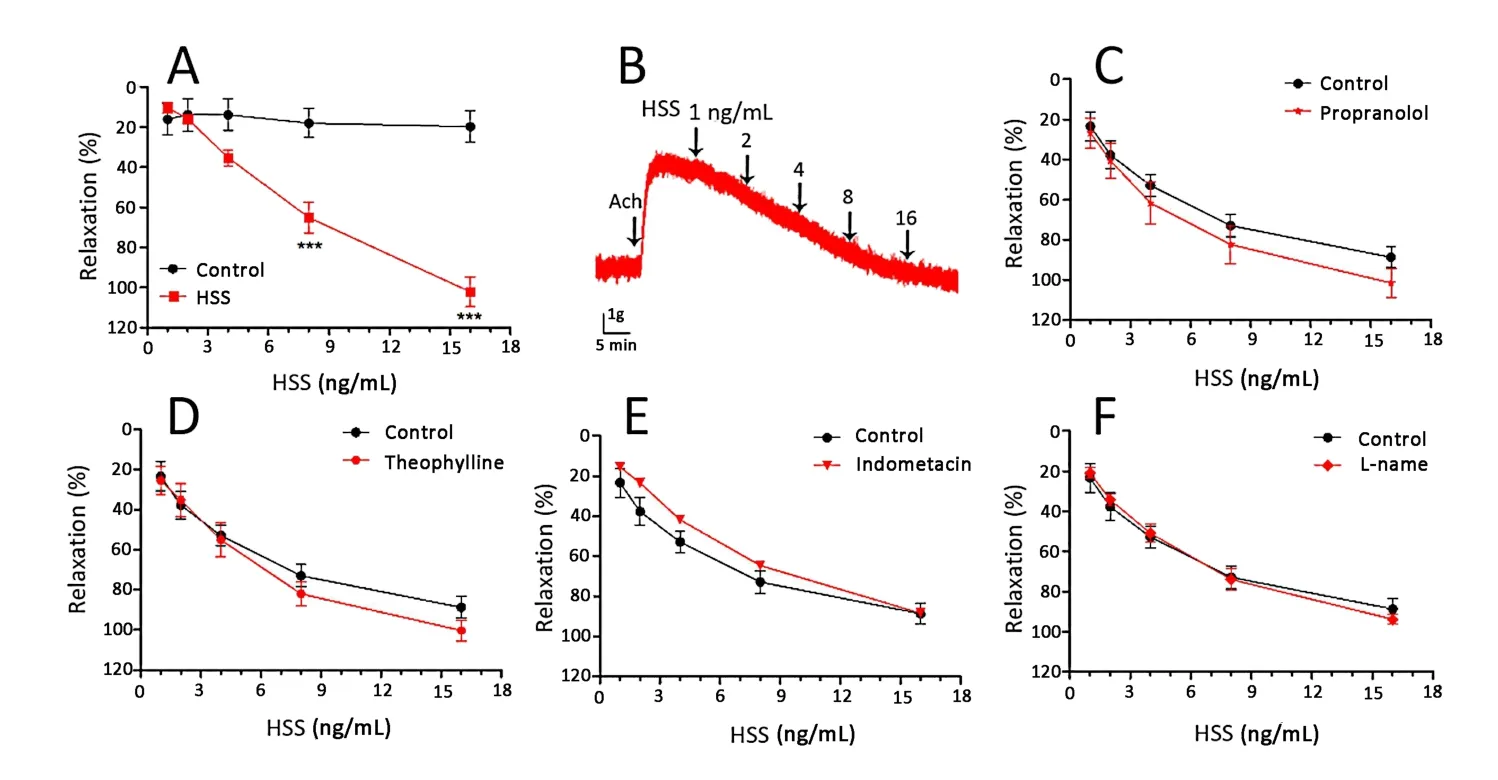
Figure 3 Concentration-response relaxant effect of HSS on Ach(10 mM)induced contraction of trachea smooth muscle.(A)Effect of Ach 10 µM on HSS-induced relaxation.(B)Effect curve of Ach 10 µM on HSS-induced relaxation.(C)Effect of propranolol(nonselective β-adrenergic antagonist,10µM)on HSS-induced relaxation.(D)Effect of theophylline(PDE unspecific inhibitor,10µM)on HSS-induced relaxation.(E)Effect of indomethacin(COX inhibitor,10 µM)on HSS-induced relaxation.(F)Effect of L-NAME(NOS inhibitor,10 µM)on HSS-induced relaxation.Compared with control,* p <0.05,** p <0.01,*** p <0.001.HSS,huashanshen;L-NAME,n(omega)-nitro-L-arginine methyl ester;Ach,acetylcholine;PDE,phosphodiesterase;COX,cyclooxygenase;NOS,nitric oxide synthase.
HSS affected the potassium channel,the influx and release of calcium
The Ca2+-activated channel inhibitor TEA,KVchannel inhibitor 4-AP and KATPchannel inhibitor glibenclamide as different pharmacological K+channel blockers were used to evaluate the relaxant effects of HSS on the tracheal smooth muscle.As illustrated in Figure 4A-D,TEA had an effect on the HSS-induced response(38.56 ± 4.33%,p<0.001).However,4-AP and propranolol had no effect on the HSS-induced response.The results showed that HSS influenced the KCa channel to relax ASM.
To determine whether the bronchodilation of HSS was mediated by Ca2+influx,the effect of HSS on the CaCl2curves was observed.It was found that HSS(Huashanshen extract,16 ng/mL)caused the CaCl2curves to become parallel to the right,reducing the maximum contraction value of CaCl2by 36.98 ± 14.96%.Pre-incubation with nifedipine(4 ng/mL)also significantly decreased the maximum contraction to 44.90 ± 3.40%(Figure 4E).The above two contractions were both lower than that of the control(91.12 ±9.55%,p<0.001),which illustrated that HSS inhibited the Ca2+influx to relax ASM.To determine the influence of the sarcoplasmic reticulum calcium release,HSS was measured by tracheal rings.Ach induced the release of intracellular Ca2+to the tracheal smooth muscle contraction.As shown in Figure 4F,pre-incubation with HSS(Huashanshen extract,16 ng/mL)for 15 min dramatically decreased the Ach-induced contraction to 76.63 ± 7.12,which was higher than that of the control(11.22 ± 1.23%,p<0.001).This showed that HSS inhibited the Ca2+release to relax ASM.
Effects of the potassium channel inhibitors,the influx and release of calcium on atropine,anisodamine and scopolamine induced relaxation
Subsequently,the alkaloids in plasma(including atropine,anisodamine and scopolamine)were measured to ensure the active components for asthma in the K+channel and calcium influx or release.As shown in Figure 5A-C,TEA had an effect on the anisodamine(18.55±2.16%vs 70.69±9.55%in control,p<0.001)and scopolamine(83.24 ± 8.99% vs 113.75 ± 10.21%,p<0.01)-induced response,but had no effect on the induction of atropine.Atropine,anisodamine and scopolamine influenced the influx and release of calcium(Figure 5D-E).
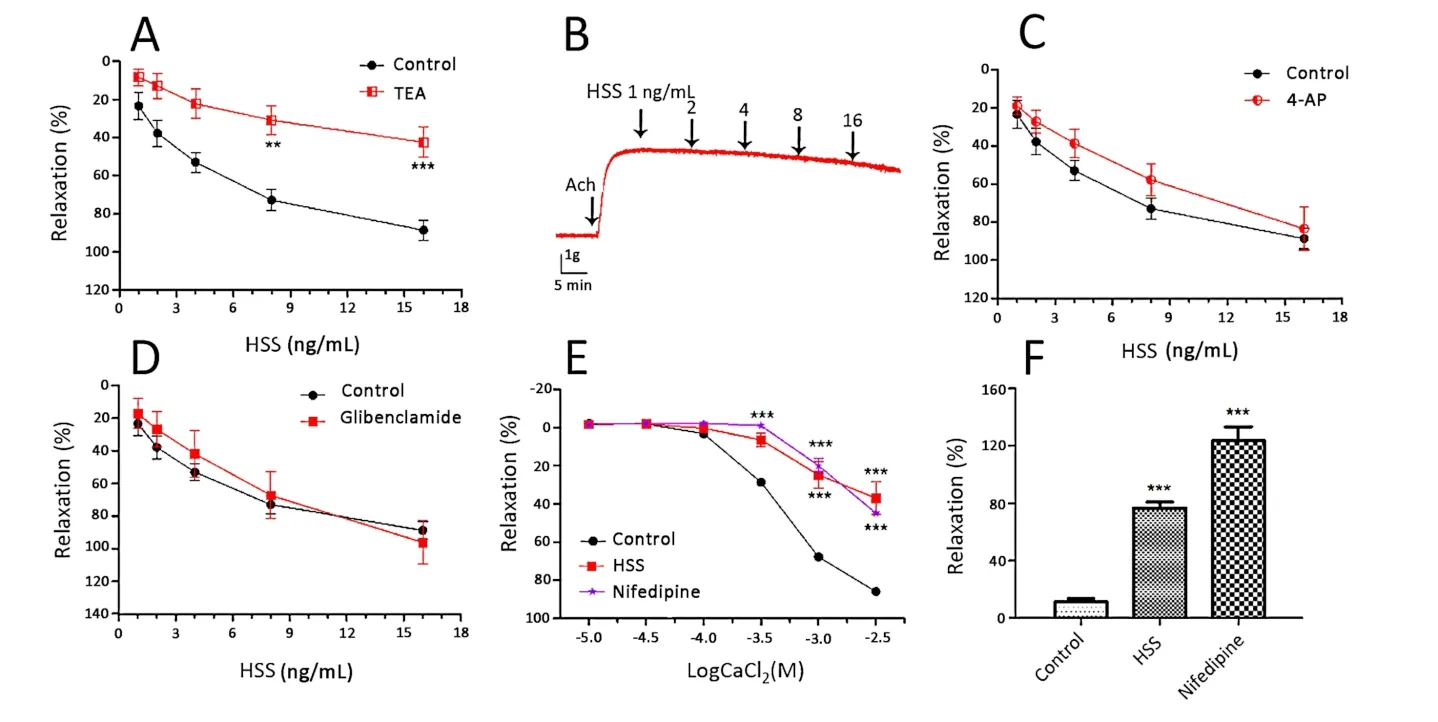
Figure 4 Effects of potassium channel inhibitors,the influx and release of calcium on HSS-induced relaxation in Wistar rats tracheal rings.(A)Effect of TEA(KCa inhibitor,1 mM)on the HSS-induced relaxation.(B)Effect curve of TEA(1 mM)on the HSS-induced relaxation.(C)Effect of 4-AP(KV inhibitor,100 µM)on the HSS-induced relaxation.(D)Effect of glibenclamide(KATP inhibitor,10 µM)on the HSS-induced relaxation.(E)Dose-effect curves of CaCl2 on Wistar rats tracheal rings in the absence and presence of HSS.(F)Bronchodilator effects of HSS on Ach pre-contracted tracheal rings in Ca2+-free solution.Compared with the control *p <0.05,**p <0.01,***p <0.001.HSS,huashanshen;TEA,tetraethylammonium;Ach,acetylcholine;4-AP,4-aminopyridine;ATP,adenosine triphosphate.
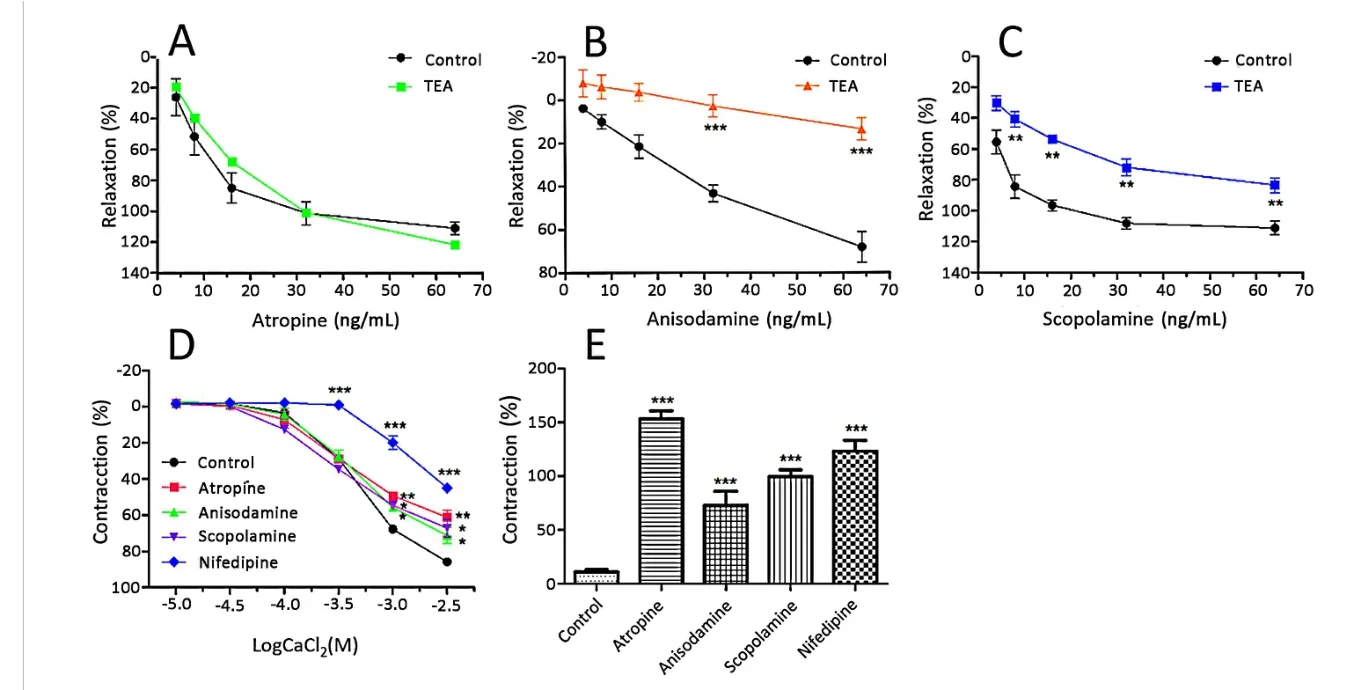
Figure 5 Effects of the potassium channel inhibitors,the influx and release of calcium on atropine,anisodamine and scopolamine induced relaxation in rat tracheal rings.(A)Effect of TEA(KCa inhibitor,1 mM)on the atropine-induced relaxation.(B)Effect of TEA(1 mM)on the anisodamine-induced relaxation.(C)Effect of TEA(1 mM)on the scopolamine-induced relaxation.(D)Dose-effect curves of CaCl2 on tracheal rings in the absence and presence of atropine,anisodamine and scopolamine.(E)Bronchodilator effects of atropine,anisodamine and scopolamine on Ach pre-contracted tracheal rings in Ca2+-free solution(n=4).*p <0.05,**p <0.01,***p <0.001,compared with control group.*p <0.05,**p <0.01,***p <0.001,compared with the control group.TEA,tetraethylammonium;Ach,acetylcholine.
Discussion
In China,HSS has been widely used in asthma.However,the anti-asthmatic mechanism was rarely researched.In a previous report,29 tropane alkaloids were identified from Huashanshen,while the HSS pill contained 11 alkaloids[12,13].Atropine was widely used as a relaxant for the gastrointestinal tract,pre-anesthetic agent and ophthalmic solutions[21].Scopolamine had a wide range of antispasmodic,anesthetic,and analgesic effects[20].Anisodamine was commonly used in the treatment of infectious shock,hepatitis,and nephritis patients[22].Anisodamine was also reported to reduce the eosinophilic airway inflammation and airway hyper-reactivity[15].
In this study,the OVA-induced allergic asthma was established(Figure 1).In the model,the asthma mice moved slowly,while HSS improved these behaviors(Figure 2A1-4 and B1-3).The main characteristics of allergic asthma include airway eosinophilic inflammation and bronchial hyperresponsiveness[23].As Figure 2C1-4 showed,HSS dramatically inhibited the airway inflammation,trachea contraction and the structural change of the airway wall in the asthmatic mice lung.The airway inflammation and smooth muscle spasm were triggered by IL-4,IL-5 and IgE.It was reported that T-lymphocyte cells played a critical role in the development of allergen-induced airway inflammation caused by the infiltration of eosinophils[5,24].Allergens presented by antigen-presenting cells to Th0 cells resulted in Th1 and Th2 cell differentiation[1,5].The Th1/Th2 paradigm was described over 20 years ago,and allergic asthma was found to be a result of an imbalance in the Th2 response[25].The Th2 cells released IL-4,which stimulated B cells to release IgE and upregulated the high-affinity IgE receptor on the surface of the mast cells.IgE was then bound to the mast cells,releasing histamine,prostaglandins,and leukotrienes that led to the ASM constriction and propagated the air inflammatory response[1,26].Simultaneously,the Th2 cells also released IL-5,a cytokine involved in the activation,migration and survival of eosinophils to the airways,promoting bronchial inflammation[4,27].However,INF-γ was produced by Th1 cells,which controlled the balance of the development of Th1 and Th2 to inhibit Th2 cell-mediated eosinophilic inflammation[4,5].In the present study,the levels of IL-4,IL-5,and IgE were increased in the BALF of asthmatic mice,while INF-γ was decreased.The results clearly showed that HSS inhibited the airway inflammation via decreasing cytokine levels,including IL-4,IL-5,and IgE,increasing the level of INF-γ and improving the balance of Th1/Th2.In addition,it reduced the amount of protein in the BALF(Figure 2D1-D4 and D6).
ASM contraction is a feature in asthma.According to the analysis of lung tissue and ET-1 in BALF,the tracheal contraction obviously occurred in asthma(Figure 2C and D5).To assess the bronchodilator effects of HSS,the isolated tracheal rings were studied.Ach,a parasympathetic neurotransmitter changing the intracellular free Ca2+and inducing bronchoconstriction via muscarinic receptors,was chosen as a stimulant in the experiments[28].The result showed significant relaxant effects of HSS in the tracheal rings contracted by Ach(Figure 3A and B).
Adrenergic receptors,NOS,PDE and prostaglandins are involved in the regulation of the ASM.In a previous report,β2-agonists acted on theβ2-adrenoceptor,whichproducedcyclicadenosine monophosphate(cAMP)and activated protein kinase A,thus reducing the affinity of Ca2+-calmodulin for myosin light chain kinase to relax ASM[29].NO had a relaxant effect on the ASM.With the receptor stimulated,inducible NOS was activated to produce NO.NO-activated guanylate cyclase increasesintracellular cyclic guanosine monophosphate(cGMP)production,which leads to relaxation of the smooth muscle by regulating intracellular Ca2+[30,31].PDE relaxed the ASM via hydrolyzation of cAMP and cGMP.Each PDE had substrate specificity.Some PDEs specifically degraded cAMP,whereas others specifically degraded cGMP[32].The prostanoids were metabolites of arachidonic acid,such as prostaglandin E2,which was the main product of the COX synthesized by almost all types of lung cells[33-35].Depending on the profile of the prostaglandin E receptor,prostaglandin E2 either mediated the inhibition of smooth muscle tone and cell activation or stimulatory effects by decreasing cAMP or increasing calcium[33].To explore the possible mechanisms,the effects of bronchodilation were studied in the presence of the PDE,β-adrenoceptor,COX and NOS inhibitors.As shown in Figure 3B-F,propranolol,theophylline,indomethacin and L-NAME did not significantly change the relaxation,suggesting that the β-adrenoceptor,PDE,prostaglandins and NO were not involved in the bronchiectasis of HSS.
K+channels in the membrane of the ASM cell participated in the regulation of the ASM[36].The opening of the K+channels induced hyperpolarization by K+efflux to relax the ASM[37].The results are shown in Figure 4A-D.In the presence of 4-AP(a KVchannel blocker)or glibenclamide(a KATPchannel blocker),the effect of HSS did not change compared with the absence of the channel blockers.However,the tracheal rings were incubated with TEA(a KCa channel blocker),suggesting that the HSS relaxing mechanism was closely related to the KCa channel.
The G-protein coupled receptor is primarily modulated by Ca2+pathways to promote ASM contraction,which stimulated the activation of phospholipase β.In the Ca2+mobilization,phospholipase C hydrolyzed phosphatidyl diphosphate inositol 2 into inositol trisphosphate and diacylglycerol,which increased intracellular Ca2+[8,38].The influx process of Ca2+involved extracellular Ca2+entry through voltage-dependent Ca2+channels(VDCC)that allowed extracellular Ca2+into the cell,receptor-operated Ca2+channels(ROCC)that were activated by agonists and store-operated Ca2+channels,which were activated by sarcoplasmic reticulum Ca2+depletion[39].Intracellular Ca2+was bound to calmodulin to regulate the activity of myosin light chain to change the ASM contraction or relaxation[8,40].The results indicated that HSS dramatically inhibited the CaCl2-induced tracheal ring contraction in the Ca2+-free Krebs solution containing 60 mM KCl,blocked VDCC and ROCC channels to inhibit extracellular Ca2+entry,and reduced the Ca2+released from the intracellular stores(Figure 4E-F).This suggested that HSS acted as a calcium antagonist.
To determine which component acted on the KCa,VDCC and ROCC channels in the ASM cells,we first studied anisodamine,atropine,and scopolamine.The results are shown in Figure 5A-E.Atropine did not modify the relaxation in TEA,while anisodamine and scopolamine were related to the KCa channel.Meanwhile,atropine,anisodamine and scopolamine influenced the influx and release of calcium.Thus,atropine,anisodamine and scopolamine might be the active compounds in HSS.
Conclusion
In the present study,D-anisodamine,L-anisodamine,scopolamine and atropine were separated and identified in rat plasma by HPLC-QTOF-MS analysis.Through the study of asthmatic animals and isolated tracheal tissue,it was clear that the HSS inhibited the release of inflammatory mediators,regulated the balance of Th1/Th2 to reduce the airway inflammation,and relaxed the tracheal smooth muscle by controlling the KCa channel,Ca2+influx and release to reduce airway hyperresponsiveness.Atropine,anisodamine and scopolamine might be active compounds of HSS.Thus,this study may aid in understanding the mechanism of HSS in the treatment of asthma,and laying the foundation for future research.
 Traditional Medicine Research2022年3期
Traditional Medicine Research2022年3期
- Traditional Medicine Research的其它文章
- Traditional Chinese medicine:an important broad-spectrum anti-coronavirus treatment strategy on COVID-19 background?
- Application of network pharmacology in the prevention and treatment of COVID-19 by traditional Chinese medicine
- Pharmacological efficacy of the traditional Chinese medicinal formula Kun-Tai-1A in the treatment of letrozole-induced polycystic ovary syndrome
- Recent advances in research on natural product inhibitors of SREBPs
- Integrated UHPLC-MS and network pharmacology to explore the active constituents and pharmacological mechanisms of Shenzao dripping pills against coronary heart disease
- Effects of Qingwen Baidu decoction on coagulation and multiple organ injury in rat models of sepsis
