Metabolomic profiling reveals hypoglycemic effect of Jinqi Jiangtang tablet on type 2 diabetic rats
Zheng-Yuan Shi,Li Bao,Chun-Jing Yang,Xi-Qiao Xu,Dan Yan,De-Chun Jiang*
1Department of Pharmacy,Beijing Shijitan Hospital,Capital Medical University,Beijing 100038,China.2Beijing Key Laboratory of Bio-characteristic Profiling for Evaluation of Clinical Rational Drug Use,Beijing 100038,China.3Department of Pharmacy,Beijing Friendship Hospital,Capital Medical University,Beijing 100050,China.
Abstract Background:Si-Miao Sun wrote about the Jinqi Jiangtang tablet(JQJT)that is derived from the traditional Chinese herbal medicine Huanglian(Coptis Chinensis Franch)pills in the Essential Recipes for Emergent Use Worth A Thousand Gold in C.E.652.The tablet is used to treat diabetes in China owing to its powerful hypoglycemic properties.However,little is known about the metabolic mechanisms underlying these properties.Methods:Ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight tandem mass spectrometry-based metabolomics approach was performed to explore the metabolic mechanism of JQJT in the treatment of type 2 diabetes mellitus(T2DM).Results:The metabolomic pathway analysis and the Kyoto Encyclopedia of Genes and Genomes database were used to screen out 25 potential biomarkers and construct pertinent metabolic pathways.Five metabolites,such as allantoic acid and taurine,were downregulated and 20 metabolites were upregulated in the urine of T2DM rats.The biomarkers in the JQJT group exhibited a good callback trend.It is speculated that JQJT can alleviate T2DM by regulating the disorders of taurine,hypotaurine,phenylalanine,ascorbate,and aldarate metabolism,as well as pentose and glucuronate interconversions.Conclusion:This study will be meaningful in the clinical application of JQJT and will be valuable for further exploration of the metabolic mechanisms underlying its properties.
Keywords:Jinqi Jiangtang tablet;type 2 diabetes mellitus;metabolomics;UPLC-QTOF-MS;traditional Chinese medicine
Background
Diabetes is a chronic metabolic disease characterized by hyperglycemia,its main clinical feature.In recent years,several studies have shown that diabetes is often accompanied by metabolic disorders,which lead to a variety of cardiovascular and cerebrovascular diseases,critically affecting the quality of life and safety of patients[1,2].Glucose,lipid toxicity,and insulin resistance due to metabolic disorders play key roles in the pathogenesis of a variety of metabolic diseases such as type 2 diabetes mellitus(T2DM).It is,therefore,critical to effectively improve diabetic metabolism disorders and explore their underlying mechanism of action.
Jinqi Jiangtang tablet(JQJT),a three-herb traditional Chinese medicine(TCM)formula containing Huanglian(Coptis ChinensisFranch),Huangqi(Astragalus Mongholicus),and Jinyinhua(Lonicerae JaponicaeFlos)[3-6],is a proprietary Chinese medicine for the treatment of T2DM[7-9].It was developed by the Institute of Materia Medica,Chinese Academy of Medical Science,based on the traditional ancient prescriptions ofCoptis Chinensispills,which containsCoptis ChinensisFranch and Shengdi(Rehmannia glutinosa(Gaetn.)Libosch.ex Fisch.et Mey.).It was the first novel Chinese patent medicine approved by the National Medical Products Administration in China for the treatment of diabetes(approval number:Z10920027).The traditional ancient prescription,mainly included in,is recorded in theEssential Recipes for Emergent Use Worth A Thousand Gold,the compendium of herbal medicine by Si-Miao Sun in C.E.652 during the Tang Dynasty of China(C.E.618-C.E.907).Recently,some studies have revealed that JQJT has a definite clinical effect,which primarily comprises four categories:alkaloids,organic acids,flavonoids,and saponins[10-12].Pharmacological studies have shown that JQJT can improve metabolism in diabetic animal models,has the action characteristics of insulin sensitization agents,reduces blood pressure,serum resistin concentration and blood glucose content,and does not increase body weight[13-16].It won the third prize for scientific and technological progress from the Ministry of Health in China in 1993.Compared to the unsatisfactory hypoglycemic effects of Western medicine and its several side effects,TCM plays an important role in the clinical treatment of T2DM.Chinese patent medicines are extracted and processed using Chinese medicinal materials as raw materials.As one of the treatment measures in traditional medicine,Chinese patent medicines should be used by referring to the theory of syndrome differentiation,with patients’ clinical syndromes as the breakthrough point.Modern pharmacological studies have found that JQJT can significantly improve glucose tolerance and glucose metabolism in patients with T2DM,reduce blood glucose levels,alleviate the symptoms of “three more and one less” caused by hyperglycemia to a certain extent,can improve the body’s sensitivity to insulin action,improve insulin resistance,and can also correct lipid metabolism disorders,playing a role in reducing serum triglyceride levels[17].Chinese patent medicine has advantages over western hypoglycemic drugs in improving the clinical symptoms of patients with diabetes,delaying the occurrence of diabetic complications,and improving the quality of life of patients,which is the direction of our clinical research in the future.After more than 20 years of clinical testing,JQJT has become a classic Chinese patent medicine for treating T2DM in China.However,little is known about its underlying metabolic mechanisms.
The use of JQJT in the treatment of diabetes has been verified in many clinical and experimental studies[18].It has been reported that JQJT can improve the metabolism disorders of diabetes[19].However,from the perspective of endogenous substances,there is still a lack of relevant research on the mechanism through which the tablet treats diabetes.The metabonomic analysis is becoming increasingly targeted.Screening target components with obvious correlation with diseases from small molecular metabolites can not only reduce the difficulty of analysis and improve accuracy but also make biomarkers easier to use in clinical and drug evaluation.
The development of metabonomic technology has provided the possibility of prediction,early diagnosis,and elucidation of the mechanism of T2DM drug treatment[20-24].It seeks potential biomarkers of the disease through the analysis of differential metabolites[25,26].The purpose of this study was to identify the altered expression of biomarkers associated with JQJT therapy in T2DM rats,analyze possible metabolic pathways,discuss the effect of JQJT on metabolic disorders of T2DM,and clarify the mechanism of action of JQJT from the perspective of metabolomics.
Methods
Chemicals and reagents
JQJT tablets were obtained from Tianjin Zhongxin Pharmaceutical Group Co.Ltd.(Tianjin,China).Streptozotocin(STZ)was purchased from Sigma-Aldrich Co.,Ltd.(batch number:415G034;purity >98%;St.Louis,MO,USA).Citric acid monohydrate and sodium citrate dihydrate were purchased from Sinopharm Chemical Reagent Co.,Ltd.(batch number:20170109,20161209;Shanghai,China).STZ was freshly dissolved in citric acid and sodium citrate buffer(0.1 M,pH 4.5).Ultrapure water was obtained using a Milli-Q water system(Millipore,Billerica,MA,USA).Acetonitrile was obtained from Thermo Fisher Scientific(Chemical Abstracts Service:A955-4;batch number:135867;Waltham,MA,USA).Formic acid was obtained from ANPEL Laboratory Technologies,Inc.(Chemical Abstracts Service:64-18-6;batch number:J6460250;purity ≥98.0%;Shanghai,China).Metformin hydrochloride was obtained from Shanghai Yuanye Bio-Technology Co.Ltd.(batch number:K17D9Y77394;Shanghai,China).
Animals and experimental design
Six-week-old male Sprague-Dawley rats(200 ± 10)g,specific pathogen free grade,were raised in individually ventilated cages with 4 rats in each cage.The laboratory temperature was maintained at 22°C and the humidity was maintained at approximately 50%.All animal experiments were approved by the ethics committee of the Beijing Shijitan Hospital,Capital Medical University(permission number:sjtkyll-lx-2021(4)).
After one week of adaptive feeding,the rats were randomly selected from either the normal group fed with a normal diet or the diabetes-induced group fed with a high-sugar-high-fat diet.After four weeks,40 mg/kg of the STZ solution(freshly prepared with citrate buffer)was injected into the diabetes-induced rats.The rats fed with a normal diet were injected with an equal volume of citrate buffer.After injection of the STZ solution for three days,the concentrations of fasting blood glucose(FBG)were measured,and the rats with fasting blood sugar FBG of more than 11.1 mM were considered diabetic.
A total of 24 rats on the normal diet were divided into a normal control group and a normal administration group(normal JQJT group).They were administered normal saline and TCM by gavage at a fixed time every day.A total of 36 T2DM rats that were fed the high-sugar-high-fat diet were randomly divided into three groups,with 12 rats in each group.These were the model group,the positive drug group,and the TCM group(JQJT group).They were administered by gavage once daily for four weeks.The TCM group and normal administration group were administered 5.3 g/(kg·d)of JQJT by gavage at a fixed time every day(1 mL/100 mg body weight).The conversion method of rat dose was based on the methodology of a pharmacological experiment edited by Professor Xu[27].The clinical dose was 0.28 g/(kg·d),whereas the rat dose was three times the clinical equivalent dose(0.28 × 70 kg × 0.018 × 5 × 3 = 5.292 g/(kg·d))[28].The positive drug control group was administered 200 mg/(kg·d)(1 mL/100 mg body weight)of metformin hydrochloride by gavage[25,28].The normal control group and model group were both administered distilled water by gavage at 1 mL/100 mg body weight.
Sample collection and preparation
After 4 weeks of JQJT treatment,all the rats were housed in metabolic cages(one per cage)and were overnight fasted for 12 h.Urine samples were collected and centrifuged at 3,500 rpm at 4 °C for 10 min,immediately transferred,and stored at-80 °C until analysis.After the last administration,the rats were fasted overnight,anesthetized with 5% chloral hydrate,and blood was collected from the abdominal aorta.
The urine samples were removed from the refrigerator and placed at room temperature until the samples were fully thawed(1 h)and then centrifuged for 20 min(14,000 rpm,4 °C).The supernatant(50 µL)was transferred to a 1.5-mL plastic centrifuge tube,mixed with 150 μL of ultrapure water and vortexed for 5 min.The clear supernatant was subsequentlyanalyzedusingultra-high-performanceliquid chromatography coupled with quadrupole-time-of-flight tandem mass spectrometry(UPLC-QTOF-MS).
Quality control(QC)samples preparation
Aliquots from each sample were collected and pooled as QC samples and were used to evaluate the stability and repeatability of the system during sample collection.The pretreatment method for QC samples was the same as that for the other samples.First,five blank samples were used to balance the column,and then,six QC samples were used to balance the column conditions.One QC sample was inserted into every six samples to monitor the repeatability and stability of the entire detection system.Unsupervised principal component analysis(PCA)was used to analyze the QC and other experimental samples.
UPLC-QTOF-MS analysis
The following chromatographic conditions were used for the UPLC-QTOF-MS analysis:ACQUITY UPLC HSS T3 column(100 mm×2.1 mm,1.8 μm;Waters Company,Milford,MA,USA);column temperature:40 °C;injection volume:2 μL;mobile phase:water containing 0.1% formic acid(phase A)and acetonitrile containing 0.1% formic acid(phase B);flow rate:0.4 mL/min;gradient elution conditions:0-1 min,1% B;1-12 min,1%-55% B;12-14 min,55%-99% B;14-16 min,99% B;16-18 min,99%-1% B.
The analysis further used the following mass spectrometry(MS)conditions:electrospray ion ionization source,the temperature of ion source was 120 °C,the temperature of the desolvated gas was 400 °C,the flow rate of the cone gas was 50 L/h,the flow rate of the desolvated gas was 800 L/h,the cone voltage was 40 V,the capillary voltage was 3 kV in positive ion mode and-2 kV in negative ion mode.
Statistical analysis and metabonomic data processing
Statistical analysis of the FBG,body weight,and biochemical indicators among different groups was performed using analysis of variance to investigate alterations.The data were then presented as the mean ± standard deviation,number(%),or median(range).Statistical significance was defined asP< 0.05.
All collected data were processed using the Progenesis QI software(version 2.0),including the steps of importing the original data,peak alignment,peak extraction,normalization,and finally forming a table of retention time,mass charge ratio,and peak intensity.The extraction time was in the range of 1-8 min.The metabolites were identified by matching with the Human Metabolomes Database(www.hmdb.ca)[29],and the chemical structures of the metabolites were identified using accurate mass and data from the MS/MS secondary sections.
An accuracy error of 5 ppm was set in both the MS and MS/MS data to confirm the tentative identification of metabolites.PCA and orthogonal partial least squares-discriminant analysis(OPLS-DA)were performed using the SIMCA-P software(version 14).An independent sample non-parametric test was used to test for significant differences between the groups(P<0.05),and the variables of important values in the OPLS-DA model were used to identify potential biomarkers.MetaboAnalyst 4.0(www.metaboanalyst.ca)was used to sort the metabolites with significantly altered expression associated with JQJT therapy.
Results
Effects of JQJT on FBG,body weight,and biochemical parameters
The FBG levels and body weight are presented in Figures 1A&B,and the serum biochemical parameters are summarized in Table 1.Compared with the control group,hemoglobin A1C and FBG levels in the model group were significantly increased(P<0.01).However,there was a significant decrease(P< 0.01)in the levels of hemoglobin A1C and FBG in the JQJT group compared with those in the control group,suggesting that JQJT had a good hypoglycemic effect.Compared with the control group,there was a significant increase(P<0.01)in the levels of total cholesterol,triglyceride,andlow-density lipoprotein cholesterol in the model group and a significant decrease(P<0.05)in the level of high-density lipoprotein cholesterol.After treatment with JQJT,all the above indicators were significantly reversed(P<0.01,P<0.05).
Analytical method assessment
The UPLC-QTOF-MS analysis technique was used to perform the metabolomic analysis.The base peak intensity chromatogram of urine in the positive ionization mode is presented in Figure 2.The components of the QC samples were the same and were aggregated in the PCA score chart.It can be observed from the PCA scores that the system has good repeatability and the collected data are worthy of further study.Simultaneously,the coefficient of variation of the metabolites extracted from the QC samples was calculated,and metabolites with a coefficient of variation of more than 30% were excluded.
Multivariate data analysis
The results of the PCA score plot are presented in Figure 3,where the model group was clearly separated from the normal group,indicating that endogenous substances in the model group had changed from those in the normal group.Furthermore,it was found that the JQJT group was able to adjust the abnormal metabolism to the normal state.The OPLS-DA score plots for both positive and negative ion modes are presented in Figure 4.Significantly separated clusters were observed between the metabolite profiles of the two groups.As shown,the R2and Q2intercept values provided no evidence of overfitting,and the models were reliable with higher general applicability.
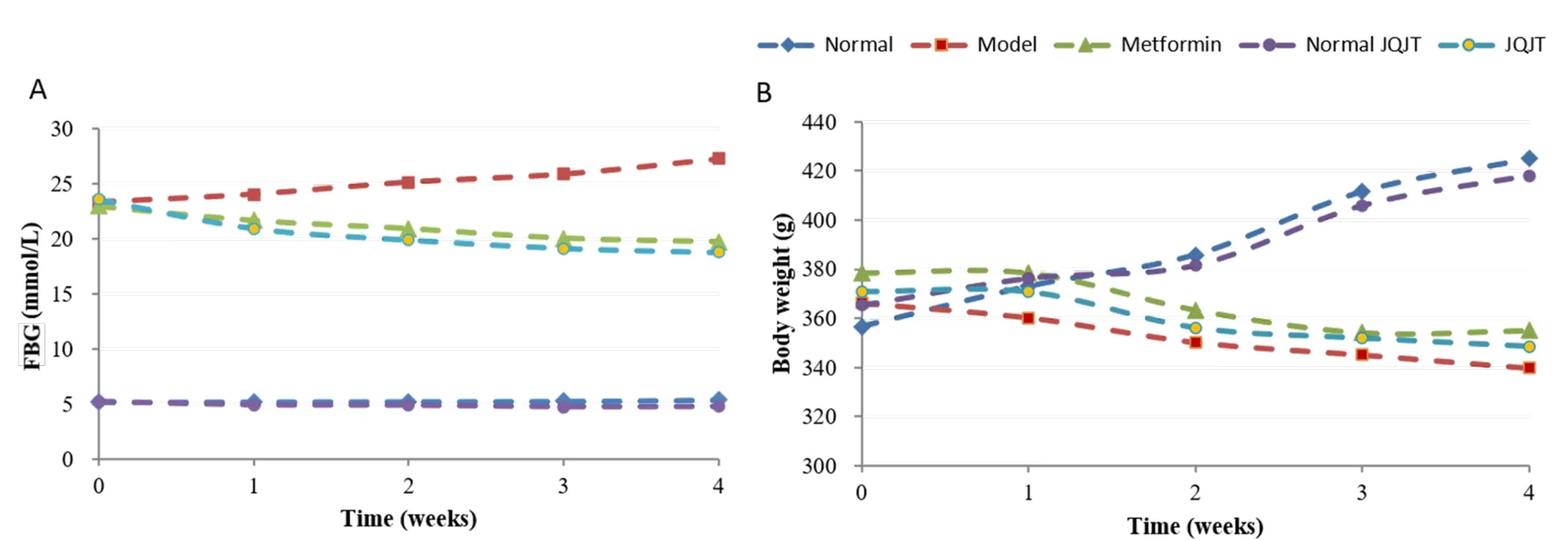
Figure 1 Effects of JQJT treatment on FBG(A)and body weight(B)of rats.JQJT,Jinqi Jiangtang tablet;FBG,fasting blood glucose.
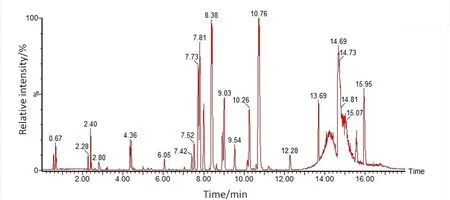
Figure 2 The base peak intensity chromatogram of urine sample of JQJT group in positive ionization mode gained from UPLC-QTOF-MS.x axis represents chromatographic peak retention time;y axis represents relative intensity of chromatographic peak.JQJT,Jinqi Jiangtang tablet;UPLC-QTOF-MS,ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight tandem mass spectrometry.
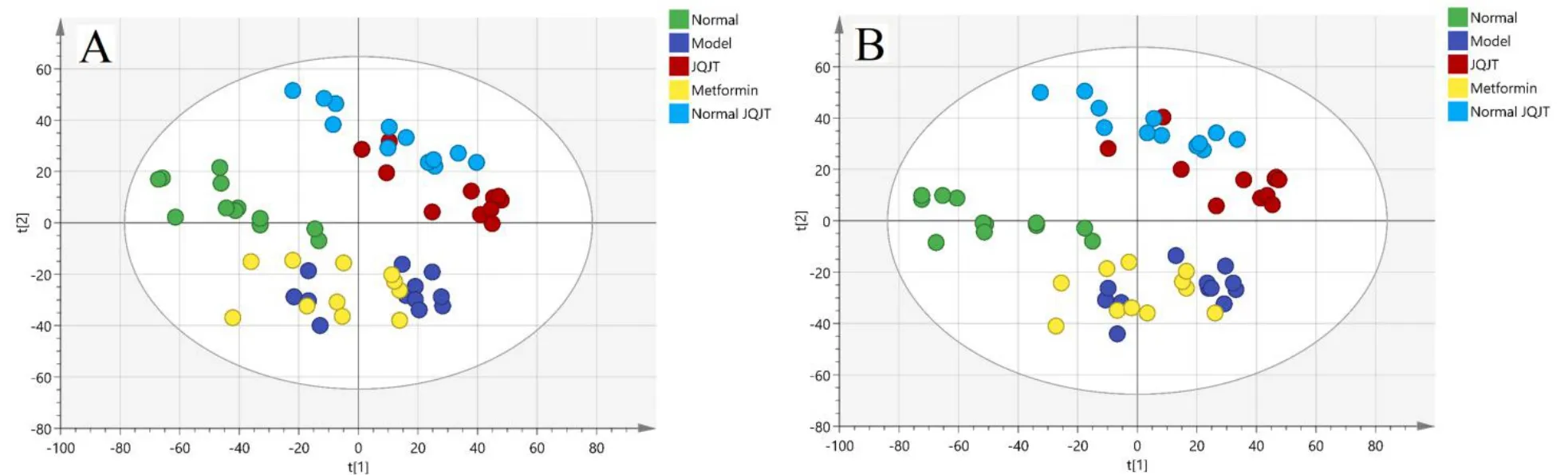
Figure 3 Sample scatter plot of PCA.(A)positive mode;(B)negative mode.x axis represent the first principal component,y axis represent the second principal component.PCA,principal component analysis;JQJT,Jinqi Jiangtang tablet.
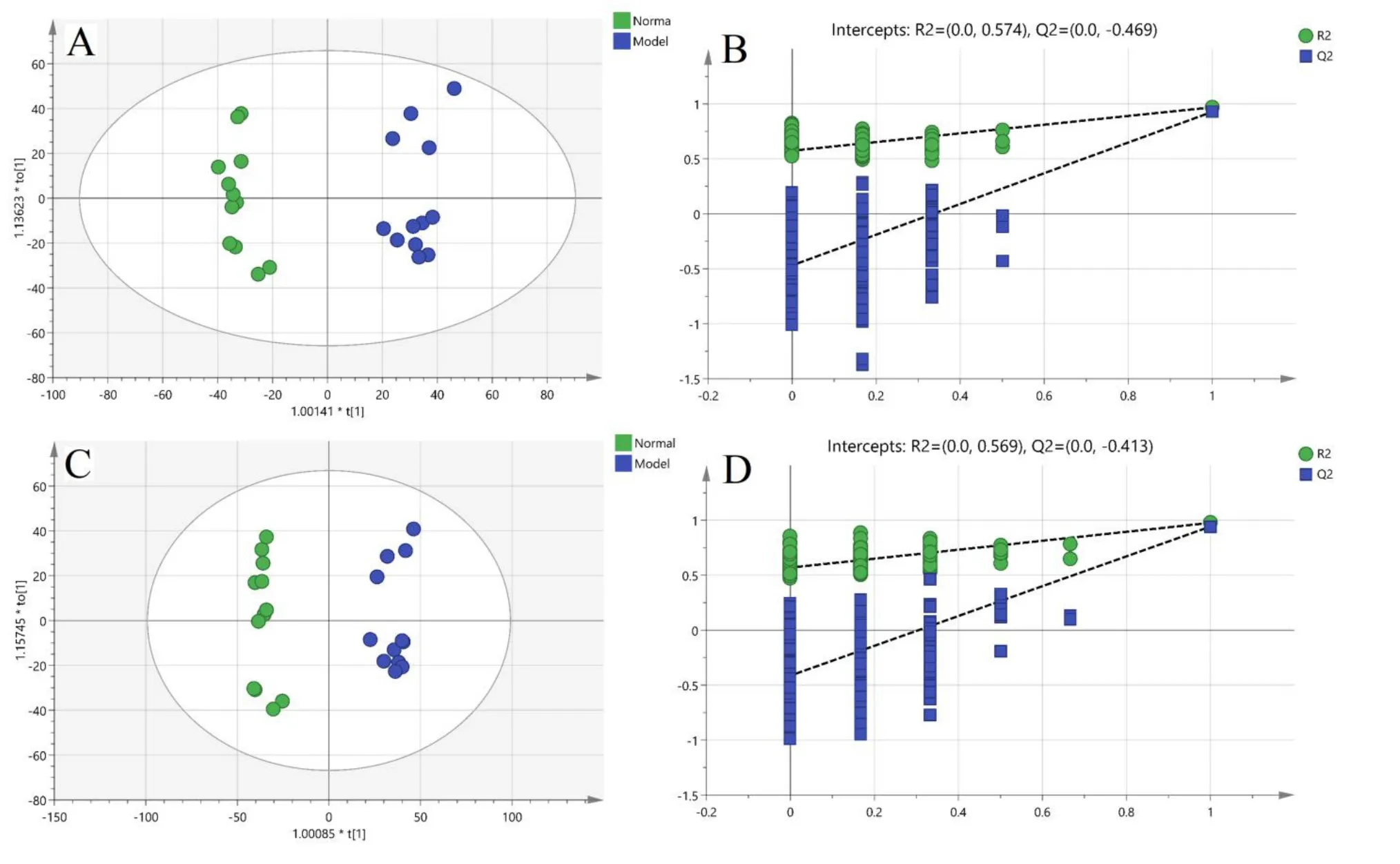
Figure 4 The scatter plot of OPLS-DA and permutation test.(A)positive mode;(B)permutation test of positive mode;(C)negative mode;(D)permutation test of negative.(A)and(C)x axis represent the score value(t)of the main component;y axis represent score value(to)of the orthogonal components;(B)and(D)x axis represent the accuracy of the model;y axis represent the frequency of the accuracy of 200 models in 200 permutation tests.R2 describes the goodness of fit,and Q2 estimates the predictive power after cross-validation.OPLS-DA,orthogonal partial least squares-discriminant analysis.
Screening and identification of different metabolites
In this experiment,the variables of importance value of the OPLS-DA model(threshold >1)and theP-value of thet-test(P<0.05)were used to identify the differentially expressed metabolites.An online retrieval Human Metabolomes Database(compared mass-to-charge ratio(m/z)or accurate molecular mass,error limit 0.01 Da)combined with a local database was used for the qualitative methods of different metabolites.Finally,25 potential biomarkers were screened,which may be related to the mechanism of JQJT in the treatment of T2DM,as presented in Table 2.
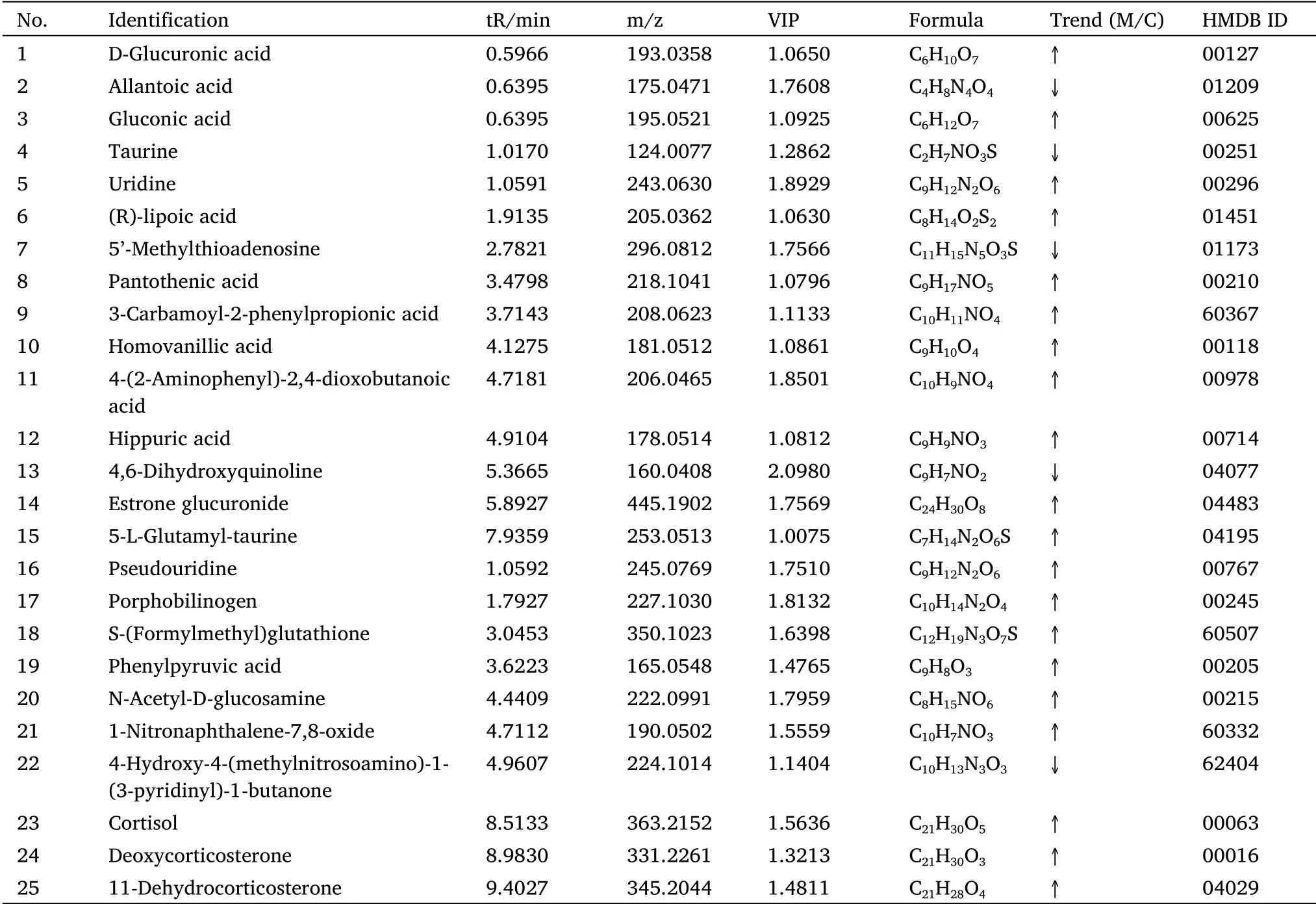
Table 2 Urine potential biomarkers of T2DM rats
Metabolic pathway analysis
The metabolic pathway was analyzed using MetaboAnalyst 4.0,and the most relevant metabolic pathway was selected by combining the results of the pathway enrichment analysis and topology analysis.A total of 25 related differential metabolites were screened from the urine samples.A metabolic pathway analysis diagram of 25 common metabolites is shown in Figure 5,including taurine,hypotaurine,phenylalanine,ascorbate,and aldarate metabolism,as well as pentose and glucuronate interconversions.
To compare the changes in the content of potential biomarkers,the sample content was converted into a visualized heat map,as presented in Figure 6.JQJT treatment significantly reversed the abnormal changes in these metabolites,improving the metabolic pathways.This proved that JQJT has a good therapeutic effect on T2DM.
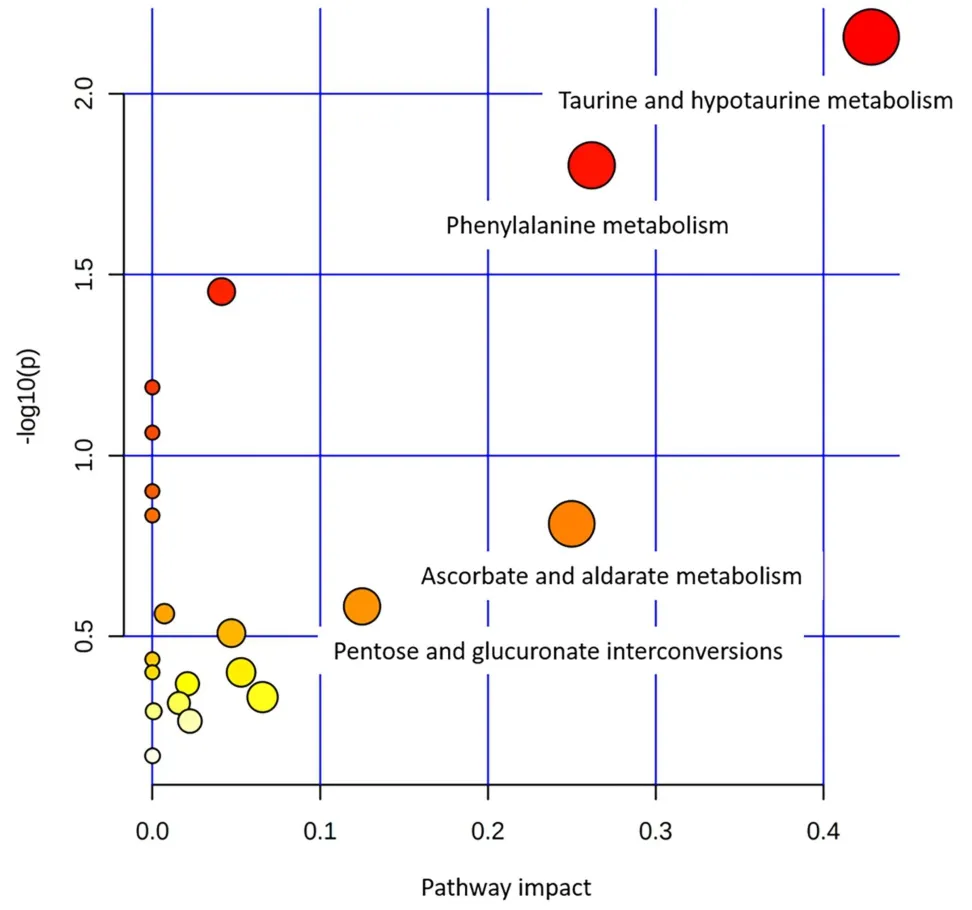
Figure 5 Urine pathway analysis of type 2 diabetes mellitus rats.x axis represents the pathway enrichment factor,y axis represents the P-value of the test.
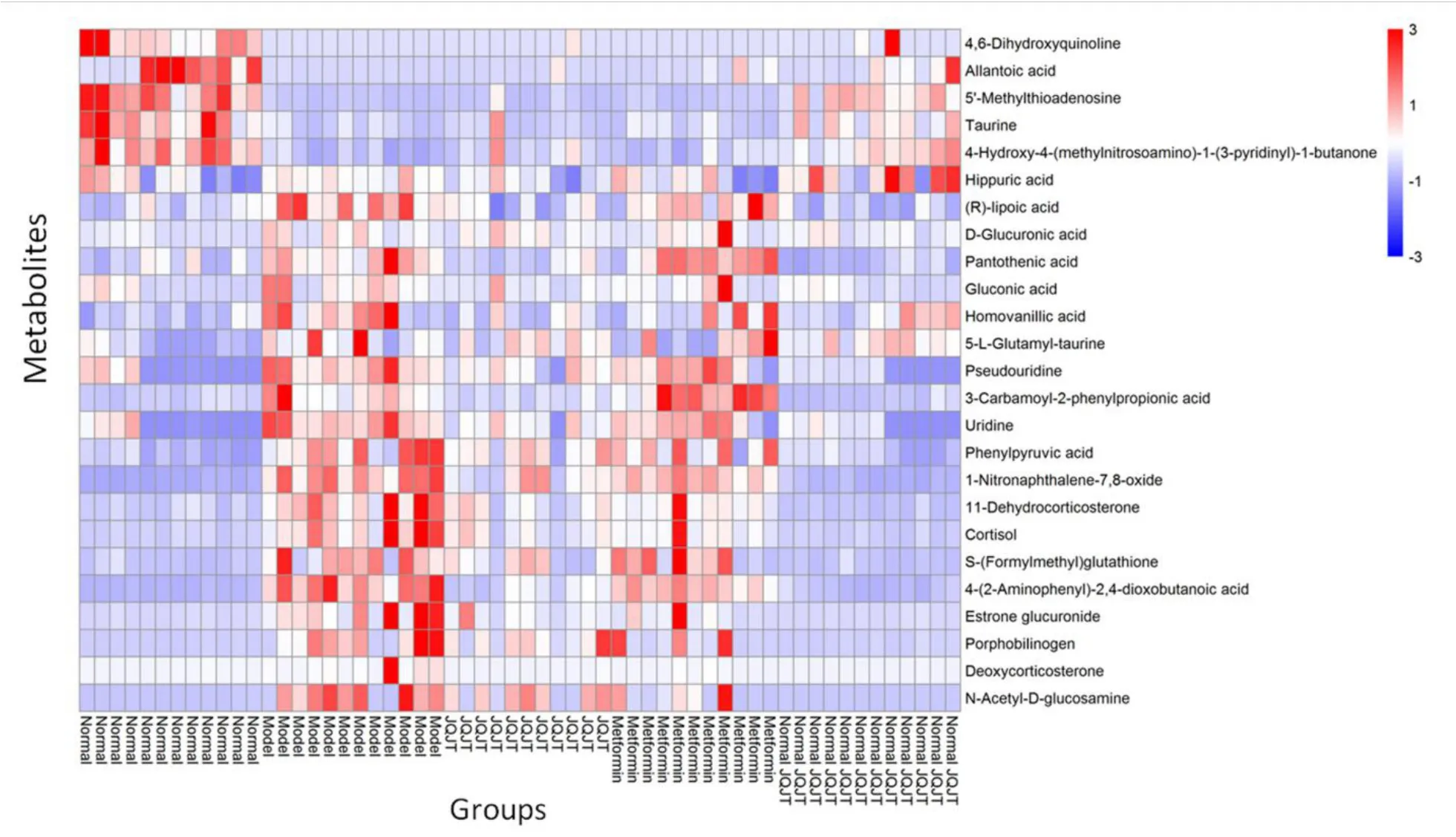
Figure 6 Heatmap of potential biomarkers in urine.The darker red color indicated the higher expression level of the metabolite and the darker blue color indicated the lower expression level of the metabolite.
Discussion
In this experiment,the changes in metabolites were studied in model rats.The results showed that 25 metabolites in the model group demonstrated significant changes compared to those in the normal group.Compared with the model group,the metabolites in the JQJT group showed a significant callback trend.It is suggested that JQJT may improve the metabolic disorder of T2DM by regulating these metabolites,and the metabolic pathway is primarily related to taurine,hypotaurine,phenylalanine,ascorbate,and aldarate metabolism,as well as pentose and glucuronate interconversions.
Taurine has a wide range of pharmacological effects and is a powerful free-radical scavenger.Tsuchiya et al.performed a glucose tolerance test by administering 10 mmol/L of taurine in rats and found that taurine could reduce blood glucose levels and improve hyperglycemia[30].Taurine is known to improve the metabolism of blood glucose and blood lipids in diabetes[31].However,whether taurine can improve the metabolism of blood glucose and blood lipids is related to its regulation of oxidative stress is unknown.In this study,the taurine content in the urine of model rats decreased significantly,probably because the taurine content was relatively low in vivo.After JQJT treatment,taurine excretion significantly increased,suggesting that JQJT may play a significant role in the occurrence and development of T2DM through the metabolic pathway of taurine and hypotaurine.
Phenylalanine is an essential aromatic amino acid found in the human body.Under normal circumstances,phenylalanine can participate as an amino acid residue in the synthesis of various proteins in human tissues and cells.It can also produce tyrosine in the liver,and tyrosine can promote the secretion of hormones and neurotransmitters(suchasdopamine,epinephrine,and norepinephrine)[32].Phenylalanine metabolism may contribute to the development of the liver,nervous system,and other diseases[33].
Lipoic acid can be synthesized by itself in an organism.It is also a powerful antioxidant.It can improve the sensitivity of skeletal muscles and red blood cells to insulin,reduce peroxidation,eliminate oxygen free radicals,regulate the metabolism of blood lipids,and inhibit inflammatory reactions.Therefore,α-lipoic acid can reduce the oxidative stress reaction in T2DM and assist in enhancing the hypoglycemic effect.
Studies have found that uridine can regulate glucose metabolism by affecting the glycosylation levels of insulin signaling pathway-related proteins insulin receptor substrate,phosphoinositide-dependent protein kinase-1,Akt protein,forkhead box transcription factor O subgroup 1,and glucose transporter 4[34].In conclusion,uridine is closely related to glucose and lipid metabolism.However,the specific mechanism requires further study.
Conclusion
In this study,urine samples from T2DM rats were analyzed using ultra performance liquid chromatography-MS/MS metabonomics.The results showed that the contents of 25 metabolites in the model rats were abnormal.JQJT intervention could reverse the disorder of 25 metabolites,indicating that JQJT has a good therapeutic effect on T2DM,which may be related to the regulation of taurine,hypotaurine,phenylalanine,ascorbate,and aldarate metabolism,as well as pentose and glucuronate interconversions.With regard to the changing trend of endogenous substances before and after administration of JQJT,the possible metabolic pathway for treating T2DM was discussed.This provides an experimental reference for the research on the mechanism of treating diabetes with TCM and is of great significance for fully understanding the nature of drugs and mastering their application rules.
 Traditional Medicine Research2022年3期
Traditional Medicine Research2022年3期
- Traditional Medicine Research的其它文章
- Traditional Chinese medicine:an important broad-spectrum anti-coronavirus treatment strategy on COVID-19 background?
- Anti-asthmatic mechanism of the Huashanshen dripping pill via suppressing contraction of the airway smooth muscle
- Application of network pharmacology in the prevention and treatment of COVID-19 by traditional Chinese medicine
- Pharmacological efficacy of the traditional Chinese medicinal formula Kun-Tai-1A in the treatment of letrozole-induced polycystic ovary syndrome
- Recent advances in research on natural product inhibitors of SREBPs
- Integrated UHPLC-MS and network pharmacology to explore the active constituents and pharmacological mechanisms of Shenzao dripping pills against coronary heart disease
