A valid evaluation method for UPLC fingerprint analysis and moisture ratio prediction model:application to microwave vacuum drying of Radix isatidis extract
You-Lu Li,Guo Qing,Ning Zhang,Yong-Ping Zhang,2,3,Yan-Yan Miao,2,3,Guo-Qiong Cao,2,3,Jian Xu,2,3*
1College of Pharmaceutical Sciences,Guizhou University of Traditional Chinese Medicine,Guiyang 550025,China.2National Research Center of Miao Medicine Engineering Technology,Guiyang 550025,China.3Guizhou Research Center of Traditional Chinese Medicine Processing and Preparation Engineering Technology,Guiyang 550025,China.
Abstract Background:Drying is a necessary component of traditional Chinese medicine extracts.The heating principle of microwave vacuum drying is different from that of the conventional heat method.However,at present,there is paucity of information on the drying process of traditional Chinese medicine extract by microwave vacuum drying,and the results of such process are unclear.Methods:To study the dynamic changes in the chemical characteristics of microwave vacuum drying under different drying conditions,ultrahigh-performance liquid chromatography fingerprint profiles were established using Radix isatidis extract as a model drug and analyzed using similarity analysis,partial least squares-discriminant analysis,and semi-quantitative analysis.In addition,a backpropagation artificial neural network model was developed to predict the moisture ratio of the drying process.Results:Qualitative results showed that the similarity between different drying conditions was greater than 0.95,and 2 amino acid components(peaks 5 and 6)affected by process fluctuations were screened out.The quantitative results showed that the mass concentration of component 1 fluctuated after drying,while that of component 2 increased.The optimal backpropagation artificial neural network model structure used to predict the moisture ratio was 5-4-1,with regression and mean squared error values of 0.996 and 0.0003,respectively,after training,which were well fitted and had a strong approximation ability.Conclusion:Upon comparison of fingerprints and the evaluation of statistical methods,common components of Radix isatidis extract had little variation under different drying conditions,and the selected components provided a reference for the establishment of process evaluation indexes.The establishment of backpropagation artificial neural network provides a theoretical basis for the application of microwave vacuum drying technology and online monitoring of moisture ratio.
Keywords: Radix isatidis extract;microwave vacuum drying;ultrahigh-performance liquid chromatography fingerprint analysis;moisture ratio prediction
Background
In China,traditional Chinese medicine(TCM)extract is an important raw material for many TCM preparations,such as TCM pills,granules,and tablets,with long historical applications and great clinical effects[1,2].Radix isatidis,one of the commonly used TCM,is a commonly used antipyretic antidote with antiviral,anti-endotoxin,antipyretic analgesia,and other effects[3,4].Radix isatidisextract is a series of refined and purified products containing many effective components.However,in the content determination item of theChinese Pharmacopoeia(2020 edition)[5],only the content of(R,S)-goitrin was detected,which has certain limitations.At present,many researchers have investigated the quality and processing ofRadix isatidisextract[6-8],but almost all of them have focused on evaluating the drying process or the quality of the extract with some index(composition or efficacy).Some scholars have established highperformance liquid chromatography fingerprint analysis methods forRadix isatidis[9,10],but there are few studies on the differences of the fingerprints of extracts before and after drying.
In fact,the drying of TCM also has a long history of development,described in as early as ancient books of Chinese medicine,HuangDi’s Canon of Internal Medicine(221-220 B.C.E.)[11],Fifty-Two Diseases(475-221 B.C.E.)[12],andMaster Lei’s Discourse on Drug Processing(the Southern and Northern Dynasties,420-589 C.E.)[13]This books recorded the drying methods of TCM,which emphasized the importance of the drying process.Of particular concern is the TCM extract with high water content and viscosity,which makes water diffusion difficult and inefficient,and prone to quality degradation[14,15].Many studies have shown that vacuum drying,though simple in structure and convenient to use,is time-and labor-intensive,but can directly produce dried extract powder and omit the mashing process.However,the phenomenon of particle adhesion often occurs,resulting in material loss[16].In contrast,microwave vacuum drying(MVD)has the advantages of a shorter time,reduced energy consumption,and uniform heating[17].This is because the drying method combines microwave heating and vacuum drying,hence,achieves low temperature and rapid drying,especially allowing time to remove the moisture via intermittent microwave energy supply[18,19].In summary,the MVD method can effectively improve the drying performance.
Microwave technology has been used as an energy source since the late 1960s[20],and has been developed for a long time for use in the field of TCM.However,the heating principle of microwaves is different from that of the conventional heat method.It has the characteristics of being highly efficient,energy-saving,and economical,but it also has some problems for further research.At present,there is paucity of information on the evaluation methods of the drying process of TCM extracts by MVD,and the results of such process are unclear.
TCM extracts contain several active components with a wide spectrum of biological and pharmacological activities.Studies have shown that components are affected by different drying processes and their operating parameters[21-23],thus a comprehensive analysis of the quality control of the drying process is necessary.TheChinese Pharmacopoeia(2020 edition)only determined the content of(R,S)-goitrin[5],which does not fundamentally reflect the effect of MVD on the chemical composition ofRadix isatidisextract.Notably,fingerprints,especially chromatography fingerprints combined with multivariate statistical analysis methods,are widely used for process quality control[24-26].Therefore,we can handle this problem using the proposed analysis methods.
In actual operation,moisture content is also considered a critical parameter for identifying suitable drying endpoints[27].Accurate and timely monitoring of the moisture content of the TCM extract can conserve energy and reduce thermal damage caused by avoiding excessive drying,but also enter the next process smoothly by avoiding insufficient drying.Unfortunately,in China,the control level of the TCM extract drying process is relatively nonprogressive,mainly relying on experience,compounded by a lack of moisture content and dynamic changes in the drying process[28].An artificial neural network(ANN)is also one of the statistical analysis methods,as a computational modeling tool,that has been frequently predicted moisture content changing trend under a range of drying conditions[29].Accurate prediction is helpful for operators to adjust the drying process and improve the quality of dried products,but little data are available on this aspect.Therefore,the suitability of ANN for predicting moisture content deserves further attention.
In this study,the chemical fingerprint ofRadix isatidisextract from the MVD method under four different conditions was investigated using ultrahigh-performance liquid chromatography(UPLC).Similarity analysis(SA),partial least squares discrimination analysis(PLS-DA),and semi-quantitative analysis were performed to compare the overall changes in the chemical components ofRadix isatidisextract before and after drying from both qualitative and quantitative perspectives.Another work is to establish a back-propagation artificial neural network(BP-ANN)model to predict the moisture ratio(MR)in the MVD process,which is useful for obtaining the accurate time and moisture content required for drying under a given condition,thus reducing the possibility of energy loss and product quality degradation.Moreover,the model was used to improve the references for other TCM drying methods.
Materials and methods
Materials
Radix isatidispieces(batch number:20200515,origin:Hebei,China)were purchased from Guizhou Tongjitang Herbal Pieces Co.,Ltd.(Guiyang,China)and authenticated by Prof.Xu Jian at Guizhou University of Traditional Chinese Medicine(Guiyang,China).Voucher specimens(No.BLG2021001)was deposited in the School of Pharmacy,Guizhou University of Traditional Chinese Medicine(Guiyang,China).Cytidine(batch number:PCS2261,purity >98%),guanosine(batch number:PCS2261,purity > 98%),and(R,S)-goitrin(batch number:PCS0060,purity > 95%)were purchased from Chengdu Zhihua Pure Biotechnology Co.,Ltd.(Chengdu,China).Uridine(batch number:110887-201803,purity > 99.7%)and adenosine(batch number:110887-201803,purity >99.5%)were purchased from China Food and Drug Verification Research Institute(Beijing,China).Chromatography-grade methanol was purchased from Thermo Scientific Co.,Ltd.(Waltham,MA,USA).
Preparation of Radix isatidis extract
Radix isatidispieces were decocted twice using a Chinese Herbal Boiler(Donghuayuan YJ13/2B-G,Beijing,China),with the first decoction for a ten-fold volume of water for 2 h,and then for an eight-fold volume of water for 1 h.Next,the collected solution was filtered through a filter paper and concentrated to a thick liquid.The initial moisture content ofRadix isatidisextract was determined by theChinese Pharmacopeia Method II(oven-drying method),which was calculated to be 37%.
Drying experiment
We designed 11 different experimental groups including a total of five factors.The initial moisture content was adjusted to 40,50,and 60%.Instrument parameters were set as follows:microwave power(0.3,0.5,1.0 kW),vacuum level(0.03,0.05,0.07 Mpa),and intermittent drying time(1,2,and 3 min drying off,kept constant for 2 min drying on).
Radix isatidisextract was loaded onto a ceramic plate with dimensions of 22 × 13 × 7 cm,and subsequently placed into a microwave vacuum dryer(Xinqi WBZ-10,Guiyang,China)to obtain the dynamic change of the drying process.During each intermittent period,the sample was quickly removed from the drying chamber,and then weighed using an electronic balance(Yingheng YHC,Ruian,China)with a precision of 0.01 g.Masses were recorded before and after drying to determine the moisture content.The drying process ended when the moisture content was less than 10%.Finally,all extracts were crushed using a high-speed grinder(Huanyatianyuan 6202,Beijing,China)and mixed to obtain the dried extract powder.Each experimental group was repeated three times,and the average value was used to calculate the moisture content.
UPLC measure and analysis
The extracts.0.15 gRadix isatidisextract and 0.1 g dried extracts of different drying conditions were weighed separately,dissolved in 10 mL of purified water,centrifuged at 13,000 rpm for 5 min and 0.22µm filtered.
Preparation of mixed reference solution.Appropriate amounts of cytidine,uridine,guanosine,and adenosine were weighed and dissolved in water.(R,S)-Goitrin was weighed and dissolved in methanol.The reference solution was filtered through a microporous membrane(0.22 µm).
Chromatography conditions.The appropriate amount of adenosine standard was weighed,dissolved in water,and filtered through a microporous membrane(0.22 µm).UPLC was performed on a Waters Acquity UPLC H-Class system equipped with a sample manager-FTN,quaternary solvent manager,photodiode array detector,and Empower software(Waters,Milford,MA,USA).The column was a Waters Acquity UPLC BEH shield RP18(2.1 × 100 mm,1.7 µm),kept at 37 °C,and employed at a flow rate of 0.3 mL/min.The mobile phases were water(solvent A)and methanol(solvent B)delivered in a 12 min gradient consisting of the following:0-2 min,1-3% B;2-4 min,3-13% B;8-12 min,13-70% B.The detector wavelength range was set at 254 nm and the injection volume was 2µL.
Validation of the UPLC method.TheRadix isatidisextract sample(initial moisture content of 50%)was used in the validation test.Precision was determined by injecting the same sample solution six times.Repeatability was determined by analyzing six independent sample solutions extracted from the same sample.The stability test was performed by injecting the same sample solution at 0,4,6,8,10,and 24 h after preparation.
Establishment of SA and PLS-DA.The chromatographic fingerprints were evaluated using chemometrics methods.SA was performed using the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine(version 2012A,National Committee of Pharmacopoeia,China).PLS-DA was performed using SIMCA(version12.0,Umetrics Inc.,Kinnelon,NJ,USA).Principal component analysis was performed using SPSS(version 20.0;SPSS Statistics,Chicago,IL,USA).
Establishment of semi-quantitative analysis.The samples were prepared in solutions of different mass concentrations,with the mass concentration as the abscissa(x)and each chromatographic peak area as the ordinate(y).To quantitatively analyze the corresponding compounds in the sample,a semi-quantitative standard curve of each component was drawn and calculated using equation(1)[30]:

where Cmrepresents the mass concentration ofRadix isatidisextract(mg/mL),y represents the chromatographic peak area,and a and b represent the slope and intercept of the standard curve calibrated by the mass concentration,respectively.
BP-ANN design
During the experimental process,the MR was calculated using equation(2)[6]:
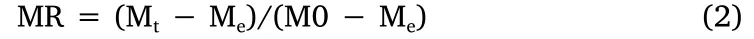
where,Mtis the moisture content at the sampling point(g water/g dry solid),M0is the initial moisture content(g water/g dry solid),and Meis the equilibrium moisture content(g water/g dry solid).Under vacuum conditions,in comparing Mtand M0with Me,the values of Mecan be approximately considered as 0,and the formula was simplified as the following equation(3):

A total of 244 units of data were collected from the start of the drying process until the end under different drying conditions.A total of 70% of the data were randomly selected to train the model;30%of the data were used for validation,and the remaining data were used for model testing.To ensure the accuracy of the analysis,the data were normalized between values of 0 and 1.This formula is expressed according to equation(4):
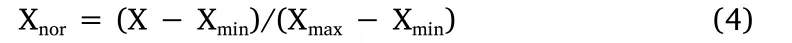
where Xnorand X represent the values of the data before and after processing,respectively,and Xmaxand Xminrepresent the maximum and minimum values,respectively.
The structural design of BP-ANN is a multilayer feedforward network model composed of an input layer,a hidden layer,and an output layer.Four drying conditions(initial moisture content,vacuum level,intermittent heating time,and power)were all factors affecting the drying process.In addition,the drying time varied with time.Therefore,five factors were considered as the input nodes.The output node was real time MR.In the hidden layer,the number of node settings can have a strong impact on the prediction accuracy and complexity level of the trained model,which were empirically chosen to yield the best performance.The algorithm used is shown in equation(5)[31].

where m represents the number of hidden nodes,a represents the number of input nodes,b represents the number of output nodes,and n represents a range of 1-10 adjustable constants.In this study,a =5 and b= 1;from this,m was between 4 and 13.
For this purpose,all data were analyzed using MATLAB neural network tool-box version 9.10.0.1577097(R2021,The MathWorks Inc.,Natick,MA,USA).A Levenberg-Marquardt algorithm was used to train the BP-ANN.
BP-ANN model performance evaluation:the higher the R and the lower the mean squared error(MSE),the better the accuracy of the index.To validate the performance of the BP-ANN model,the real results were compared to those predicted using statistical methods.
The best-fitting model was based on the MSE and the regression values(R).These statistical parameters are shown in equation(6),equation(7)[32].
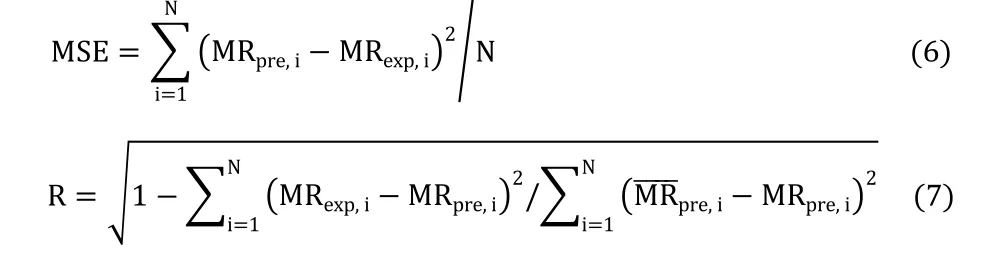
where MRexp,iand MRpre,iare the ith experimental and predicted MR,respectively,and N is the number of observations in each run.
Results
Fingerprint analysis of different drying condition
UPLC establishment.A total of 13 samples were obtained from the dried extracts obtained under 12 different drying conditions and from the extract before drying for chromatographic analysis.The chromatographic peak information of the five reference substances was compared with that of the sample and the chromatographic peaks were identified.By analyzing the full wavelength scanning results of the unidentified chromatographic peaks,unconfirmed 3 and 4(peaks 5 and 6)were presumed to be amino acid components,as shown in Figure 1.
Validation of the UPLC fingerprint method.For all methodology validation studies,the retention time and peak area of peak 10 were chosen as the reference,and the relative standard deviations of the relative retention time and relative peak area of the ten common peaks were calculated.The results showed that the relative standard deviations for precision,repeatability,and stability was less than 5%(Supplementary Table S1),indicating that the method is valid and reliable for the analysis of chromatographic fingerprints.
Result of SA.To increase credibility,the chromatogram ofRadix isatidisextract before drying was set as the reference map,and the fingerprint of the dried extracts of different drying processes was establishedusingtheSimilarityEvaluationSystemfor Chromatographic Fingerprint of Traditional Chinese Medicine,as recommended by the State Food and Drug Administration.
The common peaks of theRadix isatidisextract and different dried extract samples were corrected according to the fingerprints generated by the evaluation system and the fingerprint of the reference substance.The overlapping chromatographic fingerprints generated by the 13 groups of samples are shown in Figure 2.Ten common chromatographic peaks were calibrated with obvious characteristics,good reproducibility,and stability.The similarity values between the generated reference fingerprints and the sample fingerprints were calculated(Supplementary Table S2).In comparison with the reference chromatogram,the similarities of the 13 groups of samples were greater than 0.95(0.959-0.997)with high similarity.The results showed that there was no increase or decrease of the components in the whole drying process ofRadix isatidisextract,and the overall profile did not change.Peak 10(adenosine)was chosen as the reference(S),and the relative standard deviations values of the relative retention time and the relative peak areas of the other chromatographic peaks were 3.159-3.401% and 2.401-41.805%,respectively(Supplementary Table S3).From this,it was considered that there was high consistency among different drying conditions in the composition of the component,albeit with some differences in the content of the component.
PLS-DA,as a supervised recognition pattern,can amplify the differences between groups and help screen the feature components responsible for classification.This method is widely used in the differentiation and identification of TCM to emphasize their similarities and differences[33].In this study,to reflect the“homogeneity” ofRadix isatidisextract under various conditions,the signature difference components closely related to the drying process were screened.The common peak area data of 13 batches of samples as variables were imported into SIMCA(version 13.0)for PLS-DA.
A scatter plot of the scores is shown in Figure 3.All samples were divided into three categories,which indicated that the effective components of the dried extracts were impacted to varying degrees by different levels of MVD drying conditions.Overall,fresh samples(extract before drying)were classified into one category;vacuum level at 0.07 Mpa and microwave power 0.3 kW and 1 kW into another category;and the other samples into another category(Figure 3A).The variable importance projection(VIP)of the PLS-DA model was used to determine the symbolic difference components among the three groups of samples,as shown in Figure 3B.The higher the VIP value,the greater the contribution to the sample classification.As shown in Figure 3B,a VIP value >1 was used as the screening standard to identify two signature difference components,unconfirmed 3 and unconfirmed 4(x5and x6),which were key components affecting the sample classification.This result also suggests that the above compounds were more sensitive to the drying process,and therefore should be given more attention during the MVD process.
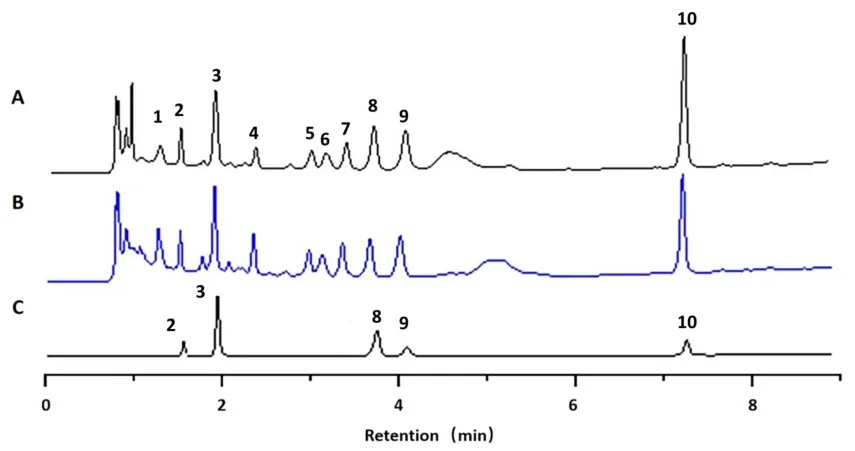
Figure 1 UPLC chromatogram.(A)The UPLC chromatogram of Radix isatidis dried extracts.(B)The UPLC chromatogram of Radix isatidis extract.(C)The UPLC chromatogram of mixed standards.2,cytidine;3,uridine;8,guanosine,9,(R,S)-goitrin;10,5-adenosine;unconfirmed chromatographic peaks were named unconfirmed 1-5.UPLC,ultrahigh-performance liquid chromatography.
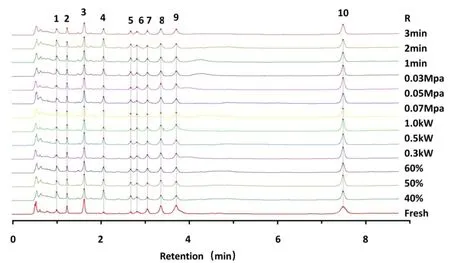
Figure 2 UPLC fingerprints of test samples under different microwave vacuum drying conditions.R,control fingerprint;3 min,2 min,1 min-intermittent heating time at 3 min,2 min,1 min;0.07 Mpa,0.05 Mpa,0.03 Mpa-vacuum level at 0.07 Mpa,0.05 Mpa,0.03 Mpa;1.0 kW,0.5 kW,0.3 kW-microwave power at 1.0 kW,0.5 kW,0.3 kW;60%,50%,40%-initial moisture content of extract at 60%,50%,40%;fresh-extract before drying.1-10,common peaks of fingerprint.
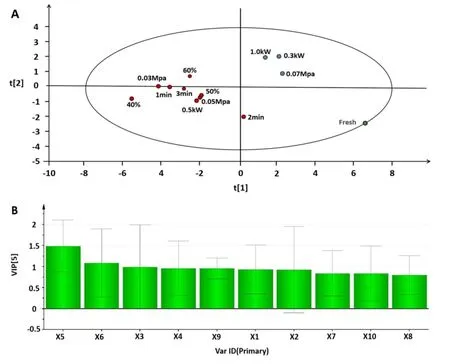
Figure 3 PLS-DA results of 13 test samples.(A)PLS-DA score plots.(B)VIP plots.t[1],t[2]-the scores t1 and t2 are new variables summarizing the all independent variable variables,and identifies which are orthogonal,i.e.,completely independent of each other;X1-X10,common peaks of fingerprint.PLS-DA,partial least squares discrimination analysis;VIP,variable importance projection.
Results of semi-quantitative analysis.The regression equation was obtained using the peak area value as the ordinate(Y)and the mass concentration of the sample as the abscissa(X).The regression equations and linear ranges of differential component 1 and differential component 2 were Y = 2149.3X-5016.6(r = 0.9939)and 8-30 mg/mL,and Y = 2166.2X-2346.2(r = 0.9931)and 8-30 mg/mL,respectively.The results showed that the linear relationship was good and could be used for the quantitative analysis of various components.
The results calculated using equation(1)are listed in Table 1.After drying,the mass fractions of the differential components 1 and 2 in the extract were 13.089-23.968 mg/mL and 25.020-29.603 mg/mL,respectively.Compared to the fresh sample(extract before drying),the mass fraction of differential component 1 increased or decreased,while that of component 2 increased significantly.The above results show that appropriate process conditions are more conducive to the quality of dried extracts and are closer to the quality before drying.
BP-ANN modeling analysis
Number of neurons in hidden layer.In the process of modeling,the number of different neurons in the hidden layer was calculated using MATLAB neural network tool-box version 9.10.0.1577097(R2021,MathWorks Inc.,Natick,MA,USA)to test the optimal topology.
According to equation(2),when the number of hidden layers was determined to be four(Supplementary Table S4),the BP-ANN had the best training effect.Therefore,the model with the 5-4-1 structure was used to predict the MR of theRadix isatidisextract.The network structure diagram is shown in Figure 4.
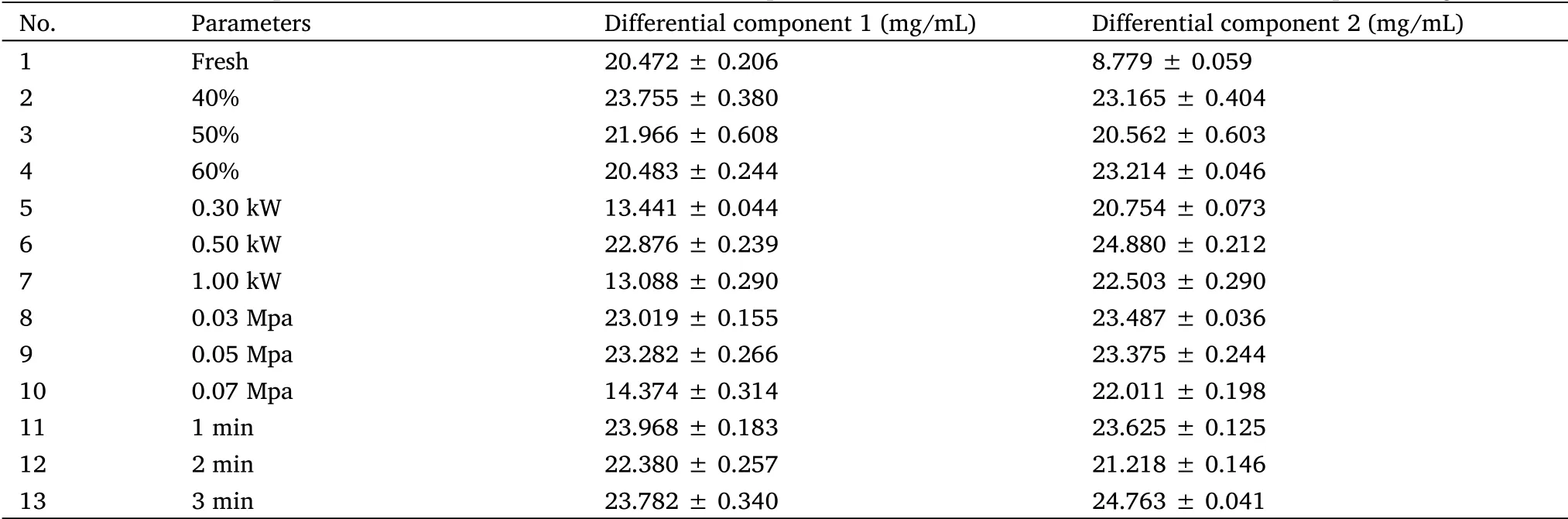
Table 1 Comparison of contents of two differential components in Radix isatidis extract before and after processing
Table 2 BP-ANN model topology for prediction of MR values of weights and bias obtained for optimal network
The BP-ANN performance.R and MSE can be used as verification indicators.As shown in Figure 5A,the minimum MSE was obtained by training the BP-ANN model with approximately 43 training epochs.In Figure 5B,it is more intuitive to see that the individual data have deviations,but the overall fitting degree is high,indicating that the currently designed BP-ANN model has high accuracy and credibility,and therefor can be used to estimate and predict the MR value ofRadix isatidisextract during MVD.
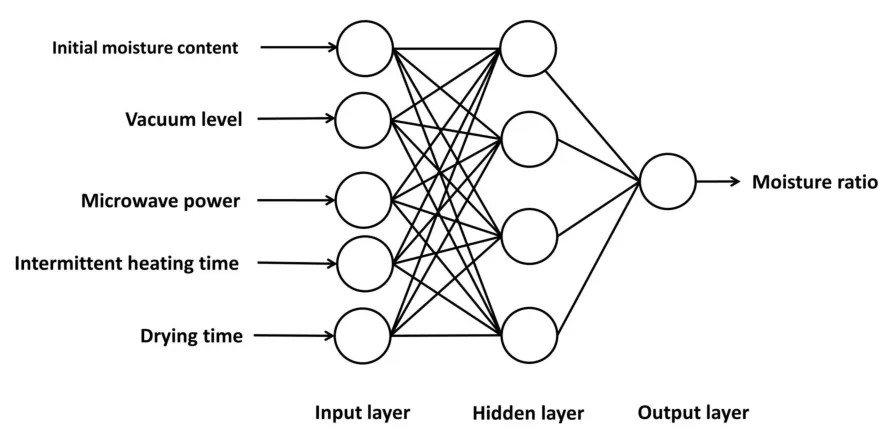
Figure 4 Optimal topology structures of the BP-ANN model.Each circle represents the network node.The network includes an input layer,a hidden layer,and an output layer.The input layer includes the drying conditions(initial moisture content,vacuum level,microwave power,and intermittent heating time)and drying time,and the output layer is the MR.BP-ANN,back-propagation artificial neural network;MR,moisture ratio.
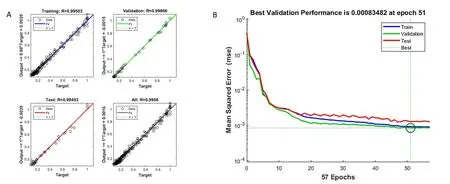
Figure 5 The results of BP-ANN performance.(A)The linear regression of targets relative to output.(B)The plotperform plots error vs.epoch for the training,validation,and test performances of the training record plotperform returned by the function train.BP-ANN,back-propagation artificial neural network.
The weights and bias estimation model data were obtained using the MATLAB neural network toolbox,as shown in Table 2.The BPANN model accurately predicted the MR ofRadix isatidisextract during the drying process.In this study,the BP-ANN model was selected not only because of its accuracy but also because of its universality,which can predict the dehydration curve behavior of the entire test range[19].The model parameters and other defined parameters are almost certainly useful for the application of the model to the prediction of moisture content changes in other TCM[34].
Discussion
To establish an ideal fingerprint,chromatographic conditions such as the mobile phase,column temperature,and volume flow rate were repeatedly investigated.The volume flow rate was finally determined to be 0.3 mL/min,the column temperature was 37 °C,and the injection volume was 2 µL,which gave the best number of peaks,peak shape,and separation.The fingerprints of the sample were analyzed in the full wavelength range(190-400 nm)using a photodiode array,and the highest number of peaks and high response values were found at a wavelength of 254 nm.The combinations of acetonitrile or methanol as the organic phase and water as the aqueous phase were also compared.Methanol and aqueous solutions were identified as the mobile phases,and the elution gradient was optimized to determine the elution gradient for fingerprinting.
First,UPLC fingerprints ofRadix isatidisextract under 12 drying conditions were established and compared.Using the fingerprint of the extract before drying as a reference,five common chemical components were identified.The results of the qualitative analysis proved the similarity of the overall chemical components of theRadix isatidisextract under different drying conditions.In addition,according to the results of PLS-DA and semi-quantitative analysis,the contents of the two amino acids varied completely,indicating that there were other component transformations in the drying process.This may also be related to the heating time and temperature of the microwave drying.In this experiment,Radix isatidiswas extracted using water as the solvent,and polar solutes were retained.Its main chemical components are sulphur-containing alkaloids,nucleosides,and amino acids.Some sulfur-containing alkaloids((R,S)-gotrin)and nucleosides(guanosine,cytidine,adenosine,and uridine)have also been identified.The unconfirmed 3 and 4(peaks 5 and 6)are thought to be amino acid components,as inferred from fullwavelength scanning and literature data[35-37],but there are still some shortcomings in the above studies,and their structural types need to be further verified by physical and chemical properties and spectral data.
Amino acid components are the key components ofRadix isatidis,which are thermally unstable and directly affect the subsequent product quality;therefore,they can be considered as evaluation indices of the drying process.The MVD of licorice extract showed that licorice and licorice extracts are sensitive to the drying process because they are rich in saponins and flavonoids with poor stability[38].A high drying temperature,low microwave power,and high vacuum level rise can easily damage these components[39].Therefore,subsequent experiments will qualitatively analyze the different components to understand the influence of MVD on the component structure ofRadix isatidisextract.In addition,from the perspective of extraction method,it only contains water extract,but not water extracting-alcohol precipitating solution and alcohol extraction.From the perspective of prescription composition,there are only single drugs and lack of TCM compound.Therefore,further experimental studies are warranted.
By analyzing and simulating the MR of the drying process,it is important to deepen the understanding of the drying process and grasp the laws of process changes,which are important for improving the quality of the products and drying efficiency[29].Research shows that BP-ANN technology can simulate the change in the water ratio during the drying process of the extract[40].This experiment developed a BP-ANN model for MR prediction during the MVD process with a highly accurate model structure of 5-4-1,which can provide a new modeling method that can better guide the actual production.In future research,different types of extracts and drying methods can be combined to ensure a more accurate prediction of the drying process.
Conclusion
The chemical composition ofRadix isatidisextract before and after MVD was relatively consistent.It is necessary to incorporate more physicochemical property indicators for evaluation to determine the best process conditions.BP-ANN modeling of MR during MVD for a variety of TCM,intermediates,and preparations may be a challenge worth studying may be a worthwhile challenge to work upon.
 Traditional Medicine Research2022年3期
Traditional Medicine Research2022年3期
- Traditional Medicine Research的其它文章
- Traditional Chinese medicine:an important broad-spectrum anti-coronavirus treatment strategy on COVID-19 background?
- Anti-asthmatic mechanism of the Huashanshen dripping pill via suppressing contraction of the airway smooth muscle
- Application of network pharmacology in the prevention and treatment of COVID-19 by traditional Chinese medicine
- Pharmacological efficacy of the traditional Chinese medicinal formula Kun-Tai-1A in the treatment of letrozole-induced polycystic ovary syndrome
- Recent advances in research on natural product inhibitors of SREBPs
- Integrated UHPLC-MS and network pharmacology to explore the active constituents and pharmacological mechanisms of Shenzao dripping pills against coronary heart disease
