Advances of biodegradable magnesium-based implants for orthopaedics
Zhao-Yang Ran, Wu-Fei Dai, Kai Xie, Yong-Qiang Hao*
Abstract Stainless steel, titanium alloys, cobalt-chromium alloys and other metal materials are the most widely used orthopaedic implants.However,there are still some problems in clinical application,including a mechanical mismatch between metal and bone,inflammation and secondary operation.As a new generation of medical metal materials, magnesium (Mg) and its alloys have attracted much attention due to their excellent biodegradability.Biodegradable Mg-based metals have good mechanical and osteogenic properties,and are expected to become implant materials to treat challenging orthopaedic diseases.However,the rapid corrosion rate is still one of the main challenges restricting its clinical application.Alloy and surface modification are effective methods to control the corrosion rate of Mg alloys.This paper reviews the mechanical and biological properties of biodegradable Mg alloys and the problems when they are applied clinically , emphasizing the latest progress of Mg-based metals in alloying and surface modification.The status of the application of Mg-based implants in orthopaedics are also described.
Keywords:Mg alloys;orthopaedic implants;corrosion;alloying;surface modification
Introduction
With the extension of life expectancy and the intensification of social aging, the incidence of aging-related traumatic bone diseases, especially fractures, is increasing [1].Fracture fixation is the key to fracture treatment.Therefore, there is a great demand for all kinds of orthopedic implants in clinic.In 2013, the global market for such devices was $5.7 billion and continued to grow at the rate of 7.2% [2].The implant must firmly fix the broken bone to avoid the slight movement under the influence of external forces, which may affect the healing of the bone.Unlike biomaterials applied in soft tissue repair such as peripheral nerves [3], metal materials have become the first choice for orthopaedic load-bearing applications because of their excellent mechanical strength and fracture toughness, and are often used to repair or replace the diseased or damaged bone tissues[4].
Traditional orthopaedic metal implants are mainly made of inert stainless steel, titanium (Ti) and its alloy, cobalt-chromium (Co-Cr) alloy and other materials, which have good biocompatibility, high wear resistance and corrosion resistance and can maintain long-term structural stability in vivo [5, 6].Therefore, they are widely used in the field of orthopaedics, such as bone substitutes for joint replacement, fixations and stabilization devices [7, 8].However, traditional metal implants also have some shortcomings.Firstly, due to the mismatch of mechanical properties between metal materials and natural bones, stress shielding may occur in clinic, resulting in the absorption of surrounding bone tissues and reducing implant stability [9, 10].Secondly, toxic metal ions and/or particles may be released through corrosion or wear process, causing an inflammatory cascade reaction that biocompatibility reduction and tissue loss [11-13].Thirdly, because of the permanent existing of traditional non-degradable metal biomaterials in the human body after implantation,the plates and screws that fix severe fractures often need to be removed by a second operation after the tissues are fully healed.Otherwise, clinical complications such as pain or impaired function may occur.The second operation increases the cost of the health care system and imposes additional trauma, economic, and time costs on the patients.In addition,due to beam hardening and related imaging artifacts, these traditional metal materials will seriously affect the diagnostic accuracy of X-ray and computed tomography images[14].
In order to solve these problems, biodegradable metal materials are attracting more and more interest from researchers.Biodegradable metals are a kind of material that degrades slowly in vivo, and the degradation products released by them can trigger appropriate host reactions.After completing the task of supporting tissue regeneration, the biodegradable metal can disappear completely, leaving no implant residue and eliminating the need for a second operation to remove the implant [15, 16].Therefore, they are considered a better choice for orthopaedic loadbearing applications[17].
As a new type of biodegradable metal materials, Mg and Mg alloys have been new research hotspots in biomedical materials because of their excellent mechanical properties, biocompatibility and biodegradability[18 - 23] (Figure 1).Because of these perfect properties, Mg-based implants are very suitable for application in the field of orthopaedics.To clarify the application prospect of Mg in the field of orthopaedics, this article provides a comprehensive review of advances of biodegradable Mg-based implants for orthopaedics.
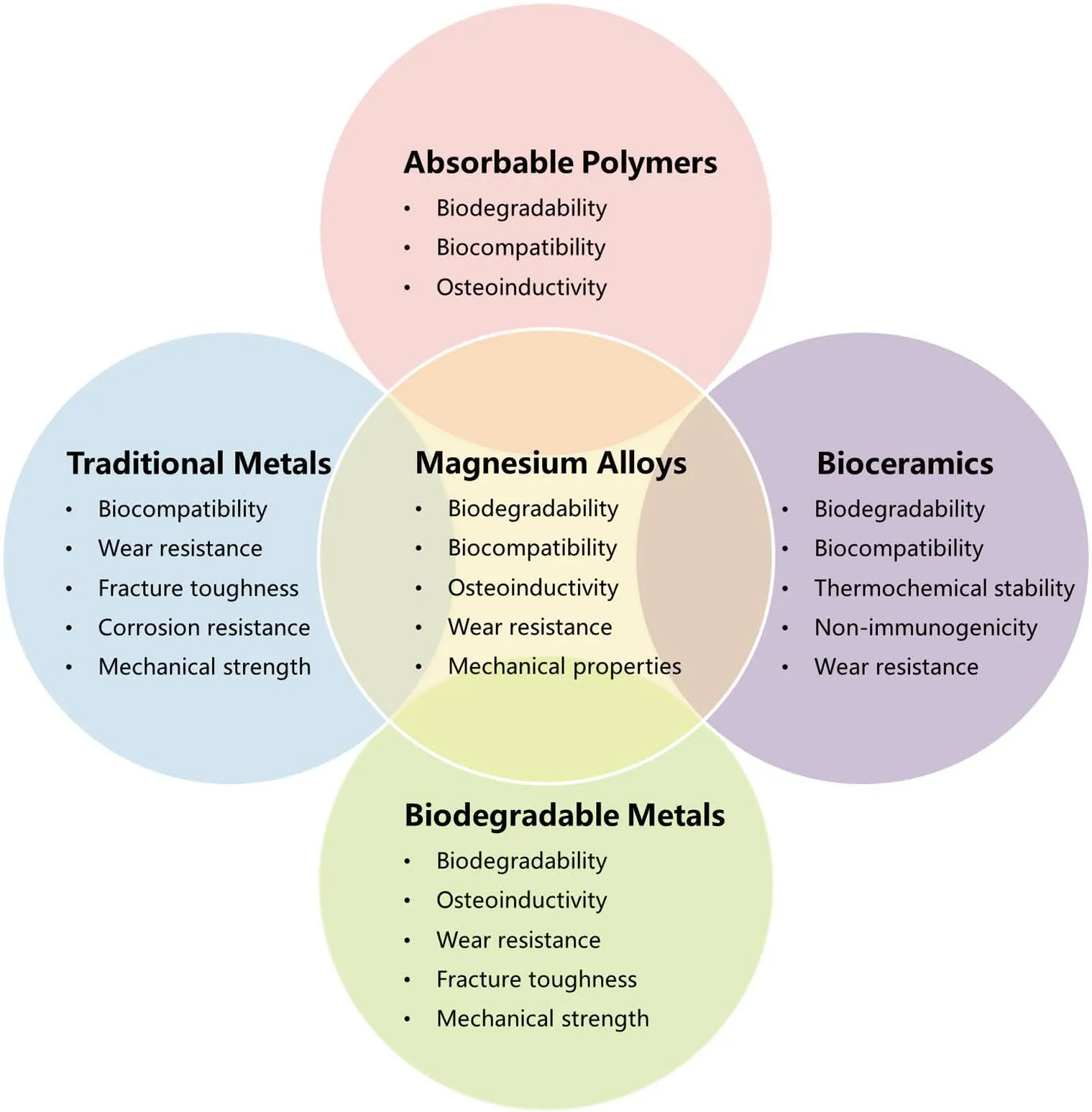
Figure 1.The relationship between magnesium alloys and other biomaterials in biomedical properties.
Properties of biodegradable Mg alloys
Mechanical properties.
As shown in Table 1, Mg and its alloys have excellent comprehensive mechanical properties, including lightweight, elastic modulus, density close to the natural bone(1.8-2.1 g/cm3), and high strength to weight ratio(158 kN·m/kg) [24].The density of Mg alloy is about 1.74-2.0 g/cm3,which is much lower than that of biomedical Ti alloy (4.4-4.5 g/cm3).In contrast, the density of aluminum and steel is 1.6 and 4.5 times higher than that of Mg alloy, respectively [25].Compared with traditional metal materials such as stainless steel (~200 GPa), cobalt-based alloy (~230 GPa)and Ti alloy (~115 GPa), the elastic modulus of Mg (40-45 GPa) matches better with that of natural bone (3-20G Pa) [8].This suggests that Mg implants can play a unique role in reducing stress shielding during load transfer between the implant and bone interface.Furthermore, in contrast with ceramics and polymer biomaterials, Mg alloys have a higher strengthto-weight ratio, more excellent fracture toughness and higher tensile strength [26, 27].Hence, Mg-based materials serve as appropriate choices for biodegradable orthopaedic implants, considering matching with bone mechanical properties.

Table 1 Mechanical properties of bone tissues and common biomaterials for orthopaedic implants
Biological properties.
Biocompatibility.Mg and its alloys have high biocompatibility and safety in the human body.The recommended daily intake of Mg for adults is 240 to 420 mg [28].This value is almost 50 times the recommended intake of iron (8-18 mg/day) and zinc (8-11 mg/day) [29].Mg is involved in hundreds of biochemical reactions and is essential in bones and soft tissues [30].It is estimated that a normal adult weighing 70 kilograms has 1 mole of Mg stored in his body, more than half of which is stored in bone tissue [31].As the fourth highest content cation in the human body, Mg ion participates in apatite formation in the bone matrix and many metabolic processes of the human body, which is essential for human metabolism[32].
Additionally, Mg is also a cofactor of many enzymes that can stabilize the structure of DNA and RNA [33].The Mg content in extracellular fluid ranges from 0.7 to 1.05 mmol/L, and the balance of Mg in the body is maintained through the regulation of the kidney and intestinal tract [30].Serum Mg levels over 1.05 mmol/L may cause muscle paralysis,hypotension, respiratory distress, and even cardiac arrest [34].Nevertheless,due to the effective excretion of the kidney and intestine, the incidence of high Mg is very low.Excess Mg ions will be transported through the circulatory system and quickly excreted through the urine and feces without any adverse effects [35].Therefore, the degradation of Mg-based implants in the human body generally does not lead to the increase of serum Mg concentration.It is well known that the unnecessary ions dissolved by metal implants in the human body have always been problems due to the potential of causing allergies.However, it was found that Mg alloys(AZ31, AZ91, WE43, and LAE442) do not cause allergenic reactions in skin application tests according to the histomorphological criteria[36].It is also proved that Mg alloys have good biocompatibility.
Osteoinductivity.Mg is an essential element for bone health, with about 60% of the body’s Mg stored in the bone matrix [37].Mg deficiency may lead to osteoporosis[38].It was found that when Mg-based implants were used to support human fractures, the mineral apposition and bone mass around implants increased while the callus formation time decreased compared with the polylactic acid (PLA) control group [39, 40].Similarly,some studies have shown that Mg-based implants may promote fracture healing and bone formation and increase mineral attachment, bone mass and bone mineral density around Mg-based implants [41-44].This phenomenon is not common in bioabsorbable polymers or permanent metal implants.
The periosteum reaction caused by Mg ions released by the implant helps to form more bone around the cortical side of the cortical bone[37].This may be achieved by stimulating the Mg ions in the dorsal root ganglion to up-regulate the expression of calcitonin gene-related peptide(CGRP) in the periosteum.Researchers found that the osteogenic effect of Mg ions was significantly reduced by surgically removing the periosteum or using small hairpin RNA (shRNA) to knock out the CGRP receptor(calcitonin receptor-like receptor (Calcrl) or receptor activity modifying protein 1 (RAMP1)).In contrast, the overexpression of Calcrl or RAMP1 in rats after Mg implantation significantly promoted the formation of new bone around the periosteum because the increased level of CGRP promotes the osteogenic differentiation of periosteal-derived stem cells when Mg implants release Mg ions [45].The increase of bone regeneration induced by Mg-based implants may also be related to the production of corrosion product magnesium hydroxide (Mg (OH)2).Studies have shown that Mg (OH)2, the main corrosion product of Mg alloy, was the main factor for the increase of bone regeneration and the temporary decrease of bone resorption in vivo.Authors also suspected that the enhancement of bone regeneration was mainly due to local Mg ion concentration or local alkalosis accompanied by Mg (OH)2dissolution [46].In addition, Belluci et al.evaluated the effect of Mg2+restriction on osteoclasts.The study suggested that Mg2+deficiency augmented osteoclastogenesis while it appeared to inhibit the activity of these cells [47].Mg supplementation can prevent osteolysis caused by wear particles in vivo by inhibiting NFATc1 (nuclear factor of activated T cells 1) and activating NF- κB(nuclear factor κB), suggesting that Mg has the effect of anti-osteoclast formation [48].Therefore, it is speculated that Mg ions may enhance the osteogenic effect by inhibiting the formation of osteoclasts.
Biodegradability.As is known to all, pure Mg and Mg alloys are prone to corrosion in the aqueous medium [49].The electrochemical reaction between Mg and water is the cause of the rapid degradation of Mg metal, resulting in Mg(OH)2and hydrogen(H2)[50].
The following chemical reactions exhibit the corrosion mechanism of Mg in an aqueous solution(Figure 2):

Figure 2.Schematic diagram illustrating the degradation mechanism of Mg-based implant in body fluid for different time.(a)The beginning of degradation.(b)A period after degraded.Mg(OH)2 is easy to react with chloride ions to form high solubility MgCl2,which accelerates pitting corrosion and causes high local stress,resulting in cracks and pits on the surface of Mg-based implants.
Anodic reaction:Mg(s)→Mg2+(aq)+2e-
Cathodic reaction:2H2O(aq)+2e-→2OH-(aq)+H2(g)
Overall reaction:Mg(s)+2H2O(aq)→Mg(OH)2(s)+H2(g)
Existence of chloride ions: Mg(OH)2(s) + 2Cl-(aq) →MgCl2(s) +2OH-(aq)
Soluble Mg (OH)2will form a slightly effective oxidation protective film on the surface of Mg, which can prevent it from further corrosion in water [51, 52].However, when the chloride ion concentration in the corrosion environment exceeds 30 mmol/L, Mg (OH)2will react with chloride ion to form highly soluble magnesium chloride (MgCl2) and hydrogen [37, 53].The chloride ion concentration in the physiological environment is about 150mmol/L, which is far higher than the concentration that Mg (OH)2can withstand [49, 54].As a result, Mgbased implants will inevitably undergo severe corrosion and rapid degradation in vivo.Mg (OH)2also releases hydroxide ions (OH-) when it reacts with chloride ions, which leads to a local increase of pH value near the host tissue[55,56].
Since the body fluid does not contain only water, the corrosion of Mgbased implants in body fluid is more complex than that in pure water.The corrosion in body fluid is affected by various factors in the body, such as pH, ion concentration and type, protein adsorption and biochemical activities of surrounding tissues [57-59].Mg corrodes slowly in pure water and alkaline solutions.However, in chloride-containing liquids and acidic solutions, the corrosion rate is quite high.The concentration of bicarbonate ions (HCO3-) in simulated body fluids (SBFs) can change the corrosion rate of pure Mg due to the rapid formation and precipitation of a protective layer of carbonate on the surface of Mg [60].In addition to chloride ions and bicarbonate ions, there are also calcium ions and phosphate ions in body fluids.The interaction between Mg ions, calcium ions and phosphate ions will generate calcium phosphate and calcium Mg phosphate [61].These complex bioactive mineral products can form a sedimentary layer on the surface of Mg to inhibit its further corrosion and the increase of pH value.
Besides various inorganic components, body fluids also contain organic components such as biomolecules, proteins, cells and even bacteria,which may adsorb or adhere to the surface of Mg, thus affecting the dissolution behavior of Mg.The concentration of Mg ions released by pure Mg in pure salt solution was tens of times higher than that in culture medium, and the addition of fetal bovine serum could reduce the leaching of Mg ions.The adsorption of proteins and the precipitation of insoluble salts in the solution effectively delay the degradation of Mg because the adsorbed proteins form an insoluble, dense and effective anti-corrosion barrier [35].The properties of the degradation products strongly affect the subsequent degradation steps on the surface of Mg and the biological response of bone tissue.Some researchers believed that the formation of corrosion products as a protective layer might partly explain the lower degradation rates observed in vivo than in vitro experiments [62].These studies reveal the importance of the interaction between organic components and implants on the degradation behavior of Mg-based implants.Therefore, it is possible to avoid the contact of inorganic components such as water and chloride ions and organic components such as protein in body fluid with Mg-based implants by modifying the surface of implants, or to change the structure and phase distribution of metals by adding different alloying elements, so as to change the degradation properties and control the degradation rate of Mg-based implants.These two methods are described in detail below.
Corrosion and related problems in the clinical application of Mg alloy
Loss of mechanical integrity caused by excessive degradation rate.
Significant progress has been made in studying Mg alloys as bone implants in the past 20 years, but the rapid degradation of Mg alloys in the human body remains a major obstacle to their clinical application.In orthopaedic applications, implants are often required to carry a certain load during injured bone tissue healing [63].Although biodegradable implants are eventually allowed to degrade, they still need sufficient mechanical strength during use.An ideal situation is that the degradation rate of the implant matches the healing rate of the bone tissue.The healing process of bone tissue usually includes an early inflammatory phase lasting for 3 to 7 days, a healing phase lasting about 3 to 4 months, and a remodeling phase lasting from months to years [64-66].For this reason,Staiger considered that the orthopaedic implants must remain stable in the body for at least 12 weeks while the bone tissue heals, eventually being replaced by natural tissue[37].
However, owing to the rapid conversion of Mg (OH)2to highly soluble Mg chloride under physiological conditions (pH 7.4-7.6 and high concentration of chloride), the degradation rate of Mg alloy is too high in a complex physiological environment [67-69].The rapid degradation of Mg alloy implants will bring about the loss of mechanical strength [70, 71].In addition to the decrease in strength, other mechanical properties of Mg,such as ultimate tensile strength (UTS), are also compromised with degradation [72].Mg alloys are prone to corrosion fatigue during degradation for the following reasons.The first is the formation of corrosion products and pits, which act as the starting point of cracks.The second is that Mg corrosion releases hydrogen and leads to hydrogen embrittlement under dynamic load in modified simulated body fluids[73].Moreover, the combination of chloride in the environment and Mg ions dissolved from the anode will further accelerate pitting corrosion and cause high local stress, resulting in the formation of cracks [72, 74].Nonuniform and local degradation behavior can also give rise to stress corrosion cracking, reduction of mechanical strength, and unexpected breakage before the expected healing time, which adversely affects the durability of implants [75, 76].The mechanical properties of the implants are insufficient before the host tissue is fully healed, which eventually leads to the loss of mechanical integrity and failure of the implants.
Hydrogen release and local alkalization.
In addition to the premature loss of mechanical integrity, rapid degradation of Mg also releases large amounts of hydrogen [77].Hydrogen generation occurs at an early stage of Mg alloy degradation, which depends on the corrosion rate and is associated with a reduction in implant volume [78,79].The corrosion rate of Mg is not similar at all anatomical locations,depending on the local blood flow before implantation [80].When the gas is produced too fast for the host tissue to process, it will accumulate in the tissue cavity or forms a subcutaneous gas cavity.Rapid accumulation of hydrogen can delay bone healing, form prominent tissue calluses, block blood flow and even lead to tissue necrosis, as the gas cavity separates the tissue layer [81-83].The experimental results showed that the bubbles almost filled the medullary cavity eight weeks after the rapidly degraded Mg alloy (ZX50) was implanted into the femur of rats.This leads to irregular bone shape, even after the Mg alloy nail is wholly degraded, but this phenomenon is not observed in slowly degraded Mg implants (WZ21)[84].Song et al.considered that the hydrogen release rate of 0.01 ml/cm2/day was tolerable to the human body and would not pose a severe threat[26].
The severe dissolution of Mg will also lead to local alkalization(increased pH) on the surface of Mg implants [85, 86].Because of the rapid corrosion of Mg-based implants, local alkalization is a common phenomenon.Though the human body automatically adjusts the pH values of body fluids and blood, the amount of OH- produced by degradation is far more than can be absorbed or transported by tissues, so local alkalization is inevitable around rapidly corrosive Mg implants [26].This may affect the balance of pH-dependent physiological responses near the implant and cause alkalosis if the local pH exceeds 7.8.In most in vitro experiments, Mg corrosion can significantly increase the pH of the immersion solution [87].Animal experiments revealed that two months after implantation, the inflammatory reaction around the uncoated AZ91 implant was more severe than that of the diopside coated implant and micro-arc oxidation implant [81].These results manifest that rapid corrosion will alter the chemical balance in the local biological environment and disrupt the healing process.
Methods to improve the properties of Mg-based implants
Since Mg-based orthopaedic implants have many problems such as insufficient mechanical strength and rapid degradation in clinical application, researchers have made many explorations and achieved many results in improving the properties of Mg-based implants in recent years,including adding appropriate alloying elements and surface modification.
Alloying
Pure Mg has low corrosion resistance and poor mechanical properties, and the rapid degradation of Mg in vivo may cause many clinical complications.To control the degradation rate of Mg-based implants, maintain their mechanical strength during use, and reduce the above side effects,many new biomedical Mg alloys have been designed by adding different alloying elements [88].Alloying elements have a direct impact on the corrosion resistance of Mg-based alloys [89].The addition of appropriate alloying elements can improve the mechanical properties of Mg and reduce the corrosion rate of Mg by changing the structure and phase distribution[90].Nevertheless, the cytotoxicity and long-term inflammatory consequences of these elements are also major concerns [91].Therefore, alloying elements need to be carefully selected to maintain the biocompatibility of implants, as they also dissolve in body fluids during the degradation process.
Al, Cu, Fe, Zn, Mn, Ca, Li, Ni, Sr, Y, Zr and rare earth (RE) elements are commonly used alloying elements of Mg, which have different effects on the corrosion properties of Mg alloys (Figure 3).What's more, the addition of these elements also affects the mechanical properties of Mg alloys [92].Among them, Al, Zn, Mn, Ca, Zr, Sr, Sn and most rare earth elements including Nd and Gd, have been proved to improve the corrosion resistance of Mg [29].Mg-RE alloys have good mechanical properties and corrosion resistance, usually showing the highest strength and the best elongation.In Mg-Y, Mg-Nd, Mg-Gd, Mg-Ce, and Mg-Ld and other new Mg-RE alloys, the corrosion rate of Mg-Nd alloy is much slower than that of other alloys [93].The corrosion resistance and mechanical properties of Mg-Y alloy prepared by the zone solidification method have been improved [94].Mg-Y-Zn alloy has excellent microstructure,mechanical, electrochemical and biological properties, making it a promising biodegradable implant material [95].It should be noted that there is a critical limit for the improvement of corrosion resistance of most elements, and further addition beyond the critical limit will lead to a decrease in corrosion resistance[96,97].
Common commercial Mg alloys used in biological research include the AZ (Mg-Al-Zn), WE (Mg-Zr) and ZK (Mg-Zn-Zr) alloys.AZ series alloys, especially AZ31 (Mg-3Al-1Zn) and AZ91 (Mg-9Al-1Zn) alloys have been widely studied in vitro and in vivo in recent years [98-101].The addition of aluminum increases the strength of Mg alloy.And the density increases slightly because the density of Al is similar to that of Mg, but results in the decrease of elongation [102].In addition, high concentrations of aluminum are neurotoxic, and their accumulation in the body may damage neurons leading to Alzheimer's disease and causing osteoblasts and muscle damage [103-105].WE series alloys have good biological corrosion resistance because they form a rare earth oxide film in an aqueous environment, thus delaying the degradation of Mg alloys [106].ZK series alloys, especially ZK40 (Mg-4Zn-0.5Zr) and ZK60 (Mg-6Zn-0.5Zr), have good biocompatibility because their alloy elements are zinc and zirconium and do not contain toxic elements[107-109].
Alloying is an important and effective strategy to improve the corrosion resistance and mechanical properties of magnesium-based implants.By adding different alloying elements to magnesium substrate,the corrosion and mechanical properties of magnesium are changed greatly, which can further meet the application needs of magnesium as orthopaedic implants.However, due to the high electronegative potential(-2.4V) and poor passivation tendency of magnesium, it is difficult to reduce the degradation rate only by alloying significantly.
Surface modification.
Surface modification is one of the most effective ways to control and reduce Mg’s degradation rate to improve the surface corrosion resistance of Mg [110, 111].The surface modification of Mg substrate can effectively isolate or reduce the contact surface area between the substrate and body fluid, which is helpful to maintain the mechanical integrity of Mg implant before a bone injury is completely healed [112].Compared with alloying,surface modification of Mg alloy can maintain good mechanical properties and realize the adjustment of surface corrosion resistance more simply and conveniently.Furthermore, surface modification can be used to construct multifunctional surfaces on Mg-based implants to obtain better biomedical properties.According to the different modification methods, surface modification can be divided into four categories: chemical modification, physical modification, compound modification, and other surface treatment,as shown in Figure 4.

Figure 3.Classification diagram of methods to improve the properties of magnesium-based implants.
Chemical modification.Chemical modification refers to the synthesis of a new phase covering the surface of Mg alloy by a chemical or electrochemical reaction, which can delay the initial corrosion and degradation of Mg substrate in a physiological environment by forming a barrier layer on the surface of Mg substrate.It usually includes alkali-heat treatment, fluorination treatment, anodic oxidation, micro-arc oxidation (MAO), etc.Due to strong chemical bonds at the interface between substrate and coating, the bonding strength of the coating produced by chemical modification is very high[113](Figure 5).

Figure 4.Schematic diagram of alloying, follow-up processing and implantation into human body

Figure 5.Schematic diagram of different methods of chemical modification and physical modification.(a) Alkali-heat treatment.(b) Fluorination treatment.(c) Anodic oxidation.(d)Micro-arc oxidation(MAO).(e)Physical modification:From bottom to top,they are Ca-P coating,biodegradable polymer coating(PLA),laser surface melting,and ion implantation.
Alkali-heat treatment is a surface modification method that can be used to modify Mg-based materials because the Mg(OH)2produced by the reaction forms the main corrosion protective layer.Some researchers treated AZ31 alloy with 5.66wt.% NaOH solution at 160 ℃, and Mg(OH)2coating was prepared on the surface of the substrate.The protection of Mg(OH)2film effectively reduces the corrosion rate of Mg alloy [114].Others incubated pure Mg in NaHCO3-MgCO3alkaline solution, followed by heat treatment at 773K.They showed no mass loss when the materials were soaked in SBF for 14 days compared with the untreated group [115].Since the coating formed by alkali-heat treatment is mainly composed of Mg(OH)2, the corrosion resistance of the coating may not meet the requirements when placed in the chlorine-rich physiological environment for a long time.
Fluorination treatment is one of the common chemical modification methods to improve the corrosion resistance of Mg and Mg alloys [116-118].Soaking in hydrofluoric acid is a common method to prepare MgF2coating on Mg alloy surface by fluorination.The Mg alloy substrate treated with hydrofluoric acid has improved corrosion resistance,antimicrobial properties and osseointegration compared to the untreated Mg alloy.Researchers compared the fluorination treatment effects of powder metallurgy Mg, cast Mg and AZ31 alloy and found that the MgF2coating formed on the surface of the samples slowed down the degradation rate of all samples [119].It was reported that the fluoridemodified implant surface promotes osseointegration in the early phase of healing following implant installation [120].Compared with Mg(OH)2produced by the alkali-heat treatment, the MgF2produced by fluorination treatment is more stable in a physiological environment.
Anodic oxidation is a widely used surface treatment technology for Mg alloys, and its effects on corrosion resistance, biocompatibility and bioactivity have been extensively studied [121, 122].It works by applying a voltage to form a thick and stable protective oxidation coating on the surface of the anode.The study found that the corrosion resistance of AZ91D Mg alloy was improved by electrochemical test in Ringer’s solution at 37 ℃ after anodic oxidation in molybdate solution [123].Anodic oxidation is one of the commonly used techniques to improve the corrosion resistance of Mg alloys.However, the biggest problem is that cracks may form on the surface of the coating [124].Combined with other coating or surface treatment technologies may be the solution to this problem.
Micro-arc oxidation (MAO), also known as plasma electrolytic oxidation (PEO), is also widely used to prepare porous and solid coatings on the surface of biodegradable Mg and its alloys [125].The oxide film formed by micro-arc oxidation has high metallurgical bonding strength with Mg alloy substrate.The corrosion resistance of the Mg-based metal is improved by forming a porous structure with strong adhesion on the surface of the substrate [126].Yang et al.used this technique to prepare the coating containing Mg2SiO4on the surface of ZK60 Mg alloy, which significantly improved the corrosion resistance of Mg alloy and its biocompatibility in vitro [127].Micro-arc oxidation can significantly enhance the corrosion resistance of Mg alloys.However, the porous structure of micro-arc oxidation coating limits its long-term protective effect on Mg substrate.
Physical modification.Physical modification aims to provide a physical barrier to improve the corrosion resistance of Mg substrates.Unlike chemical modification, there is no chemical bond between surface and substrate after physical modification [128].Physical modification is mainly carried out by introducing Ca-P coating, polymer coating, laser surface melting, physical vapor deposition or ion implantation[113](Figure 5).
Calcium phosphate (Ca-P) is the main inorganic component in bone tissue and one of the most commonly used Mg surface modification coatings.Its chemical composition is similar to that of minerals in mammalian bones [129-131].Ca-P coating has good biocompatibility,bioactivity, osteoinductivity and non-toxicity, and can improve Mg alloys’wear resistance and corrosion resistance [132, 133].Electrochemical deposition (ECD) is a common approach for the deposition of Ca-P coatings on Mg and its alloys.Researchers used this method to fabricate brushite, hydroxyapatite and fluoridated hydroxyapatite coatings on Mg - Zn alloy and compared their corrosion behavior in M-SBF.They found that all of these coatings decreased the degradation rate of Mg-Zn alloys [134].Wen et al.deposited HA coating on AZ31 alloy by ECD in the solution of Ca(NO3)2, NH4H2PO4and NaNO3.Electrochemical tests showed that the corrosion resistance of the coating alloy in SBF was improved[135].
Biodegradable polymer coatings, such as polylactic acid (PLA),polycaprolactone (PCL), poly (lactic-co-glycolic acid) (PLGA), and chitosan coatings, are another promising strategy for improving the initial corrosion of Mg-based materials to meet the requirements of bone tissue healing [136].Some studies have demonstrated that the polymer coatings significantly improved the corrosion resistance of the Mg substrates compared to the uncoated samples.The immersion test also confirmed that the degradation rate of PCL and PLA coating samples decreased in SBF solution [137].Xu et al.prepared biodegradable polymer coatings of poly-L-lactic acid (PLLA) and PCL on Mg by spin coating.The results show that the protective coating of PLLA and PCL can improve the initial corrosion resistance and cytocompatibility of Mg substrate [138].Whereas,because the bond formed through the physical interaction between the polymer coating and the substrate is not strong, when the implant is inserted into the bone, the coating is easy to detach from the metal substrate,limiting the clinical application of polymer coating.
Laser surface melting can effectively improve the surface properties of Mg alloys, such as wear resistance, corrosion resistance and biocompatibility [139, 140].Researchers synthesized corrosion and wearresistant aluminum coating rich in Al12Mg17intermetallic phase on AZ31B Mg alloy substrates by a highly intense laser beam.Compared with the untreated AZ31B, the laser processed samples’ corrosion resistance and wear properties were improved [141].After laser irradiation with sufficient power, the surface grains of MEZ and AZ91D Mg alloys are refined [142].The fine grain boundary precipitates are beneficial to the anchoring and maintenance of Mg (OH)2film, thus improving the corrosion resistance[143].
Physical vapor deposition (PVD) is an excellent coating process for improving wear and corrosion resistance.For Mg substrates, the most preferred and commonly used PVD technique is sputtering.Some researchers deposited Ti-29Nb-13Ta-4.6Zr (TNTZ) targets on the pure Mg and AZ31 Mg alloys by PVD method.The in-vitro corrosion tests indicated that the Ecorr values of the TNTZ coated pure Mg and AZ31 alloy were nobler about 400 mV than the uncoated Mg-based samples [144].Others investigate the bioactivity and biomineralization of an AZ91 alloy coated with nanostructured HA prepared by radio frequency (RF) magnetron sputtering in simulated body fluid tests.The results revealed the capability of the HA coating to significantly improve the corrosion resistance of the uncoated AZ91 alloy [145].The advantages of PVD include uniform and adherent layers and its convenience to control the film thickness [146, 147].However, the main disadvantage of the PVD technique is poor adhesion to the substrate than thermal spray methods.
Ion implantation is a common technology for surface treatment of Mg and its alloys, which changes the surface properties of materials, such as corrosion resistance and biocompatibility, by introducing an appropriate amount of ions into the near-surface of the materials.Researchers carried out nitrogen ion implantation on AZ31B Mg alloy and found that the corrosion resistance of AZ31B Mg alloy was greatly improved.[148].The surface of Mg alloy was modified by aluminum and oxygen double ion implantation.Al and O ion implantation formed a protective layer containing Al2O3, which improved the substrate’s corrosion resistance,and the implant’s degradation rate was slower [149, 150].Ion implantation allows different elements into the substrate without being affected by thermodynamic limitations such as solubility[88].
When the magnesium-based implants are implanted into the human body, corrosion first occurs on the implant surface.Therefore, surface modification can change the surface properties of magnesium substrate through physical and chemical methods and effectively isolate or reduce the contact surface area between the substrate and body fluid so as to improve the corrosion resistance of magnesium alloy.Compared with alloying, surface modification is cheaper, more flexible and eliminates the risk of adding potentially toxic alloy elements.However, the surface modification does not change the mechanical properties of the substrate.After the degradation of the coating, the magnesium substrate will be exposed to physiological conditions again, and the problems caused by rapid corrosion still exist.
Composite modification.Considering the limitation of single chemical or physical modification, more and more attention has been paid to compound modification involving both chemical and physical treatment.It is reported that the double-modified coating can effectively improve the biodegradation resistance of the substrate and control the degradation rate in a broader range [113].Researchers sealed PLLA onto MAO coating by physical interlocking, and a composite MAO/PLLA coating was fabricated on the surface of the WE42 alloy.The PLLA coating effectively sealed the microcracks and micropores on the surface of the MAO coating by physical interlocking to interfere with the corrosion ions.The results showed that the WE42 alloy modified by MAO/PLLA composite coating has good corrosion resistance and cytocompatibility [151].Kaseem et al.successfully deposited a functionalized composite coating with unique floweryflake structures on the surface of plasma electrolysis coating of AZ31 Mg alloy via layer-by-layer self-assembly process of 1-azanaphthalene-8-ol molecules.Compared with a single coating, the composite coating showed excellent corrosion performance [152].In addition, a chitosan/MAO composite coating was also prepared on Mg-Zn-Ca alloy.Compared with untreated Mg alloy, the corrosion resistance of Mg alloy with a composite coating is significantly improved, and the degradation rate in SBF solution is lower[153].
The simultaneous use of these methods could not only combine their advantages but also make up for their shortcomings through internal and external cooperation to further reduce the degradation rate of magnesiumbased implants and improve mechanical properties so as to accelerate the clinical application of magnesium-based orthopaedic implants.
Status of orthopaedic application of Mg-based implants
Although the research on the biomedical application of Mg continues throughout the 20th century, the relatively high degradation rate,impurities and underdeveloped processing technology of Mg-based implants hinder their further clinical application.In recent years, with the continuous progress of processing and manufacturing technology, the properties of Mg-based implants have been greatly improved, and clinical trials of Mg-based implants have been carried out in Germany, South Korea and China.
Researchers used Mg-Y-Re-Zr alloy screws (MAGNEZIX, Syntellix AG)for fixation during chevron osteotomy in patients with a mild hallux valgus in Germany.At 6-month follow-up, there were no significant differences between Mg group and the titanium group in terms of the American Orthopaedic Foot and Ankle Society (AOFAS) score for hallux,visual analog scale (VAS) for pain assessment, or range of motion of the first metatarsophalangeal joint [154].This clinical trial enabled Mg-Y-Re-Zr screws to be approved with the Communauté Européenne (CE) mark in 2013.So far, the clinical application of MAGNEZIX series screws has been extended to more than 50 countries/regions.Multicenter clinical trials of MAGNEZIX screws are still under preparation in China and the United States [1].Considering the potential health risks of alloy elements to patients, Zhao et al.have been committed to the development of highpurity Mg orthopaedic internal fixation implants.In 2013, they used highpurity Mg screws to fix vascularized bone graft in osteonecrosis of the femoral head patients in China.Within 12 months of follow-up, the patients treated with Mg screws had higher satisfactory results in functional recovery Harris hip score and bone graft displacement [155].These screws have also been successfully used for the fixation of vascularized iliac bone grafts for displaced femoral neck fractures in young adults, with better results and a lower incidence of complications such as avascular necrosis and nonunion than control group fixed with conventional implants [156].Recently in China, 9 cases of medial malleolar fractures were treated with Ca-P coated Mg-Nd-Zn-Zr alloy(JDBM) screws.The mean follow-up time was 12.2±4.9 months.After the operation, all patients achieved good medial malleolar fracture alignment,and none of them experienced breakage of the JDBM screws before fracture healing.Postoperative radiography indicated JDBM screws gradually degraded with implantation time, and obvious degradation could be observed 12 months postoperatively.At the final follow-up, the average AOFAS score was 90.4, indicating that biodegradable JDBM screws effectively treat medial malleolar fractures [157].In South Korea,it was reported in 2016 that Mg-Ca-Zn alloy (K-MET, U&I) screws were used to fix radial fractures, and the fractures healed completely 6 months after surgery.More importantly, biodegradable Mg-implants were entirely replaced by new bone within 1 year of implantation in 53 patients [158].The K-MET Mg alloy screw is the second Mg-based orthopaedic device approved by official agencies in the world, which significantly encourages clinicians and scientists to accelerate the clinical translation of new Mg implants designed for new indications[159].
Mg and its alloys have a long history as orthopaedic implants.Mgbased implants have been used to treat orthopaedic diseases as early as a century ago, and the research on orthopaedic applications of magnesium and its alloys has never stopped.With the deepening of research on magnesium-based implants, many problems hindering their clinical application have been gradually solved, so further clinical trials have been carried out in many countries.It is hoped that this potential biomaterial can be introduced into the clinic as soon as possible so that biodegradable magnesium alloy can play a more critical role in treating orthopaedic diseases and benefit more patients.
Conclusion
As new biological material, biodegradable Mg alloys have excellent mechanical and biological properties, making them auspicious orthopaedic implant materials.However, the loss of mechanical integrity, hydrogen release and local alkalization caused by rapid corrosion in the physiological environment significantly restrict its further application in biomedicine.Therefore, how to reduce the corrosion rate of Mg alloys has become the primary issue for researchers.Alloying and surface modification, two of the most widely used strategies for Mg alloys, are discussed in this paper.The latest progress in controlling the degradation rate of Mg-based implants is also reviewed.A large number of studies have proved that these measures play a crucial role in improving the corrosion properties of Mg alloys.Unfortunately, the current studies have not achieved a match between the rate of degradation of Mg implants and the rate of bone healing to achieve the optimal clinical effect.In the future, it is necessary to improve the mechanical properties and degradability of Mg alloys by alloying, and further reduce the corrosion rate of Mg alloys by combining different surface modification methods, so that this potential biomaterial can be introduced to the clinic as soon as possible for the benefit of patients.
- Life Research的其它文章
- Increased internet addiction during COVID-19 pandemics
- Intracranial hemangiopericytoma with right-sided aortic arch:a case report and summary of experience
- Low expression of novel biomarker RCSD1 predicts poor prognosis of lung adenocarcinoma
- Graft rejection after deep anterior lamellar keratoplasty in fellow eye in macular corneal dystrophy:a case report
- Utility of convalescent plasma for addressing the COVID-19 infection:brief review and case reports
- Molecular mechanism of different viruses associated with autoimmunity

