Utility of convalescent plasma for addressing the COVID-19 infection:brief review and case reports
Fazli Azim, Md Shahidul Islam, Ashraful Hoque, Muhammad Javed, Aneela Hayat, Kaniz Fatema,Molla Amiruzzaman, Md.Nasir Uddin, Kajal Kumar Karmakar, Syed A.A.Rizvi
Abstract Novel coronavirus,SARS-CoV-2 is responsible for causing a pandemic that has affected individuals worldwide,over 192 million people and about 4.1 million people died so far.The spread is ongoing and the numbers are still increasing.Numerous therapeutic approaches have been explored and developed during this pandemic.Immunotherapy with virus-specific antibodies in convalescent plasma (CP)has shown potential benefits for various pathogenic diseases.In many instances,it is the only available and safe management option for the COVID-19 patients.Here we describe two confirmed cases of COVID-19 from two different geographical areas that were managed with standard treatment modalities initially.Both of the patients were presented with high-grade fever,dry cough,and sore throat.Lab reports showed increased values of D-dimer, serum ferritin, leukocyte count (LC), Lactate dehydrogenase (LDH), and C-reactive protein (CRP).Chest X-ray showed bilateral infiltration (multifocal and bilateral ground-glass opacities and consolidations with peripheral and basal predominance), consistent with the previous reports on COVID-19 infection.The patients received conservative treatment according to the hospital's protocol.The convalescent plasma (from recovered patients) infusion was the last treatment given to both patients.After the convalescent plasma transfusion, both patients showed a reduction of viral load, an increase of anti-SARS-CoV-2 IgG and IgM antibodies, reduction in lung infiltration, with no adverse events.However, further randomized controlled trials are needed to investigate the full scope of safety and efficacy(both short and long-term)of convalescent plasma therapy for COVID-19 and other related infections.
Keywords: SARS-CoV-2, COVID-19 pandemic, convalescent plasma therapy, critical care, neutralizing antibody titer, acute respiratory distress syndrome.
Introduction
Treating infectious diseases with convalescent plasma is not a new concept.During the Spanish flu pandemic in 1918, convulsant plasma therapy showed potential benefits in treating influenza [1, 2].In 2003,amidst SARS pandemic, convalescent plasma displayed remarkable success in preventing complications and increasing survival rate [3, 4].Similar effectiveness of the convalescent plasma was noted during the influenza H1N1 pandemic in 2009,and the 2015 Ebola outbreak in Africa[5].Therapeutic benefits of plasma of a cured person from infectious diseases started in the 20th century.The first Nobel Prize in Physiology or Medicine was awarded in 1901 to Emil Adolf von Behring for the use of serum therapy in treating diphtheria [2, 6].New antiviral, antibiotics, and vaccines products are launched, nevertheless, administration of convalescent plasma can still be an important treatment (the only option in some cases)in the absence of specific treatment choices for new outbreaks [7].A systemic review and meta-analysis data on the clinical effects of convalescent plasma showed a statistically significant reduction of mortality rate for the COVID-19 treatment.In this novel coronavirus pandemic convalescent plasma could be a promising treatment option for severe cases [8, 9].The definitive pharmacological treatment protocol for severe acute respiratory dysfunction caused by COVID-19 has not been established yet.However, a few vaccines have been approved recently to prevent infection or reduce the severity [10].At present, treatment of COVID-19 is challenging due to the lack of suitable antiviral agents, even though work is underway at record speed worldwide.As such, classical and historical interventions have remerged as options for the control of this disease.The major target for convalescent plasma is to neutralize the pathogen for its eradication, that is, immunomodulation via amelioration of severe inflammatory response.Until now (February 2021), about 173 clinical trials registered at www.clinicaltrials.gov, in which the role of convalescent plasma (CP) in COVID-19 will be evaluated [11].To date,only the supportive care is available for SARS-CoV-2 infection [12], with the expectation that convalescent plasma will further help in increasing the rates of recovery with a similar therapeutic manner achieved during previous pandemics [13].Convalescent plasma has been considered as an investigational new drug for COVID-19 and rigorous clinical trials are ongoing worldwide to evaluate the safety and therapeutic efficacy.Numerous therapeutic agents have been explored or repurposed during the outbreak as trial-and-error method.Immunotherapy with convalescent plasma has been used to improve the survival rate of patients with severe symptoms of COVID-19 [14].IgG in COVID-19 convalescent plasma donors has been noted to reach peak levels around four weeks following the onset of symptoms, suggesting it is the optimal time to collect plasma,according to a new report [15].It is pertinent that the primary and secondary outcomes of the experimental convalescent plasma therapy should be set earlier in protocol for meaningful comparisons as well as parallel matched controls and a procedure of randomization.Every patient’s baseline clinical data and follow-up data after infusion of convalescent plasma should be noted and shared with the clinical trial team.Minimum criteria for convalescent plasma donors i.e., individuals who recovered from COVID-19 and remained asymptomatic for at least two weeks.While minimum criteria for convalescent plasma recipients i.e.,patient’s informed consent, COVID -19 PCR positive status, respiratory rate >30 per minutes, Oxygen saturation of <93% or less and Pulmonary infiltrate involving >30% or more lung parenchyma [16].Different parameters need to be tested before starting the therapy for optimization including the timing, volume and number of the transfusion after symptoms onset.These need to be adjusted based on body mass index and donor antibody titers.Various studies have observed that administration of transfusion of CP sooner at the onset of symptoms, leads to better outcomes[17].
Mechanism of action and related risk of the convalescent plasma therapy
SARS-CoV-2 viral dynamics suggest that viral load peaks during the onset of symptoms or a few days after, and usually disappear within about two weeks [18].After the onset of symptoms, in the second week postinfection, host immune response may become aggressive and culminate into cytokine storm leading to lethal outcomes [19].The neutralizing antibody, produced in response to an infection, is a key player in viral clearance and provides essential protection against infection from the dame pathogen.Convalescent plasma provides this neutralizing antibody to promote passive immunity [20].Nonetheless, viral eradication is determined by the level of neutralizing antibody titer (NAT) [7].The other non-neutralizing antibodies, IgG and IgM might contribute to prophylaxis and/or recovery improvement [21].The amount of specific antibody (IgG) against SARS-CoV-2 in the COVID-19 survivor’s serum,usually starts to increase around the 3rdweek and peaks around the 12thweek [22].Studies demonstrated that plasma from COVID-19 survivors after 12 weeks of onset of symptoms having the NAT level not less than 1: 160 is effective and can reduce the fatality [23].In addition,several studies further confirmed that plasma therapy is more effective in the earlier stage of disease [4].Research on SARS-CoV and MERS revealed that, neutralizing antibody binds to spike 1 receptor binding protein to inhibit viral entry, thus limiting viral amplification [24].In addition,convalescent plasma promotes antibody-mediated pathways (complement activation, antibody-dependent cellular cytotoxicity and/or phagocytosis)that could additionally contribute to the therapeutic effects [25].To date,no serious adverse effects or complications due to plasma therapy have been reported.However, fever, chills, anaphylactic reaction, circulatory overload, hemolysis, transfusion related acute lung injury are the common transfusion-related events [1].There are also some risks for transfusion transmitted infections such as human acquired immune deficiency virus,hepatitis-B virus, hepatitis-C virus and syphilis, however, donor blood products are carefully screened nowadays.
Scope of the use of investigational convalescent plasma
The passive immunization by convalescent plasma of recovered COVID-19 donors containing specific IgG and IgM antibodies against SARS-CoV-2 is an emerging solution worldwide in addressing the COVID-19.On January 15th, 2021, the US Food and Drug Administration (FDA) issued a new guidance on the use of convalescent plasma to treat hospitalized patients with COVID-19, superseding the guidance of the same title issued in November 2020.FDA recommends that health care providers and investigators use convalescent plasma under the emergency use authorization (EUA) or investigational new drug (IND) during the public health emergency.Since the use of convalescent plasma in the treatment of COVID-19 has not yet been approved by FDA, it is still regulated as an investigational product.FDA restricted the scope for the use of convalescent plasma in COVID-19 as i) Emergency Use Authorization(EUA); ii) Clinical Trials; iii) Expanded Access.Expanded Access pathway privileged the physician to use convalescent plasma for the patients with life threatening COVID-19 disease who are otherwise ineligible or unable to participate in randomized clinical trials[26].
Effects of convalescent plasma on hospital stay
In a study, 103 patients with severe or life threatening COVID-19 were randomized and 101 completed the trial allocating convalescent plasma with standard treatment and standard treatment alone.The analysis revealed the improvement within 28 days in 51.9% (27/52) of the plasma group vs 43.1% (22/51) in the control group.The study concluded that convalescent plasma with standard treatment had no significant role in clinical improvement within 28 days [27].On the other hand, there are many positive indications in favor of plasma therapy in the treatment of COVID-19 patients.For example, a case series of 5 critically ill COVID-19 confirmed patients in the Shenzhen Third People's Hospital, China, who had been suffering from severe pneumonia and supported by mechanical ventilation, subsequently treated with convalescent plasma between 10 and 22 days after admission along with antiviral therapy and methylprednisolone.Acute respiratory distress syndrome (ARDS) was resolve in 4 patients at 12 days after convalescent plasma administration,and 3 patients were weaned from mechanical ventilation within 2 weeks of treatment.Following clinical improvement, 2 patients were stable (37 days of therapy) and the rest 3 have been discharged on days 51, 53 and 55 [28].Another multicenter study conducted including 189 COVID-19 positive patients (115 in the plasma therapy group and 74 in the control group), reported that hospital discharge rates were higher in the plasma group (98.2 % vs 78.7 %).The length of hospital stay was remarkably lower in the plasma group (average 9.54 days vs 12.88 days).This clinical study provided strong evidence on the efficacy of convalescent plasma therapy in COVID-19 patients [29].A systematic review and meta-analysis were conducted by Zhiheng Xu et al.(2020), in which five prospective randomized controlled trials involved 2861patients with influenza who were treated with convalescent plasma and/or hyperimmune intravenous immunoglobulin (H-IVIG).Analysis revealed that treatment with convalescent plasma had no significant role on the reduction in the number of days in the ICU and ventilators and over all hospital stay length and even in mortality (odds ratio, 1.06; 95% CI, 0.51-2.23;P=0.87;I2=35%).The possible benefits of convalescent plasma might be the rising heme agglutination inhibition titers and reducing viral loads and cytokine levels [30].Another systematic review by Karthick Rajendran et al.(2020), included 27 patients from five studies who received convalescent plasma therapy for COVID-19.The analysis focused on different aspects including plasma dosage, mortality, clinical outcome and length of hospital stay.21 out of 27 patients were diagnosed as critically ill and 14 patients received mechanical ventilation.A total of 17 patients reported Acute respiratory distress syndrome.All the patients (n=27)received plasma therapy between Day 6 and Day 50 after the onset of symptoms or admission to hospitals.Despite the length of stay in hospital was not specified but most studies revealed data of discharge from hospital (n=15).Hence this systematic review recommended that the plasma therapy in addition to antiviral/antimicrobial drugs, could be an effective therapeutic option considering the safety, alleviation of clinical symptoms and reduction in mortality [20].Another recent report presents the results of a randomized, open-label, controlled clinical trial at 27 hospitals in Spain among 350 patients with criteria on inclusion that they must have been admitted for COVID-19 pneumonia within 7 days from the onset of symptoms and are not on mechanical ventilation at the start of clinical investigation.The authors noted a statistically significant advantage to the patients in the CP group in preventing progression to high-flow oxygen, invasive mechanical ventilation, or death at 28 days[31].
Effect of convalescent plasma on lung function improvement
Studies on convalescent plasma indicated that convalescent plasma offers immunomodulatory effects on lungs through induction of antiinflammatory cytokines and antibodies those inhibit complement cascade(C3a, C5a), inflammatory cytokines like IL-1β, IL-2, IL-6, IL-17, IL-8,TNFα and CCL2 along with some autoantibodies.These effects may preserve lungs damage involving fibrosis and reduction of pulmonary capacity, also migration of neutrophils to the lungs may lead to the damage to lungs tissues [32-34].They have an impact on the immunomodulatory property of the CP in patients with COVID-19 [11].Studies described, macrophage is a major immunological factor associated with inflammation and lung damage in various infectious diseases,especially in the case of COVID-19.Such patients suffer from a macrophage activation syndrome-like disease associated with innate immune migration to lung tissues [33].In a study, Blanco-Melo et al.reported that the inhibition of macrophage immunological pathway may help to control excessive cytokine production and prevent lungs damage(i.e., fibrosis).This study also showed that there is an up-regulation of chemokines for innate immune cells in ferrets as well as in patients with COVID-19.Furthermore, it was noted, this development mainly happened in the first 7 days post infection, whereas on day 14th, other cytokines such as IL-6 and IL-1 stayed elevated, while other observed cytokines returned to baseline [35].In the study conducted by Kozicky et al.(2018),authors noticed that macrophages treated with IV Ig raise production of IL-10, with a reduction in IL-12/23p40, thus signifying the promotion of an anti-inflammatory macrophage profile [36].In this article, we report two cases of severe COVID-19 patients in two different South Asian countries,showing a favorable clinical course after the convalescent plasma infusion.Tables 1,2 and 3 show the overall snapshot of the two Covid-19 case reports.
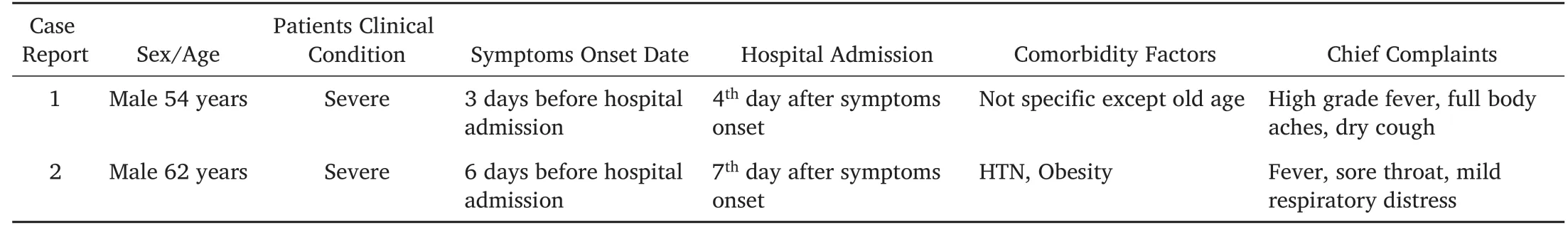
Table 1.Clinical Presentation and Treatments of Patients receiving convalescent plasma.
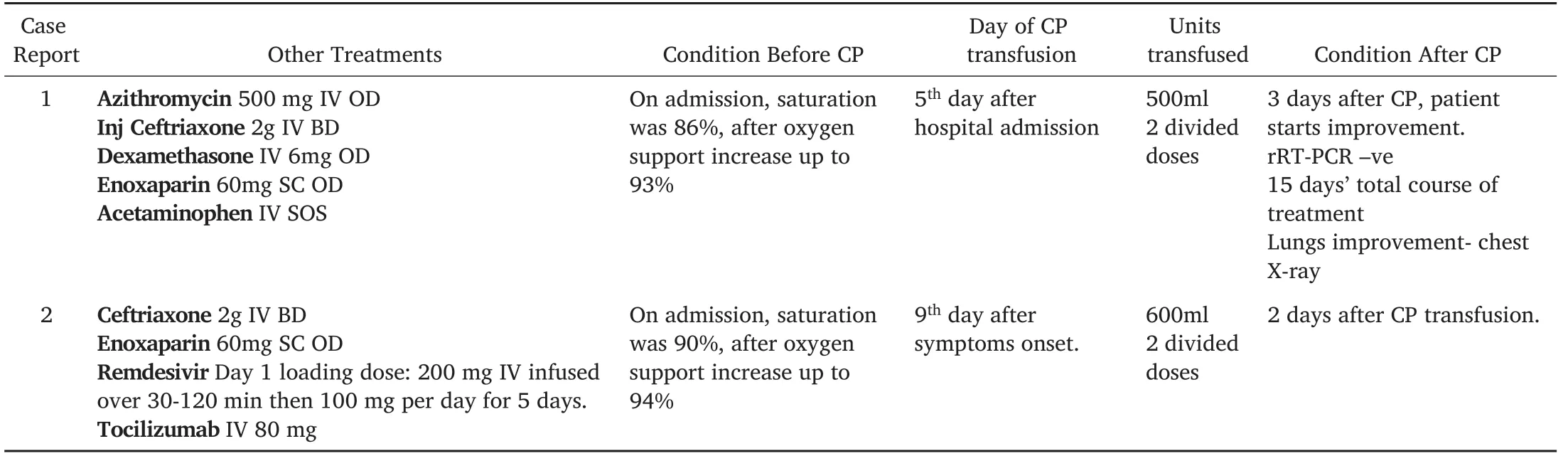
Table 2.Treatment protocol with convalescent plasma.
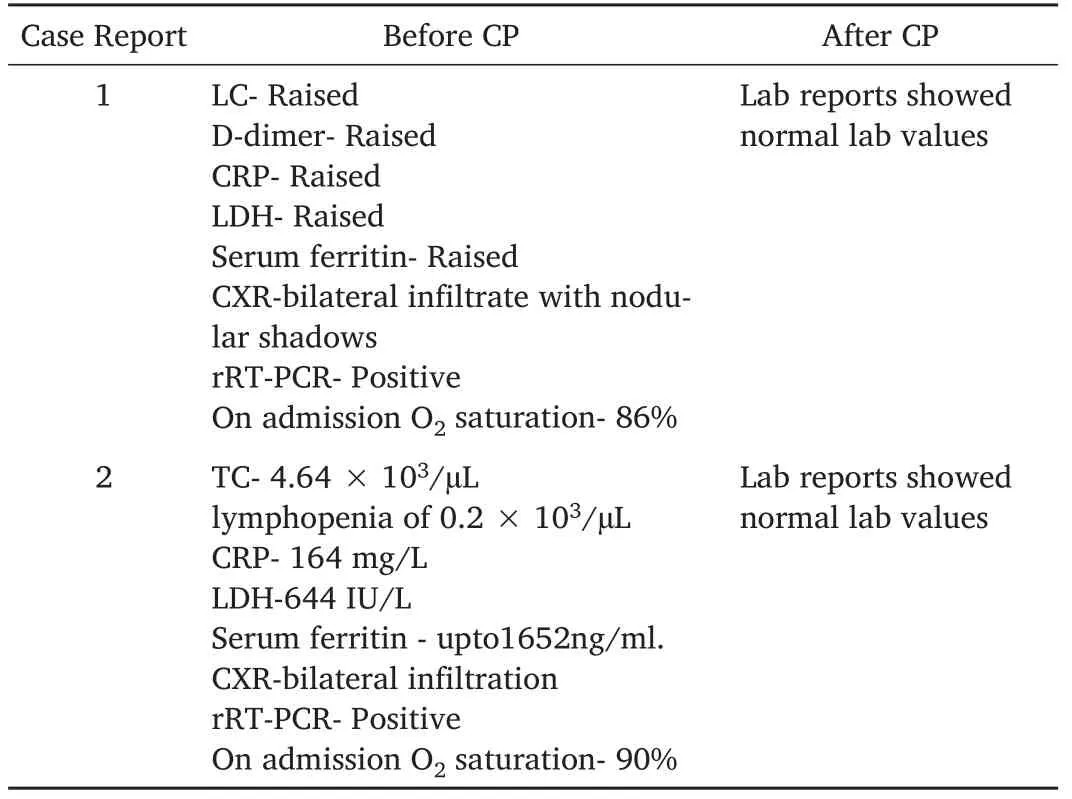
Table 3:Laboratories Reports before and after receiving convalescent plasma.
Case reports
Case presentation-1
The patient is a 54-year-old male patient from labor colony Peshawar city,Pakistan, presented to Corona isolation unit of Hayatabad Medical Complex Hospital, Peshawar with complaint of high-grade fever for 3 days associated with full body aches.The patient was complaining of a dry cough for the last 2 days with chest discomfort.Initially the patient was vitally stable without shortness of breath.In the baseline investigation, complete blood count (CBC) showed that total leukocyte count, D-dimer was raised.Serum ferritin level and lactic dehydrogenase were also raised.Chest X-ray showed bilateral infiltrate with nodular shadows.Other baseline investigations were normal.Conservative treatment for COVID-19 had been started according to the hospital treatment protocol.In this case no antiviral agents have been used.Hospital treatment protocol for COVID-19 comprised Azithromycin 500 mg iv OD, ceftriaxone 2 g IV BD, dexamethasone 6 mg IV OD, enoxaparin 60 mg sc OD, acetaminophen iv SOS.Sample from upper respiratory tract also sent for real-time reverse transcription polymerase chain reaction (rRT -PCR) test to confirm COVID-19.Oxygen saturation, which was 86% and immediate O2inhalation through nasal prong improves the saturation to 93%.On 2nd day of admission, positive rRT-PCR confirmed COVID-19.The patient’s condition was deteriorating and dropping oxygen saturation.Despite full-blown oxygen support with a non-rebreather mask, the patient was not improving.The patient was on non-invasive ventilation.The possible benefits and adverse effects of plasma transfusion therapy have been explained to the patient’s attendants and consent was taken from patient attendant successively.
On day 5thafter hospital admission, the patient received 500 ml of plasma transfusion in two divided doses at 12 hours interval along with the conservative treatment of COVID-19.Each dose was given in a continuous infusion for 1 hour.After 3 days of plasma transfusion, patient condition was much improved and started step downing of the patient from noninvasive ventilation to nasal cannula.Chest X-ray further revealed further resolution of both lungs’ infiltrates (Figure 1).On the 15thday of complete treatment (conservative therapy plus CP infusion), the patient becomes completely asymptomatic.Follow up sample for COVID-19 was sent again for rRT-PCR that reported negative result and the patient was discharged from hospital with necessary treatment and advice.
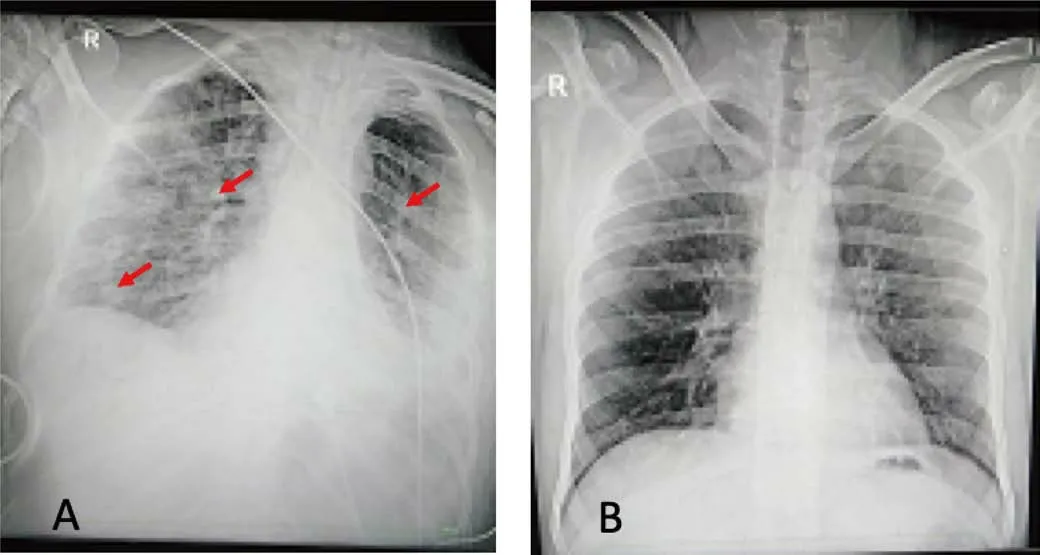
Figure 1:(A)Chest X‐ray of the patient(AP view)showing bilateral lung infiltrates.(B)Chest X‐ray of the patient(AP view)after CP treatment.
Case presentation-2
A 62 years old male patient was admitted in Dhaka Medical College Hospital, Bangladesh, with the complaints of fever, sore throat and mild
Discussion
The convalescent plasma has been employed in the prevention and treatment of various epidemic infections for more than a century.Although it is not considered as a standard or definite treatment, but has respiratory distress for 6 days.On admission, oxygen saturation was 90%on room air which was increased up to 94% by 8 L/min oxygen supplement.The patient was kept in the yellow zone of the COVID-19 unit and lab investigation was sent for evaluation.SARS-CoV-2 via rRT-PCR was positive and diagnosed as COVID-19.Then the patient was shifted to red zone for the proper care.Chest radiographs demonstrated rapidly aggravated bilateral infiltration.Routine blood tests found white blood cell (WBC) count at 4.64 × 103/μL, with lymphopenia of 0.2 × 103/μL.C-reactive protein and lactic dehydrogenase elevated up to 164 mg/L and 644 IU/L respectively.Serum ferritin raised up to 1652 ng/ml.Tocilizumab iv was administered according to body weight.Then a repeated dose was also given since satisfactory clinical improvement was not seen.
Since the oxygen saturation was in a declining trend and could not be maintained by non- rebreathing face mask, high flow nasal cannula(HFNC) was started on day 3 of admission.Remdesivir iv was started on day 09.In the meantime, laboratory report was deteriorated.Arterial blood gas (ABG) analysis showed PaO2/FiO2 of 126, consistent with severe ARDS.As planning for convalescent plasma was taken, two male donors in their thirties who had recovered from COVID-19 for 28 days.Donors were diagnosed as COVID-19 presenting fever, cough and pneumonia, however, showed complete recovery and did not have any symptoms at the time of plasma donation.Thus, meeting the blood donor eligibility criteria, including age, weight, reasonable-sized antecubital veins.In addition, allogeneic donor screening tests, defined by enforcement rules of the safe Blood Transfusion Act, were acceptable for transfusion.Donor apheresis was performed with Spectra Optia apheresis system(CMNC software; Spectra Optia IDL Tubing set; Terumo BCT, Lakewood,CO, USA), 600 mL of convalescent plasma was collected.Anti-SARS-CoV-2 IgG antibody in plasma was measured by enzyme-linked immunosorbent assay (ELISA) (Beijing Kewei Clinical Diagnostic Reagent Inc.China) and optical density (OD) ratio for IgG was Cut off Value=NC+0.15, 0.066+0.15=0.216).
The plasma was divided into two doses from two donors and administered to the patient at 24 hours’ interval.Each dose was given over for 2 hours.No adverse reaction was observed after the administration of convalescent plasma.Fever subsided and oxygen demand decreased since day 11.Supplemental oxygen dependency decreases rapidly and the patient could maintain 92% saturation on room air after 3 days of transfusion.The patient’s condition much improved with decreased CRP 90 mg/L and then gradually became normal at day 15.Chest X-ray revealed further resolution of both lungs’ infiltrates (Figure 2).The follow up rRT-PCR was done on day 14 and the result found negative.He was discharged from the hospital on day 21 after checking all parameters.Reduction of viral load, increase of SARS-CoV-2 IgG and IgM antibodies,reduction in mortality, hospital duration and no adverse events were observed.
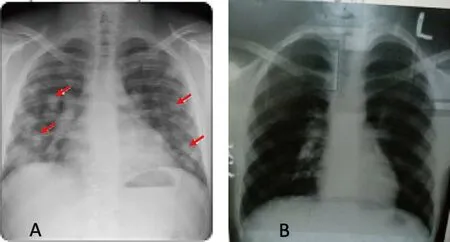
Figure 2:(A)Chest X‐ray of the patient(AP view)showing bilateral lung infiltrates.(B)Chest X‐ray of the patient(AP view)showing improvement after the CP therapy.
been useful in the absence of proper and targeted therapy.Owing to its potential benefits and positive experiences during previous viral outbreaks, MERS, SARS-CoV, H1N1 and H5N1 avian flu, the US FDA approved its use under the coverage of EUA (Emergency Use Authorization) and IND (Investigational New Drug) policy for the COVIDmanuscript, along with many previous reports indicate that convalescent plasma infusion is a safer, cheaper and widely available option to address emergent pandemic situations.
Conclusion
In this article, two clinical cases were described where patients attained dramatic recovery from COVID-19 infection after convalescent plasma therapy along with conservative treatment, which was not effective by itself.No adverse reactions were reported with the transfusion of convalescent plasma.Outcomes from this report of two fully removed patients indicate that administration of convalescent plasma might be a safe and potential treatment option for severe COVID-19.We suggest that the safety and efficacy of convalescent plasma transfusion in critically ill patients from SARS-CoV-2 should be further studied within the context of a well-designed randomized clinical trial.19.In our case study #1, a male patient of age 54 years, presented with classical COVID-19 symptoms, high-grade fever with body ache, dry cough and considerably stable without shortness of breath initially.Subsequent baseline lab investigation revealed raised values in common parameters especially leucocyte count, D-dimer and serum ferritin.The radiograph of the chest showed typical bilateral infiltration with nodular shadows.He was tested positive in rRT-PCR for COVID-19.Oxygen saturation was in declining trend despite full oxygen support and conservative treatment.While, case #2, pertains to a male patient of age 62 years, presented with fever, sore throat and respiratory difficulty and dropped oxygen saturation.He was also tested positive in rRT-PCR for COVID-19.The common lab investigation parameters were also raised especially WBC count, CRP and serum ferritin.His chest radiograph showed bilateral infiltration.He had been treated conservatively.In these two case reports, both patients received conservative treatment, in addition, the second patient received Tocilizumab and Remdesivir,however, the clinical conditions and lab parameters were not stable prior to plasma therapy.Soon after the plasma infusion oxygen saturation of both patients were improved, lab reports and chest X-rays showed promising recovery with decreased viral load and inflammatory states.There are still limitations to the use of convalescent plasma.Scientific evidence is evolving, thought still insufficient due to the lack of largescale clinical trials data.Here, these two cases reports presented in this
- Life Research的其它文章
- Palliative care for Parkinson’s disease patients and their caregivers
- Molecular mechanism of different viruses associated with autoimmunity
- Graft rejection after deep anterior lamellar keratoplasty in fellow eye in macular corneal dystrophy:a case report
- Low expression of novel biomarker RCSD1 predicts poor prognosis of lung adenocarcinoma
- Advances of biodegradable magnesium-based implants for orthopaedics
- Intracranial hemangiopericytoma with right-sided aortic arch:a case report and summary of experience

