High-performance stretchable and compressible supercapacitor based on the core-shell flower structured electrode for integration with photodetectors
SHAO Wenke,ZHANG Daohong,WANG Qiufan
(School of Chemistry and Materials Science & Key Laboratory of Catalysis and Energy Materials Chemistry of Ministry of Education & Hubei Key Laboratory of Catalysis and Materials Science,Hubei R&D Center of Hyperbranched Polymers Synthesis and Applications,South-Central Minzu University,Wuhan 430074,China)
Abstract The integration of energy storage devices with solar cells is a challenge to the development of wearable and flexible electronics with stable power output. A high-performance flexible asymmetric all-solid-state fiber supercapacitor was fabricated with Co3O4@Ni-Co-S and FeSe2 as the positive and negative electrodes,respectively. The as-prepared Co3O4@Ni-Co-S electrodes demonstrated outstanding volume-specific capacitance(5.0 F·cm-3at 0.4 mA)and excellent cycling performance(83.6% after 5000 cycles)owing to their 3D core-shell micro-chrysanthemum flower and microsnowflakes structures. Furthermore,photo-charged asymmetric supercapacitor(PSC)was prepared by using silicon solar cells for direct photo-charging. The resulting PSC could also be used to power a mini-scale flexible photodetector based on TiO2 anode and PEDOT cathode under any bending angles and under stretchable/compressible states. In addition,coreshell Co3O4@Ni-Co-S micro-chrysanthemum electrodes displayed exciting electrocatalytic hydrogen evolution reactions,showing excellent potential for the construction of high-performance flexible and wearable energy devices.
Keywords 3D core-shell;asymmetric supercapacitor;photo-charging;stretchable/compressible
With the demand for sustainable technologies,there have been considerable developments in integrating energy harvesting and storage functions in a self-powering unit[1-2]. In the meanwhile,energy conversion devices such as nanogenerators[3-4],solar cells[5],thermoelectric devices[6],biofuel cells[7],and other sustainable energy have been investigated to integrate with energy harvesting and storage devices into a self-sufficient system for improving the flexibility and convenience of energy devices.Solar energy as a widespread and renewable clean resource has been a promising alternative to conventional fossil fuel for a long time[8-9]. LIU et al[10]demonstrated hybrid SiNW/polymer solar cell and a polypyrrole supercapacitor assembled a high-performance self-charging power unit,which simultaneously achieved both photoelectric conversion and energy storage.Therefore,integrating energy storage devices with solar cells as self-powering systems may be a great idea through the simultaneous storage of the electricity and manipulation of the energy output[11-13].
Flexible supercapacitors(SCs)with desirable properties of high-power density,light-weight,portability and long-life have been considered for the application of energy storage[14-16]. However,improving the energy density is an intractable challenge for practical applications.According to the energy densityE=1/2CV2,building an asymmetric supercapacitor(ASC)with a wide operating voltage window is regarded as a promising strategy.On one hand,the high specific capacitance active materials such as Co3O4,NiCo2S2,CoSe2[17],FeSe2[18],have largely been used as electrode materials in electrochemical supercapacitors. On the other hand,regulating the electrode architecture is an effective way for the high electrochemical properties of ASCs[19]. The 3D microstructure can increase electrochemical reactive sites and enlarge the effective surface area[20]. The higher surface area can provide more effective channels for ion diffusion and electron transport at the electrode/electrolytes interface[21]. WANG et al[17]demonstrated a novel heteronanostructure of CoSe2@PPy core/shell nanoflowers provided a large electrode/electrolyte interfacial contact area as well as short paths for electron/ion diffusion in electrochemical processes,and the fiber-shaped asymmetric supercapacitor CoSe2@PPy//EACF provided a high energy density of 2.63 mWh·cm-3at 14 mW·cm-3.SHI et al[22]developed ZnO nanorods/carbon@(Ni,Co)Se2nanosheets core/shell nanostructure electrode via using synergistic effect between the ZnO NRs/C and(Ni,Co)Se2NSs,and the obtained ZnO NRs/C@(Ni,Co)Se2NSs core-shell nanostructure electrode showed the excellent specific capacity of 164.18 mAh·g-1at 1 A·g-1. For practical applications,flexible ASCs may work under stretchable/compressible states,developing high elasticity and good conductivity electrodes with stretchable/compressible ability is essential for fabricating various stretchable devices[23].
In this work,an integrated system comprising highperformance Co3O4@Ni-Co-S//FeSe2ASC,commercial Si solar cell,and the TiO2-based photodetector device was developed. The ASC was composed of the core/shell composite springlike structure of Co3O4@Ni-Co-S and CF@FeSe2that exhibited excellent performance including high specific capacitance,large energy density,long cycle life,and wonderful bending and/or compressible/stretchable stability. High-performance ASC was charged(using the integrated Si solar cells)to continuously operate the photodetector device,regardless of bending angles or compressible/stretchable states.Besides, the core-shell structure Co3O4@Ni-Co-S electrode exhibited excellent catalytic activity for the hydrogen evolution reactions. It is believed that this electrode may also provide an important platform for other multiple applications,such as sensors and batteries.
1 Experimental section
1.1 Synthesis of Co3O4 on Ti wires
In a typical synthesis of Co3O4nanosheets,2 mmol CoCl2·6H2O,4 mmol CO(NH2)2and 8 mmol NH4F were dissolved in 30 mL deionized water by stirring for 10 min.The solution was transferred into a Teflon-lined stainless steel autoclave containing the Ti wires,and then sealed and maintained at 120 °C for 6 h. After the autoclave was cooled down to room temperature,the product was collected,washed with distilled water three times,and then thermally treated at 300°C for 3 h in the air to obtain Co3O4nanosheets. All chemicals were purchased from China National Pharmaceutical Industry Corporation Ltd.
1.2 Synthesis of Co3O4@Ni-Co-S on Ti wires
Before electrodeposition, the electrolyte was prepared by dissolving of 3 mmol Ni(AC)2,6 mmol Co(AC)2,and 12 mmol CS(NH2)2in 45 mL distilled water,sonication for 10 min.Ni-Co-S was deposited on Co3O4nanosheets using an electrochemical deposition process. A three-electrode configuration was employed,in which the Co3O4was used as the working electrode,Ag/AgCl as the reference electrode,and platinum foil as the counter electrode. The Ni-Co-S film was deposited with a constant scan rate of 10 mV·s-1at the potential of 0.2 V to -1.2 V. To obtain high quality Ni-Co-Snanosheets,a series of experiments were performed to optimize the deposition parameters. The deposition cycles were varied from 2-25 cycles and obtained a series of Co3O4@Ni-Co-S-n(ndenotes the Ni-Co-S electrodeposition cycles). The Co3O4@Ni-S and Co3O4@Co-S were also obtained by the same electrodeposition method in Ni(AC)2/CS(NH2)2(1∶1 by mole ratio)and Co(AC)2/CS(NH2)2(1∶1 by mole ratio)as the electrolyte,respectively.
1.3 Characterization
The synthesized samples were identified by X-ray diffraction(XRD). The morphologies of the products were studied by scanning electron microscopy(SEM)and transmission electron microscopy(TEM). Cyclic voltammetry (CV) , electrochemical impedance spectroscopy(EIS)and galvanostatic charge/discharge(GCD)tests were carried out on an Ivium 10800 electrochemical workstation.
1.4 Preparation of Co3O4@Ni-Co-S//FeSe2 ASC
PVA/KOH gel electrolyte was prepared by mixing PVA(polyvinyl alcohol,6 g)and KOH(3 g)with deionized water(60 mL)and heated to 95 °C under vigorous stirring until it became clear. Both the Co3O4@Ni-Co-S positive electrode and the CF@FeSe2negative electrodes were coated with a layer of PVA/KOH gel electrolyte and dried at 45°C for 1 h.
2 Results and discussion
The fabricating process of Co3O4@Ni-Co-S included the growth of Co3O4flowers on a Ti wire and deposition of Ni-Co-S nanosheets.In Fig.1(a),Co3O4flowers-like nanostructures were obtained through a simple hydrothermal method. A thin layer of Ni-Co-S nanosheets was then coated on the surface of the Co3O4flowers-petals uniformly to form Co3O4@Ni-Co-S core/shell structuresviaelectrodeposition. The obtained electrode material with a unique 3D-core-shell structure enhanced reaction kinetics from the morphological characterization and electrochemical performance results.
From the scanning electron microscopy(SEM)image(Fig.2(a)),the Co3O4sample was composed of interesting bundles of aligned acicular nanowires to form flowers-like structure on 1D Ti wires. Fig.2(b)demonstrated the Ni-Co-S nanosheets were successfully deposited on Ti wires. During the growth of Ni-Co-S nanosheets on Co3O4/Ti,the Co3O4flowers were used as a template for heterogeneous nucleation,and the core/shell composite structure of Co3O4@Ni-Co-S was obtained. Fig.2(c)showed the SEM images of the product obtained after electrodeposition of Ni-Co-S,the uniform coverage on the surfaces of the Co3O4nanopetals with Ni-Co-S nanosheets were interconnected with each other to form a highly porous surface.Further,a single Co3O4@Ni-Co-S nanopetal had a mean diameter of ~300 nm(Fig.2(d)). In Fig.2(e),transmission electron microscopy(TEM)image of an individual core-shell Co3O4@Ni-Co-S composite,from which the thin nanoflakes were uniformly covered on the surface of the Co3O4nanopetals. This unique 3D-core-shell structure was beneficial to the supercapacitor electrode application on account of the superior synergistic effect of high surface area and short electron/ion transport pathways[24]. The high-resolution TEM(HRTEM)image in Fig.2(f),displayed clear lattice fringes with an interplanar spacing of 0.283 nm and 0.322 nm,which correspond to the(311)and(220)planes of Co3O4,respectively.
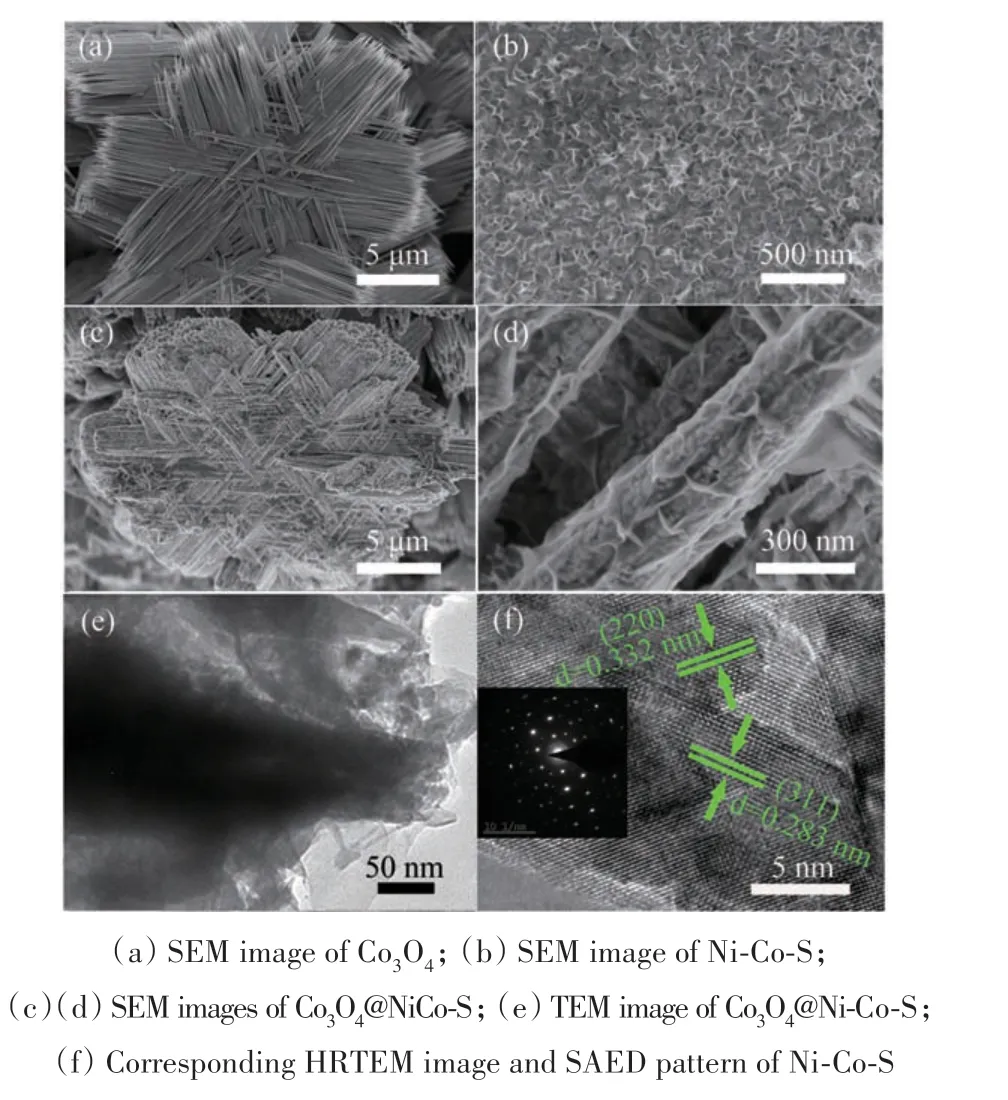
Fig.2 Morphological characterization of the electrode materials(a)Co3O4的SEM 图像;(b)Ni-Co-S的SEM 图像;(c)(d)Co3O4@NiCo-S的SEM 图像;(e)Co3O4@Ni-Co-S的TEM 图像;(f)Ni-Co-S 的相应HRTEM 图像和SAED 图图2 电极材料的形貌表征
The electrochemical properties of the electrodes were first investigated in a three-electrode configuration.Fig.1(b)compared the CV curves of the Ti@Co3O4,Ti@Ni-Co-S and Ti@Co3O4@Ni-Co-S electrodes at a scan rate of 10 mV·s-1. The CV internal area for the Ti@Co3O4@Ni-Co-S electrode was much larger than that of the Ti@Co3O4and Ti@Ni-Co-S electrodes. This was probably a result of the higher conductivity of the Co3O4@Ni-Co-S electrode. To optimize the electrodeposition cycle of Ni-Co-S,electrochemical properties CV curves were measured with different Ni-Co-S deposition cycles from 2 to 25. Obviously,the Ti@Co3O4@NiCo-S-15 electrode demonstrated much higher electrochemical properties than other Ti@Co3O4@NiCo-S-nelectrodes(ndenotes the Ni-Co-S electrodeposition cycles),including higher CV window area at 10 mV·s-1(Fig.1(c)),which exhibited that the Ti@Co3O4@Ni-Co-S-n(n=15)electrode had good capacitive behavior and a low diffusion resistance for ions[25]. It demonstrated that the electrochemical properties increased with increasing deposition cycles from 2 to 15. However,further increasing the deposition cycle to 20-25 weakened the electrode′s electrochemical performance.
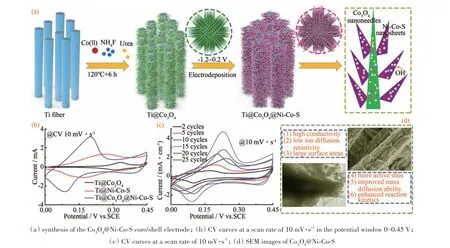
Fig.1 Synthetic route and highlight of Co3O4@Ni-Co-S core/shell electrode(a)Co3O4@Ni-Co-S核/壳电极的合成;(b)在电位窗口0~0.45 V中以10 mV·s-1的扫描速率的CV曲线;(c)在10 mV·s-1的扫描速率下的CV曲线;(d)Co3O4@Ni-Co-S的SEM图像图1 Co3O4@Ni-Co-S核-壳电极材料的合成路线和亮点
To better evaluate the practical application of Co3O4@Ni-Co-S core-shell hetero-structure electrode materials,an ASC was assembled by using the Co3O4@Ni-Co-S core-shell heterostructure as the positive electrode,the high electrical conductivity of FeSe2as the negative electrode,PVA/KOH as the solid-state electrolyte.According to the charge balance of the two electrodes,the length ratio of Co3O4@Ni-Co-S to FeSe2electrode was 4∶1,thus the 4 cm FeSe2was inset into 1 cm spiral Co3O4@Ni-Co-S coil to assemble Co3O4@Ni-Co-S//FeSe2ASC. The CV curves with redox peak(Fig.3(a))and the GCD curves both had an obvious platform(Fig.3(b)),indicating the good electrochemical performance of this ASC device.Fig.3(c)demonstrated the energy efficiency of the ASC device with increasing discharge current on 0.4 mA onwards(~70%)and reached a maximum value of 97% at 8 mA. The specific capacitance of the ASC device was 5.0 F·cm-3derived from the discharge curves at 0.4 mA and remained at 1.5 F·cm-3at a high current of electrochemical performance of the ASC could be due to the unique core-shell nanostructure of Co3O4@Ni-Co-S.The ASC exhibited an energy density as high as 1.33 mWh·cm-3at a power density of 56 mW·cm-3and an energy density of 0.30 mWh·cm-3at a high power density of 0.98 W·cm-3(Fig.3(e)).The energy density of our ASC device was much higher than the reported fiber ASCs,such as Fe2O3/HP-CF//Ni-Co-S/HP-CF(1.0 mWh·cm-3at 8 mA(Fig.3(d)). Hence,the significantly enhanced 550 mW·cm-3)[26],Ni-Co-S nanosheet decorated 3D porous Ni film@Ni wires(5.33 mWh·cm-3at 856 mW·cm-3)[27],Ni-Co-S@Fe2O3//MnO2(2.26 mWh·cm-3at 196 mW·cm-3)[28],0.5Mn-Ni-Co-S//AC HSC(2.29 mWh·cm-3at 704 mW·cm-3)[29]. As shown in Fig.3(f),the cycling durability of the Co3O4@Ni-Co-S//FeSe2ASC was tested up to 5000 cycles,at which the device retained 83.6%of its initial capacitance.
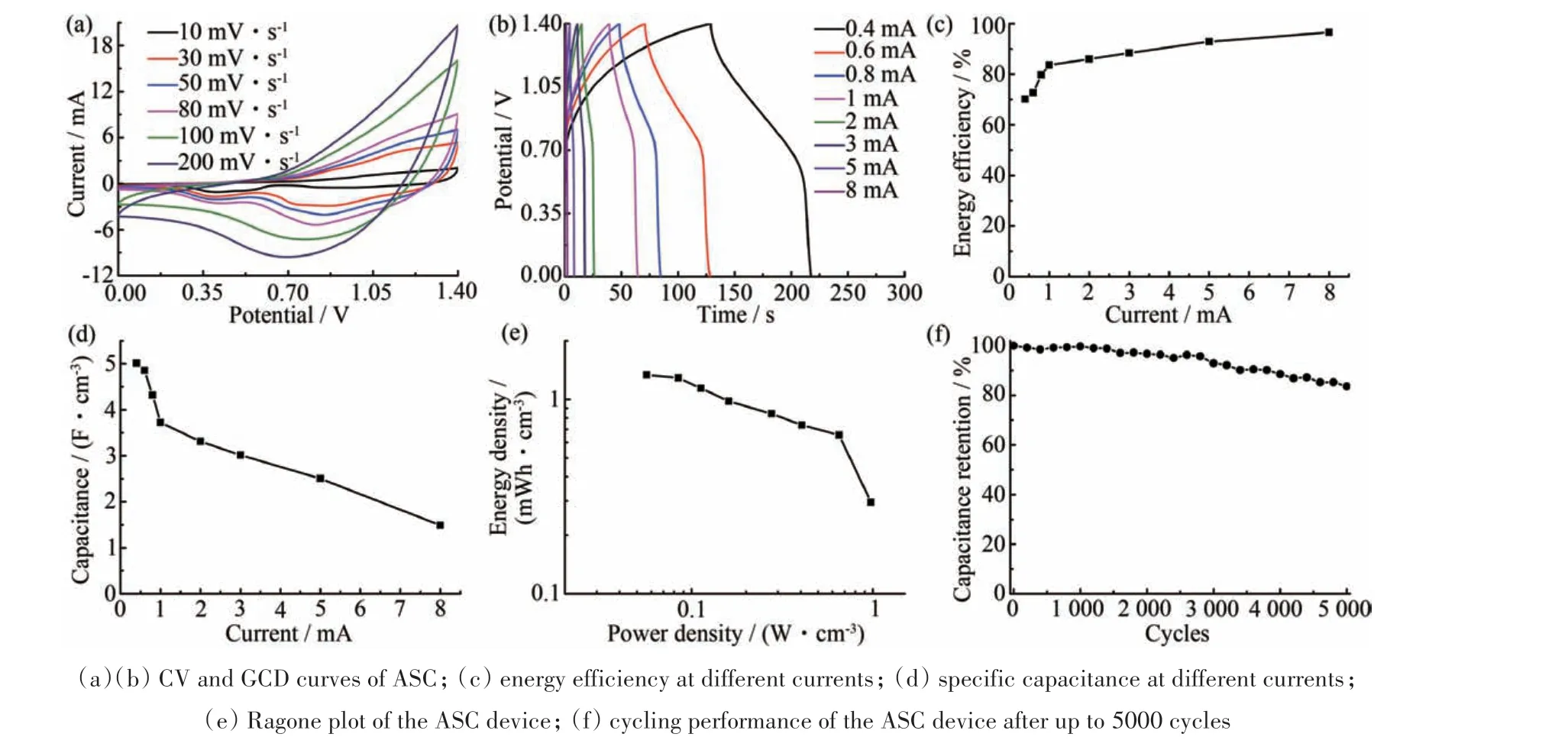
Fig.3 Performance of asymmetric supercapacitor device(a)(b)ASC的CV和GCD曲线;(c)不同电流下的能效;(d)不同电流下的比电容;(e)ASC器件的Ragone图;(f)ASC器件循环5000次后的循环性能图3 不对称超级电容器器件的性能
The mechanical flexibility of our ASC device was also investigated.Fig.4(a)demonstrated the compressible and stretchable states of the fabricated ASC. Its electrochemical performance was evaluated under different elastic lengths at a current of 2 mA(Fig.4(b)),and the GCD curves of the device showed no obvious deterioration. When the as-prepared ASC device was compressed to 40%of the original state,its capacitance retention was about 96.7% and then stretching up to 160% after return to the original state. Its capacitance retention was changed from 92.8% to 84.8%,which decreased 8%in the whole stretching process(Fig.4(d)).The EIS spectrum(Fig.4(c))exhibited negligible changes.They both indicated that the structures of the device were maintained well and thus possessed exceptional flexibility in this reciprocating process.
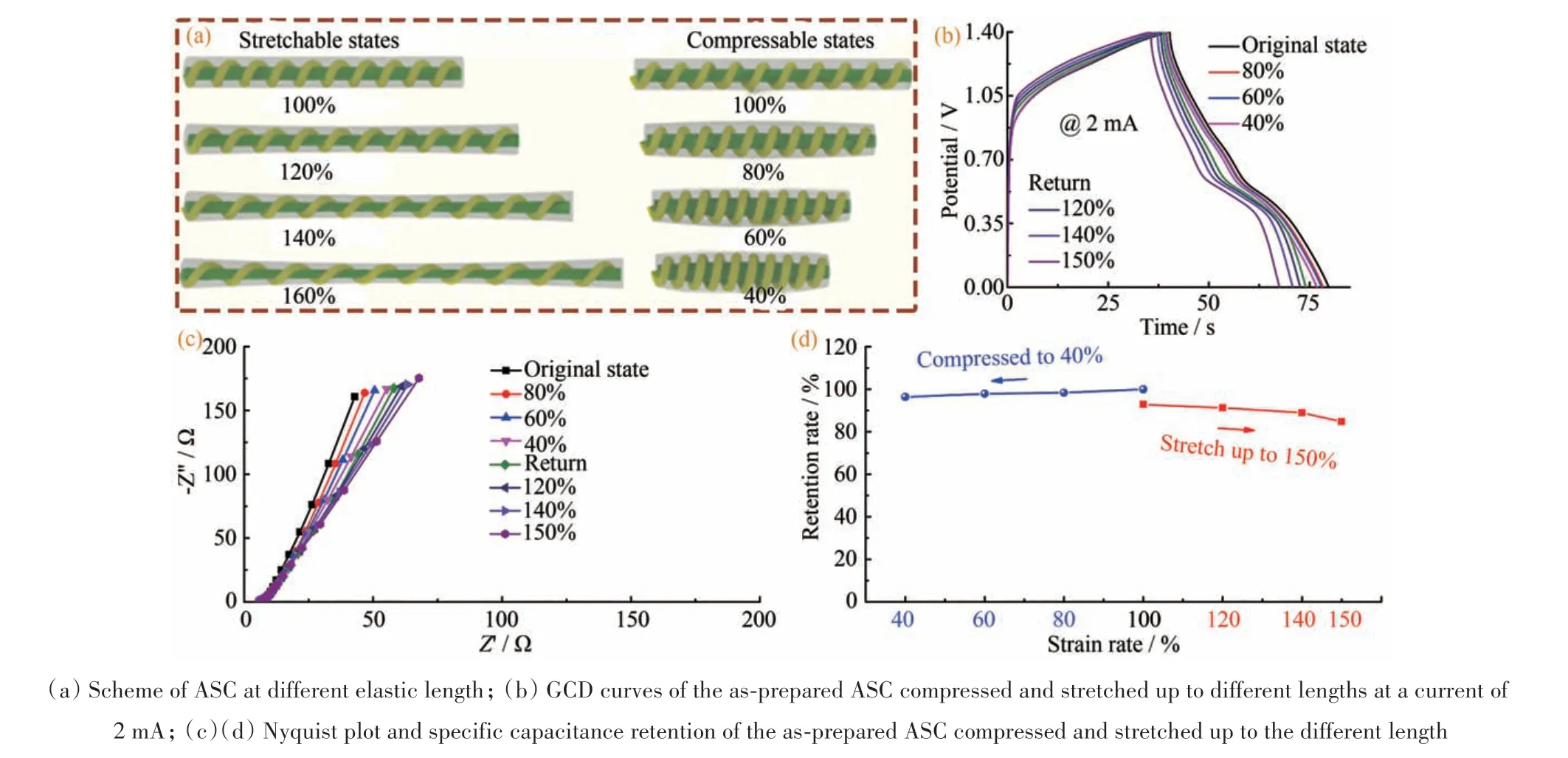
Fig.4 Mechanical flexibility of asymmetric supercapacitor device(a)不同弹性长度下的ASC示意图;(b)所制备的ASC在2 mA电流下被压缩并拉伸至不同的长度的GCD曲线;(c)(d)所制备的ASC被压缩并拉伸到不同的长度的奈奎斯特图和比电容保持率图4 不对称超级电容器器件的机械柔性
As a demonstration of the application,an integrated self-powering energy system was fabricated,as illustrated in Fig.5(a). Under visible light illumination on the Si solar cell,the PSC could be charged to 2.0 V in 10 s and then the PSC was discharged at different currents in dark(Fig.5(b)).The calculated areal capacitance was 386.7 mF·cm-3. The discharge time of the PSC was slightly longer than those of the pure SC driven by an external power source,which might be due to the delay effect of the voltage transient in the solar cell during the discharge process,as explained in the previous reports[30]. TiO2is a common active electrode material in photoelectrochemical devices because of its excellent effectiveness,low cost as well as chemical stability.When the UV light irradiates the TiO2-based photodetector,the charge separation of TiO2will take place,yielding electrons and holes. With the applied external field from the charged PSC,the electron-hole pairs are separated.Subsequently,electrons move to the positive and holes to the negative electrode,respectively.The photo-response of the photodetector device to 345,460,523 and 623 nm light was shown in Fig.5(c).Different responses to different light confirmed the visibleblind properties of the TiO2-based photodetector device.Fig.5(d)presented the typical current-time curves for the photodetector device under periodic intermittent 365 nm UV light irradiation driven by PSC.From the results,it could be seen that the photodetector showed a fast and stable response to UV light irradiation.The performance was comparable to a common flexible photodetector driven by an external workstation power supply,confirming that the PSC was a suitable power supply for a photodetector. The current response by introducing a continuous 365 nm UV light rectangle pulse with an on-off interval of 50 s remained almost unchanged at any bending angles(Fig.5(e))and compressible/stretchable states(Fig.5(f))of PSC,indicating good stability of the integrated system.
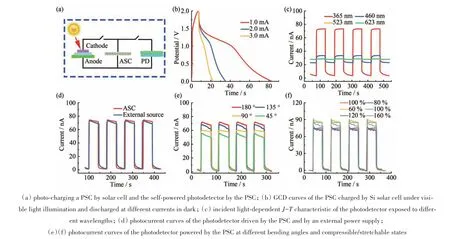
Fig.5 Integrated application of PSC and solar cell(a)通过太阳能电池对PSC进行充电,并通过PSC对自供电的光电探测器进行充电;(b)硅太阳能电池在可见光照射下充电并在黑暗中以不同电流放电的PSC的GCD曲线;(c)暴露于不同波长光下的光电探测器的取决于入射光的J-T特性;(d)由PSC和外部电源驱动的光电探测器的光电流曲线;(e)(f)在不同的弯曲角度和可压缩/可拉伸状态下,由PSC供电的光电探测器的光电流曲线图5 PSC与太阳能电池的集成应用
As the Co3O4@Ni-Co-S is a good oxygen evolution catalyst for water splitting, the self-supported Co3O4@Ni-Co-S core/shell flowers can provide a large surface area without the usage of binders,and the electron transfer can also be improved by direct electronic contact. The electrocatalytic activity of the Co3O4@Ni-Co-S core/shell electrode was evaluated. The tests were measured using the standard three-electrode electrochemical cell setup in 1 mol·L-1KOH with a scan rate of 2 mV·s-1. The Co3O4, Co3O4@Co-S,Co3O4@Ni-S,Co3O4@Ni-Co-S electrodes were employed as working electrodes,respectively. Fig.6(a)showed linear sweep voltammetry(LSV)curves on the reversible hydrogen electrode (RHE) scale. Obviously, the Co3O4@Ni-Co-S electrode exhibited much higher activity toward HER,with a current density of 10 mA·cm-2at a small overpotential of 68 mV(Fig.6(b)). The linear fitting of the corresponding Tafel slope were given in Fig.6(c),and the Co3O4@Ni-Co-S electrode displayed the smallest Tafel slope among the four working electrodes, indicating that the Co3O4@Ni-Co-S required lower overpotential to generate a specific current. The results reflected the Co3O4@Ni-Co-S was a promising catalyst for HER. The long-term stability of the Co3O4@Ni-Co-S was tested under the overpotential condition of 68 mV for 10 h(Fig. 6(d)),and a constant current density delivered the good durability of the electrode.
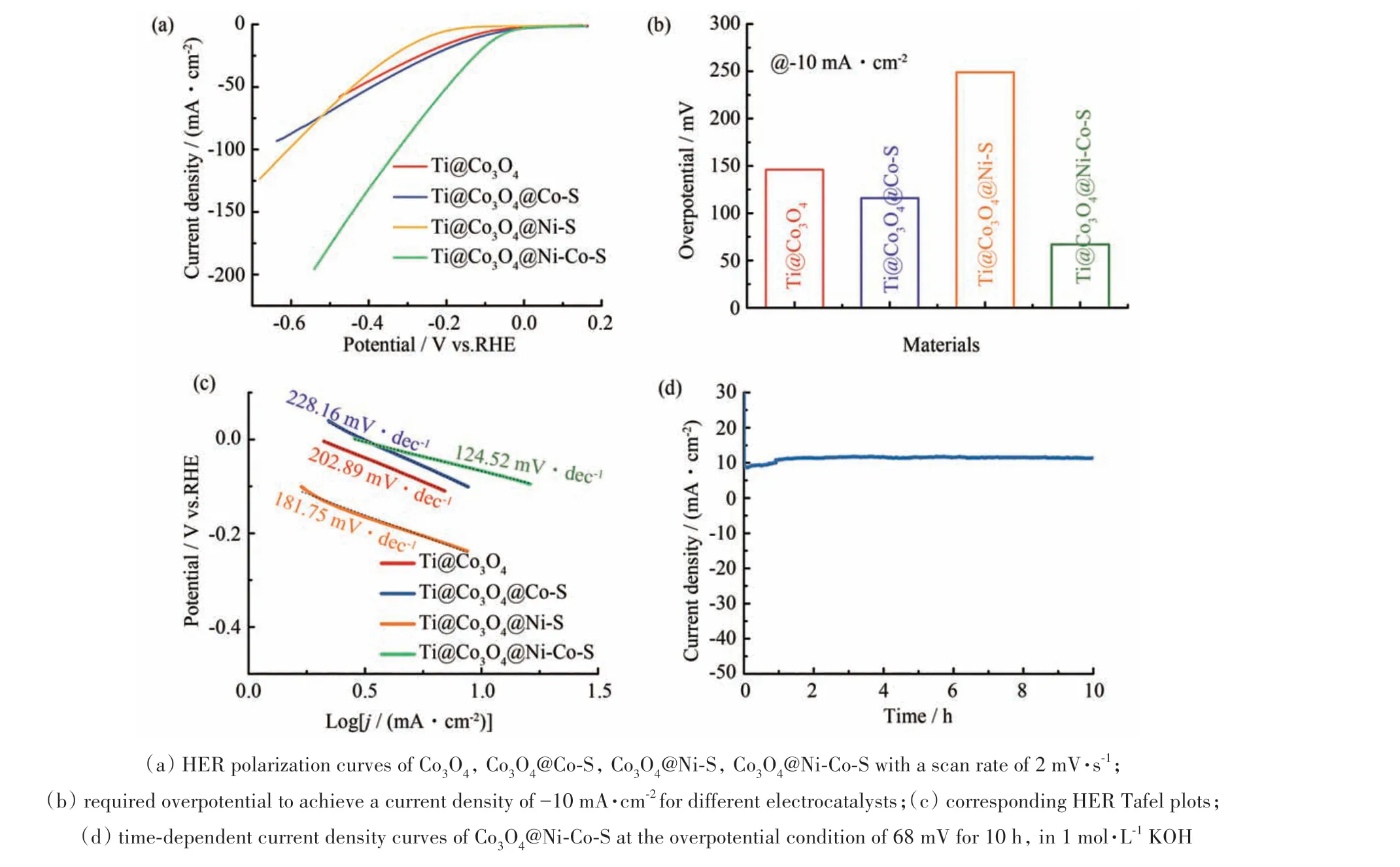
Fig.6 Oxygen-evolving performance of the electrode materials(a)扫描速率为2 mV·s-1的Co3O4,Co3O4@Co-S,Co3O4@Ni-S,Co3O4@Ni-Co-S的HER极化曲线;(b)对于不同的电催化剂,要达到-10 mA·cm-2的电流密度所需的过电势;(c)相应的HER Tafel斜率图;(d)在1 mol·L-1 KOH中在68 mV的超电势条件下持续10 h的Co3O4@Ni-Co-S的时间依赖性电流密度曲线图6 电极材料的析氧性能
3 Conclusions
In summary,an integrated system composed of Co3O4@Ni-Co-S//FeSe2ASC which is charged by the Si solar cell and discharged to drive the TiO2-based photodetector device was successfully assembled.The Co3O4@Ni-Co-S//FeSe2ASC exhibited excellent electrochemical properties including a stable voltage window of 1.4 V,a high specific capacitance of 5.0 F·cm-3at 0.4 mA and an increased energy density of 1.33 mWh·cm-3at 56 mW·cm-3. In addition,the ASC device also presented excellent electrochemical stability and good mechanical stability,its capacitance retention was 84.8% after the reciprocating process, and the capacitance retention was about 92.9% after being bent by 170° and 83.6% after 5000 cycles. It can be charged to 2.0 V within 10 s by the Si solar cell,the areal capacitance of this PSC was 386.7 mF·cm-3. In the meanwhile,the TiO2-based photodetector device demonstrated a fast and stable response to UV light irradiation. Through integrating the PSC and a photodetector device,the self-power system indicated good stability. Moreover,the electrocatalytic property of the core-shell Co3O4@Ni-Co-S micro-chrysanthemum electrode for hydrogen evolution reactions was evaluated,which also exhibited superior performance in terms of low overpotential(68 mVvs.reversible hydrogen electrode(RHE))and excellent stability.This work further presented a general and effective route in developing self-powered portable electronics storage and conversion devices.

