Environmental and biological factors affecting the abundance of Prosopis flexuosa saplings in the central-west Monte of Argentina
Cappa F.M. ,Campos V.E. ,Barri F.R. ,Ramos L. ,Campos C.M.
a INTERBIODES (Interacciones Biol'ogicas del Desierto),Facultad Ciencias Exactas,Físicas y Naturales (Universidad Nacional de San Juan),San Juan,Argentina
b CIGEOBIO-CONICET (Centro de Investigaciones de la Ge'osfera y Bi'osfera,Consejo Nacional de Investigaciones Científicas y T'ecnicas),San Juan,Argentina
c Instituto de Diversidad y Ecología Animal,CONICET - UNC,C'ordoba,Argentina
d IADIZA,Instituto Argentino de Investigaciones de las Zonas 'Aridas,Universidad Nacional de Cuyo,Gobierno de Mendoza,CONICET,Mendoza,Argentina
Keywords:Bos primigenius taurus Dry forests Equines Exotic and domestic ungulates Lama guanicoe Prosopis flexuosa Remote sensing Saplings Seed dispersal
ABSTRACT Background: Trees and forests in drylands help mitigate the challenges through provision of economic products and vital environmental services such as habitat for biodiversity,prevention of erosion and desertification,regulation of water,microclimate,and soil fertility.The condition and changes in dry forests can be assessed by using ecological indicators able to quantify spatial and temporal changes in vegetation.One of the ways to determine the condition of the forest is to study the dominant tree species and its regeneration.Our study aimed to assess whether the abundance of Prosopis flexuosa saplings is affected by environmental and biological factors.Results:To evaluate the first variables we used data from remote sensing such as satellite images and Aster Global Digital Model (GDEM).The second set of variables was about exotic and native ungulates and we used feces of these animals and camera traps to take data.We found that sapling abundance related positively to sandy substrates and negatively to Wetness Index.On the other hand,in relation to biological variables,the abundance of saplings was positively affected by density of adult trees and by number of seeds dispersed by equines,but space use by Lama guanicoe had a negative relationship with saplings.This research shows that P. flexuosa saplings are benefited from sandy substrates and the conditions around adult trees.In addition to this,we found that exotic ungulates in low densities have neutral (i.e.cattle) or positive (i.e.equines) effects on sapling abundance.Conclusions: Based on these findings,we conclude that regeneration of the population of P. flexuosa in our study area has no major problems.In addition,we corroborated that the presence of exotic and domestic ungulates in low densities does not have deleterious consequences for saplings of the dominant tree, P. flexuosa.
1.Introduction
Drylands extend on more than 40% of the earth's surface and are home to over 2 billion people who depend on the natural resources of these systems for their livelihoods (Bastin et al.,2017).These ecosystems host 1.1 billion hectares of forest,corresponding to 27% of the world's forest area(FAO,2019).Drylands and,therefore,dry forests are vulnerable to water shortage,drought,desertification,land use,degradation and climate change impacts,with dangerous ramifications for the food security,livelihood and well-being of their populations(Burrell et al.,2020).Trees and forests in these lands help mitigate the challenges through provision of economic products and vital environmental services such as habitat for biodiversity,prevention of erosion and desertification,regulation of water,microclimate and soil fertility(FAO,2019).
The condition and changes in dry forests can be assessed by using ecological indicators able to quantify spatial and temporal changes in vegetation (Lawley et al.,2015).If maintaining woodland ecological integrity is one of the goals of conservation,then it is necessary to evaluate and monitor the forest's condition.One of the ways to determine the condition of a forest is to study the dominant tree species and its regeneration (Monteiro-Henriques and Fernandes,2018).Dominant species generally occur in low abundance,are taller,provide a high proportion of biomass,and create and define ecosystem structure and processes(Grime,1998;Ellison et al.,2005).
The establishment,survival and regeneration of trees could be affected by several environmental factors (Campos et al.,2016;Méndez-Toribio et al.,2016).The abundance of saplings ofQuercussp.,for example,depends upon seed availability,grazing rate and climate variables (i.e.drought and average temperature) (Monteiro-Henriques and Fernandes,2018).In Mexican dry forests,the abundance of saplings of different species was related to soil attributes and light gaps (Vargas-Rodriguez et al.,2005).In drylands,evapotranspiration involves more than 95% of the water output and depends on atmospheric demands (i.e.incident radiation,humidity,and wind speed) and on soil moisture(see review by Magliano et al.,2017).This last variable derives from the combination of weather,vegetation,soil,and landscape attributes.Thus,low primary productivity occurs primarily during periods of minimum soil moisture and is not directly related to an absence of precipitation(see review by Schlaepfer et al.,2017).Therefore,atmospheric demands and soil moisture are of particular concern in dry forests for they,along with grazing pressure,are the main factors affecting seedling emergency,sapling establishment,and adult tree permanence(Páez and Marco,2000;Enoki and Abe,2004;van der Sande et al.,2017;Ball and Tzanopoulos,2020).
Such as occurs in temperate forests,where ungulates disperse 44%of the regional plants (Albert et al.,2015),ungulate-mediated seed dispersal is,around the world,potentially important for plant demography from one generation to the next(Wang and Smith,2002;Vellend et al.,2006),and plays a role in metapopulation dynamics (Baltzinger et al.,2019).These positive interactions could become negative with an increasing density of ungulates because the impact of their grazing on the forest is related to their density(Meier et al.,2017;Ramirez et al.,2018).Ungulate populations are increasing,both native populations (Meier et al.,2017;Ramirez et al.,2018) and introduced domestic species(Etchebarne and Brazeiro,2016;Ball and Tzanopoulos,2020).These medium and large-sized animals that are present worldwide explore large home ranges,daily cover long distances,and are important in plant dispersal(Baltzinger et al.,2019).
In dry woodlands of the Monte of Argentina,arboreal species of the genusProsopis(locally called algarrobos) are the main trees providing important nature's contributions to people from rural communities,such as forage for livestock and firewood,and facilitating establishment of other plant species under their canopies (Rossi and Villagra,2003;Álvarez and Villagra,2009;Moreno et al.,2018;Villagra and Álvarez,2019).In this ecoregion,the probability of finding these trees is higher in areas with potential moisture and a sandy substratum (Campos et al.,2016).Prosopissaplings establish in areas with removed vegetation and increased light level (Augspurger,1984).They occupy bare and arid microsites,far from tree and shrub canopies,and with high percentages of incident light (Páez and Marco,2000),because solar radiation availability improves sapling growth and development (Vilela and Ravetta,2000).While insolation rate is important for the growth of these plants,in its early stages the algarrobo also needs humidity in the surface soil to complete its photosynthetic activities(Villagra,2000).
Prosopisflexuosa,the dominant tree species in the Monte,is dispersed by animals,such as endozoochorous ungulates (guanaco,cow,donkey,horse;Campos and Ojeda,1997;Campos et al.,2008,2011).The relationship with ungulates can affect plant performance differently in the consecutive stages of the life cycle.For example,cattle move a great amount of seeds and the number of saplings is higher on grazed sites than on sites without livestock (Aschero and García,2012).However,even though cattle transport a huge amount ofP.flexuosaseeds in a spatially differential manner,trampling activity appears to have an important impact on seedling survival and sapling establishment (Campos et al.,2011).In the long term,those sites with high number of seedlings but with heavy cattle traffic,are the ones with a low occurrence of saplings(Campos et al.,2011).
Our study aimed to assess whether the abundance ofP.flexuosasaplings is affected by environmental and biological factors.Based on this,we have two predictions related to environmental variables:the abundance of saplings will be high 1) on sites with high incident radiation,and 2) on sites with more moisture content.In addition,we postulate three predictions related to biological variables:3) sapling abundance will be negatively related to the density of adultP.flexuosatrees because saplings need high solar radiation,4) sapling abundance will be positively related to the input of seeds provided by ungulate feces,and 5)on sites intensively used by ungulates,the abundance of saplings will be low because of animal trampling.
2.Material and methods
2.1.Study area
Research was conducted in a part of the Ischigualasto Provincial Park(IPP) and its area of influence (29°55′S,68°05′W,San Juan Province;Fig.1),located in the Monte of hills and closed basins ecoregion(Morello et al.,2012).The study area covers approximately 15,000 ha and lies in the zone between the park ranger's house and Los Baldecitos locality.The altitude range of this area is between 1,200 and 1,800 m a.s.l.The desert climate of the study area is characterized by high temperature amplitude that ranges from-10°C to 45°C.There are poor rain events that occur primarily between November and February (<100 mm,Poblete and Minetti,1999).
The dominant plant physiognomy is open shrub with no more than 50% of plant cover (Márquez et al.,2005).The most representative species areLarrea cuneifolia,Atriplex spegazzinii,Zuccagnia punctata,Prosopis torquata,andBulnesia retama(Acebes et al.,2010).The main tree species,P.flexuosa,is present in five of the ten plant communities(Márquez et al.,2005).
The native ungulate guanaco (Lama guanicoe) and the exotic ones,cattle (Bos primigenius taurus),donkey (Equus africanus asinus),horse(Equus ferus caballus),and mule (Equus mulus) are present in the area.These species occur in low densities,and the most abundant one is the guanaco (<0.5 ind•km-2;Baigún et al.,2008).At the beginning of the 20th century,local people worked as miners and donkeys were the main pack animals.When the mining activity decreased and people started to use motorized vehicles,donkeys became feral.Currently,some local people live on extensive livestock farming;cattle,horses and mules move freely in the Park and its influence area.
2.2.Saplings
From January to March 2019,we collected data from 38 randomly chosen sampling points,spaced at least 1 km apart within a 1 km×1 km regular grid (150 cells).Sapling abundance was recorded along four 50 m×4 m(length×width)transects beginning in the center of each cell and following each orientation (i.e.North,South,East,and West).We considered allP.flexuosaplants with height less than 1 m and a basal stem diameter less than 1 cm to be saplings(Aschero and García,2012).
2.3.Environmental factors
We computed the Local Insolation Index (LII) as an indicator of incident radiation.This index was calculated as the cosine of the aspect multiplied by the square root of slope inclination(Cingolani et al.,2010).Therefore,north-facing slopes(i.e.sunnier)had positive values,whereas south-facing slopes had negative values,without considering projected shadows(Cingolani et al.,2010).
The Tasseled Cap transformation(Kauth and Thomas,1976;Crist and Cicone,1984) results in new bands by combining the original bands of the image to enhance some features of interest.We used the Landsat 8 OLI image(30-m resolution) of the study area,acquired on January 19,2019 (path 232,row 081).The first Tasseled Cap index (Brightness Index,BI) provides data on soil signature,the second index (Greenness Index,GI) reflects vegetation characteristics,and the third index(Wetness Index,WI) captures information on the interaction of soil and vegetation moisture.We used BI to determine substratum heterogeneity because it provides information about reflectivity,particularly of the soil(Martinelli,2009;Gatica,2010;Campos et al.,2015),and WI as a measure of soil and vegetation moisture.Moreover,as an indicator of soil moisture,we computed the Topographic Wetness Index (TWI),which quantifies the role of topography in redistributing water across the landscape(Beven and Kirkby,1979).This index combines local upslope contributing area and slope,and is so able to estimate where water will accumulate in an area with slope differences.
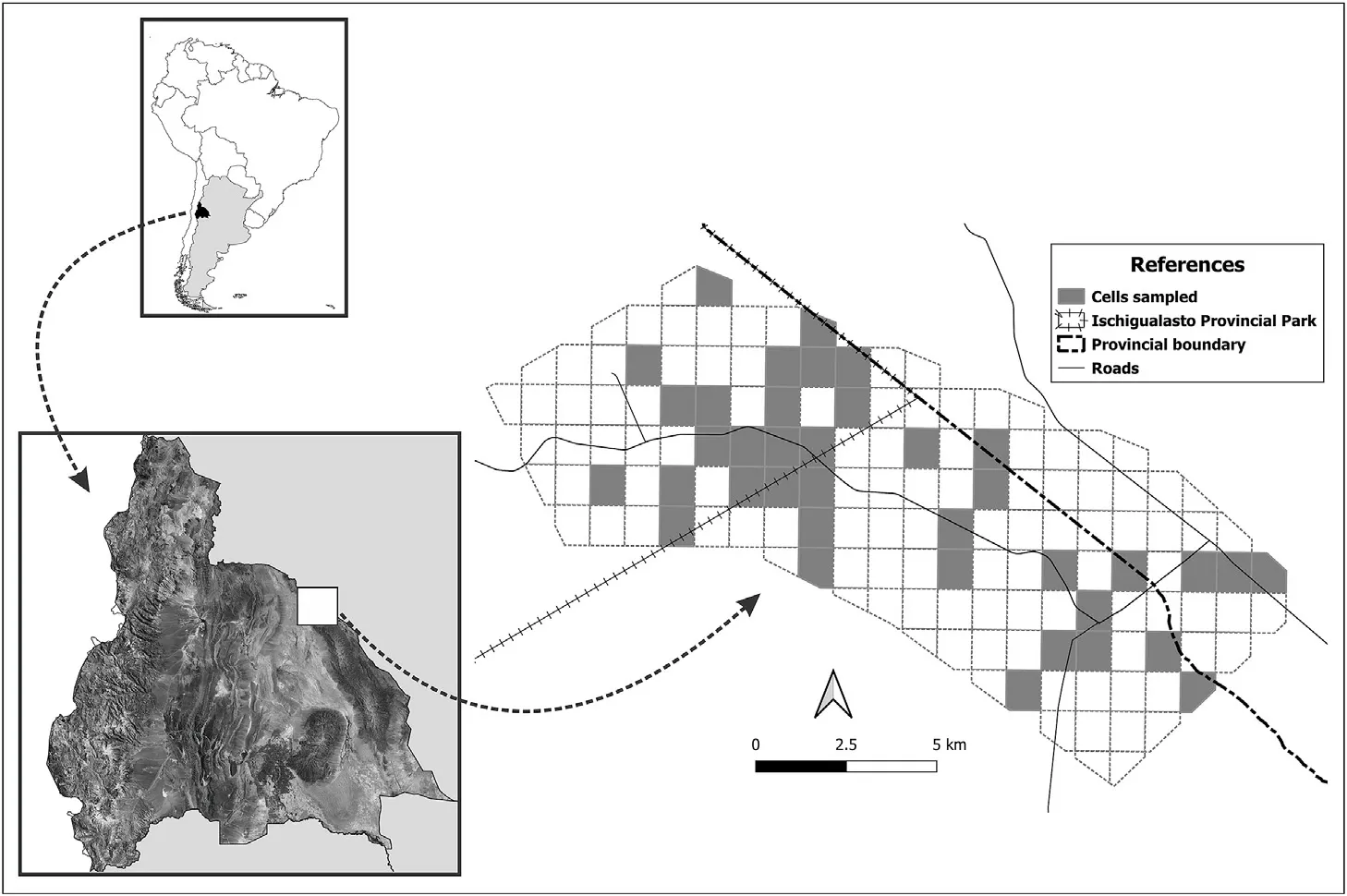
Fig.1.Geographic location of study area and cells sampled.
Both indices (i.e.TWI and LII) were computed from a GDEM (Alos Palsar-SRTM,30 m-resolution,https://www.ign.gob.ar/NuestrasActivi dades/Geodesia/ModeloDigitalElevaciones/Mapa).For BI,WI,TWI and LII indices,we took into account the pixel values of each of the 38 cells to calculate mean and standard deviation using a 5×5 moving window size(equivalent to 150 m × 150 m,area 2.25 ha).Each statistic value was assigned to the central pixel of the moving window (Haralick et al.,1973).
2.4.Biological factors
We estimated adult tree density using the nearest neighbor method(Silvy,2012) in each of 38 cells;as central tree we selected the adultP.flexuosatree nearest the centroid and its four nearest neighbors.From these four nearest neighbors,we took into account the one farthest away from the central tree to define the area for estimating tree density in each cell.We considered allP.flexuosaplants with height more than 1 m and diameter at breast height(DBH)>5 cm to be adults(Aschero and García,2012).
To assess the effect of ungulate-mediated seed dispersal on abundance ofP.flexuosasaplings,two persons walked randomly alone across each cell for 15 min and collected fresh ungulate feces.Those samples spaced at least 5 m apart were considered independent.Because the feces of donkey,horse,and mule could not be distinguished from one another,they were grouped as equine feces.In the laboratory,we quantified the seeds contained in 20% of the total feces collected.Then,taking into account the average value of seeds present in 20%of the total feces and the number of feces collected,we estimated the total number of seeds contained in feces from different ungulates for each cell.
In order to assess intensity of habitat use by ungulates,we put one camera-trap in the center of each selected cell for 30 consecutive days in the summer season of 2019.The 38 camera-traps(N=28:Moultrie 999i,Alabaster,AL,USA;andN=10:Primos Truth Cam 46) were placed at 0.4 m above the ground.Previously tested for their best setup,the camera-traps were set to take a burst of three photos per shot with a 30-s delay between them.Records were considered independent when the individuals could be differentiated or when picture events were separated by ≥60 min(Rich et al.,2017).We considered only the data from camera traps that had remained operative for at least 15 days.The photos of the donkey,horse,and mule were grouped as equines to maintain the grouping made with the feces samples.To estimate the value of habitat use intensity for each cell,we used RN models(Royle and Nichols,2003).These models include the probability of detection,which was evaluated considering distance to the first visual obstacle,camera-trap models,and distance to roads.In our models,the probability of occupancy was included as a constant(~1).
2.5.Statistical analysis
We fitted Generalized Linear Models (GLM) to assess the effect of environmental (LII,BI,WI,and TWI indices) and biological variables(density ofP.flexuosaadults,input of seed transported in feces,and intensity of habitat use by ungulates) on the abundance ofP.flexuosasaplings,which was included as response variable.For each prediction,we fitted sets of separate models for environmental and biological effects,considering all possible additive combinations between the explanatory variables.
For the environmental variables we fitted two sets of models.One of these sets included the mean and standard deviation for LII and BI as explanatory variables (prediction 1),and the other one,the mean and standard deviation of WI and TWI(prediction 2).The sets of models for assessing the effect of biological variables considered were three.The first model had only one explanatory variable,i.e.adult tree density(prediction 3).The second set of models was fitted to the number of seeds dispersed by each kind of ungulate:guanacos,cattle and equines (prediction 4),and the last set of models contained the intensity of habitat use by these ungulates as explanatory variable(prediction 5).
All explanatory variables were standardized by z-scores prior to analysis.Sapling abundance was fitted to GLMs with Negative Binomial error distribution because it exhibited overdispersion.We excluded collinear explanatory variables,i.e.with a Spearman rank correlation coefficient |ρ| >0.7 (Appendix I).Then,we assessed the variance inflation factor (VIFs) for any remaining collinearity in the full models,and excluded explanatory variables with VIFs >5 (Heiberger and Holland,2004).To test for spatial autocorrelation among sampling cells,we fitted semivariograms with the Pearson residuals of the models containing all explanatory variables (Zuur et al.,2009) (Apendix II).We found no evidence of spatial dependence affecting the models.
We used an information-theoretic approach described by Burnham and Anderson (2002) to model the data,based on the second-order Akaike's Information Criterion (AIC).Akaike's information criterion corrected for small sample size (AICc) was calculated for each model.Models were compared with ΔAICc,which is the difference between the lowest AICcvalue(i.e.,the best of suitable models)and AICcfrom all the other models.We considered an Akaike's weight of a model (wi),which determines the relative likelihood that the specific model is the best of the suite of all models.We evaluated the support for predictor variables by summingwiacross all models that contained the parameter being considered (parameter likelihood;Burnham and Anderson,2002).Parameter estimates were calculated using model-averaged parameter estimates based onwifrom all candidate models.To supplement parameter-likelihood evidence of important effects,we calculated 95%confidence interval limits(CL)of parameter estimates.Besides,to know the percentage of the variability explained by each model we estimated their adjustedR2(Zuur et al.,2009).
All analyses were done with R software (R Core Team,2020).We fitted occupancy models with the“unmarked”package (Fiske and Chandler,2011).VIFs were evaluated with the“car”package (Fox and Weisberg,2019),and spatial correlation with the“sp”and“geoR”packages(Pebesma and Bivand,2005;Ribeiro et al.,2020 respectively).The models were selected using the“MuMIn”package(Barton,2020).
3.Results
To analyze the environmental variables,we first assessed the effects of LII and BI on the abundance of saplings.We fitted 16 models and found that three of them had ΔAICc<2.This subset of best models explained,on average,33%of the variability(Table 1).Only the mean of BI did not include zero in its confidence interval (Table 2) and had a positive relationship with the response variable(Fig.2).Secondly,in reference to the effects of WI and TWI on sapling abundance,we also fitted 16 models,six of which had ΔAICc<2.On average,the variability explained by these six models was 37%(Table 1).In these best models,the mean of WI excluded zero from their confidence intervals (Table 2) and had a negative effect on sapling abundance(Fig.2).
In relation to the effects of biological variables,we found that densityof adult trees had a positive relationship with sapling abundance and explained 41% of the variability (Table 3,Fig.3).With respect to the input of seeds dispersed by each ungulate,only two models out of eight had ΔAICc<2 and explained,on average,36% of the variability(Table 3).In this set of models,only the input of seeds dispersed by equines excluded zero from its confidence interval (Table 4) and had a positive relationship with the response variable (Fig.3).Finally,in relation to intensity of habitat use by ungulates,we found that four out of eight models had ΔAICc<2.On average,these models explained 22%of the variability (Table 3).Among the explanatory variables used,space use by guanaco was the only one excluding zero from its confidence interval (Table 4).This variable had a negative relationship with abundance of saplings(Fig.3).
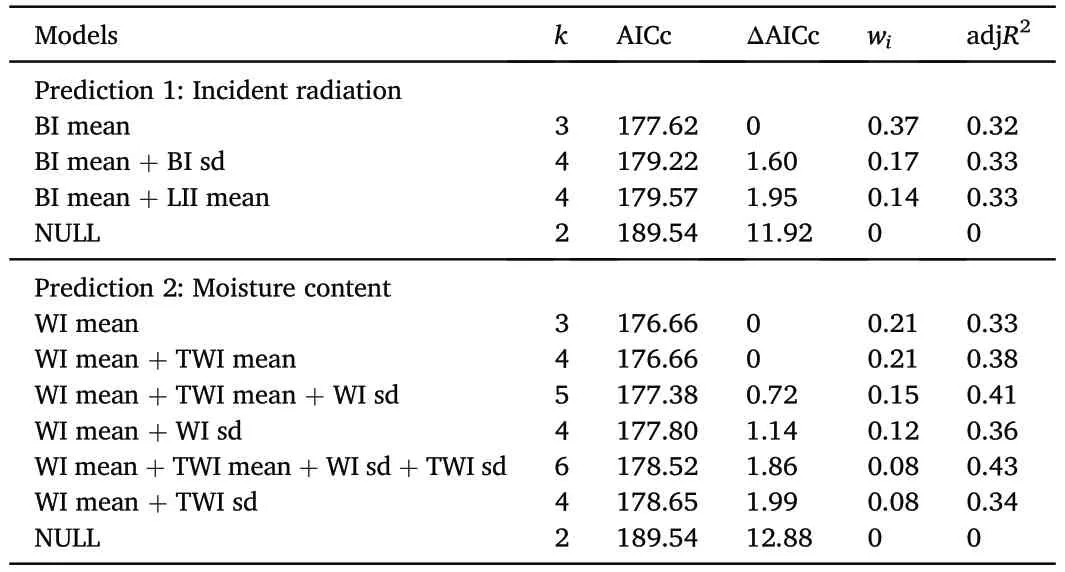
Table 1 Models with ΔAICc value lower than 2 and the NULL model for abundance of P. flexuosa saplings in relation to environmental variables.

Table 2 Parameter likelihoods,estimates(±Standard Error)and 95%confidence interval limits (CL) for biological explanatory variables that affect abundance of P. flexuosa saplings.In bold explanatory variables with CL excluding zero.
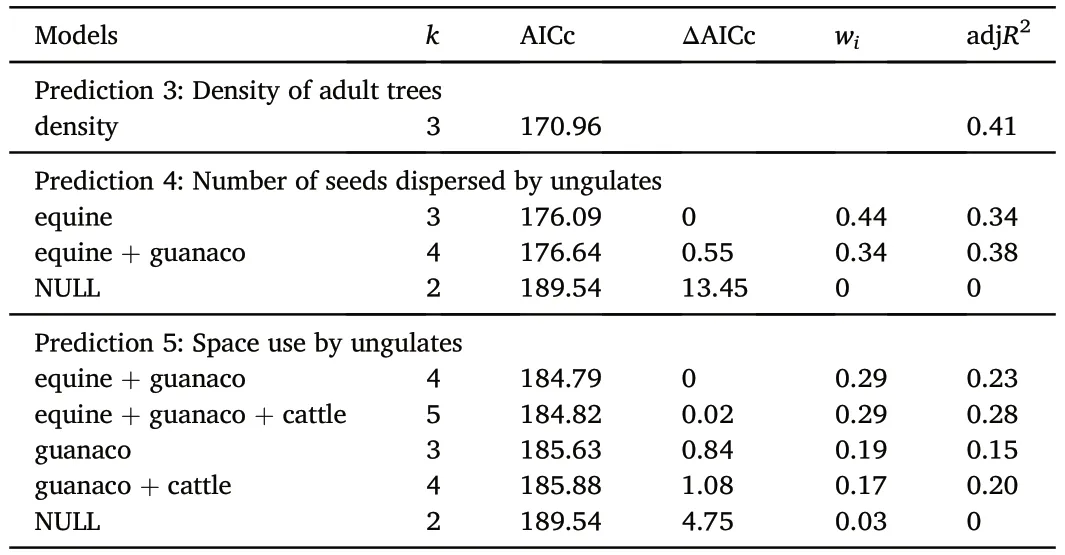
Table 3 Models with ΔAICc value lower than 2 and the NULL model for abundance of P. flexuosa saplings in relation to biological variables.
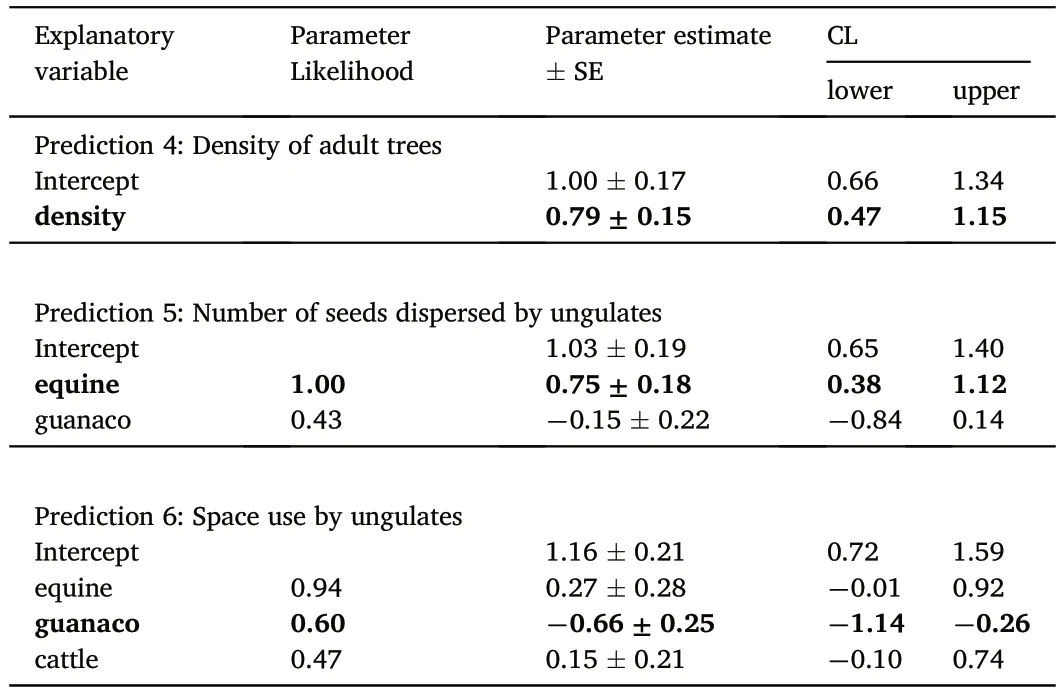
Table 4 Parameter likelihoods,estimates(±Standard Error)and 95%confidence interval limits (CL) for biological explanatory variables that affect abundance of P. flexuosa saplings.In bold explanatory variables with CL excluding zero.
4.Discussion
In this research,we explored the effect of environmental and biological variables on the abundance ofP.flexuosasaplings in the Monte Desert,Argentina.We found a mean abundance of 90 saplings per ha,a lower value than that for other areas in the Monte ecoregion in Mendoza Province,such as the~Nacu~nán reserve:620 per ha(Campos et al.,2011).Regarding environmental variables,we can say that abundance of saplings related positively to BI and negatively to WI;this confirms and rejects our first and second predictions respectively.On the other hand,in relation to biological variables and contrary to our third prediction,sapling abundance was positively affected by density of adult trees and by number of seeds dispersed by equines,this latter agreeing with the fourth prediction.Furthermore,space use byL.guanicoehad a negative relationship with sapling abundance,in accordance with our prediction.
Prosopisflexuosais a tree adapted to arid conditions.The distribution of adult individuals has been related to sandy substrate in our study area(Campos et al.,2016) and in other zones such as the Telteca Reserve in Mendoza (Giordano et al.,2011).According to this,we found that the greater abundance of saplings is positively related to these substrates(high brightness values).P.flexuosa,P.chilensisand their hybrids can grow in dry systems,although the first is the most tolerant (López Lauenstein et al.,2013).These very permeable substrates would enable it to use rainwater in addition to groundwater,which is possible because these trees have a dimorphic root system that generates independence from environmental water (Villagra et al.,2011).Because saplings still need humidity in surface soil horizons (Villagra,2000),we expected to find a positive relationship with moisture indicators,but did not find this relationship.This could be explained by the fact that saplings also develop a great net of roots and use the groundwater available(Giordano et al.,2011).
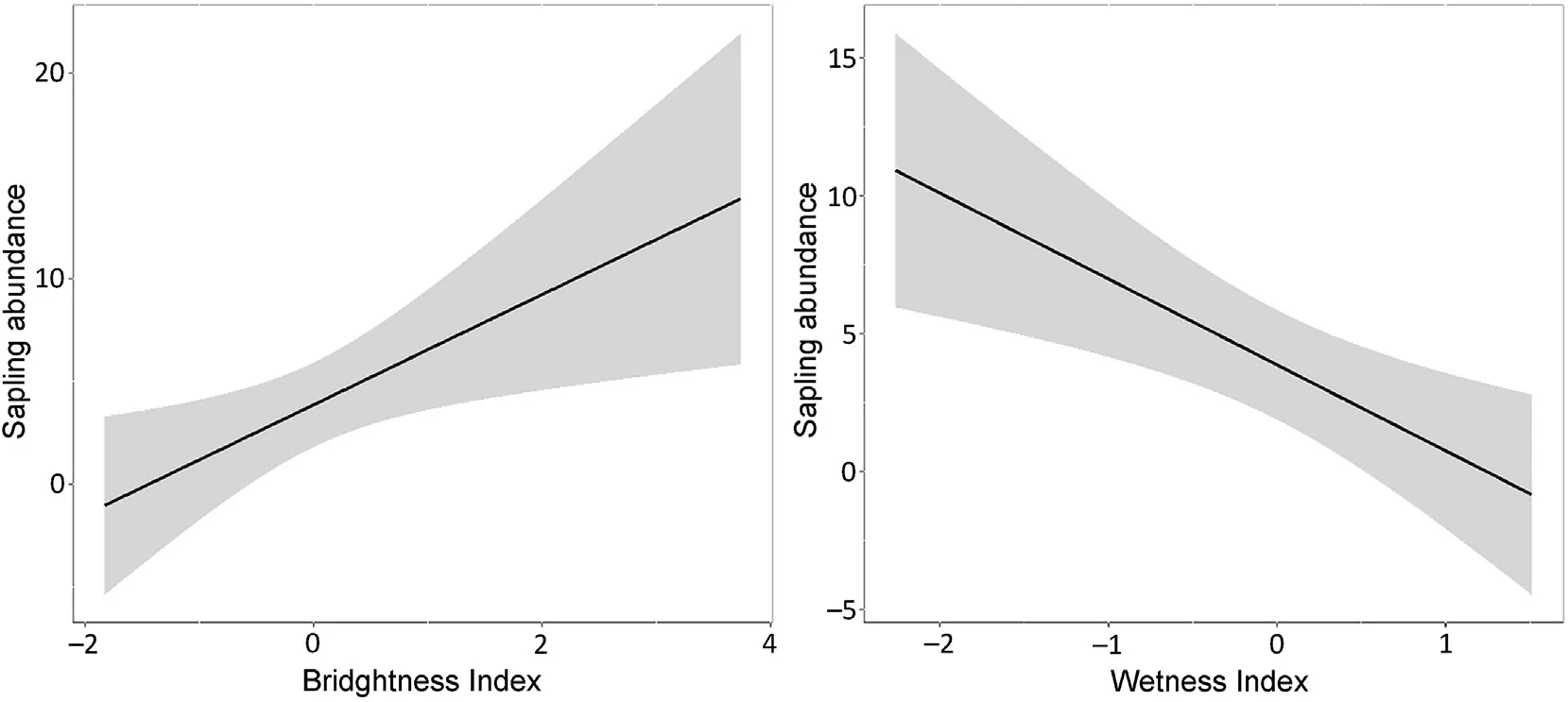
Fig.2.Main effects and 95% credible intervals for environmental explanatory variables on abundance of P. flexuosa saplings.
Although we expected a negative relationship between abundance of saplings and density of adult trees because solar radiation availability improves sapling growth and development (Vilela and Ravetta,2000),actually this relationship was positive.Probably in these open woodlands with low-density (90 ind•ha-1) of trees and few shady areas,light availability is not a limiting environmental factor.In addition,previous studies reported the importance of adult tree density to ensure seed availability (Vayreda et al.,2013;Monteiro-Henriques and Fernandes,2018).It was observed that some saplings are grown from the roots of adults such as has been found in other xerophytic trees with a vegetative reproduction (vegetative recovery from roots) (Moglia and Jofre,1998;Barchuk et al.,2006).
Previous studies showed that saplings are more abundant at grazed than ungrazed sites (Aschero and García,2012) and that sapling establishment is related to cattle activity (Campos et al.,2011).Along these same lines,we found that sapling abundance was high at sites with higher input of seeds from equine feces.These animals are consumers ofProsopispropagules and seed dispersers(Campos et al.,2008;Sánchez de la Vega and Godínez-Alvarez,2010).It was found that because equines are hindgut fermenters,i.e.non-ruminant,ingestedP.flexuosaseeds have lower retention time in their digestive tract and,thus,recovery of alive seeds is higher in comparison to a ruminant(cattle)and a“ruminant like”ungulate (guanaco) (Campagna,2018).This positive relationship between equines and saplings could be explained by the apparent low density of domestic ungulates;otherwise these animals could be trampling on seedlings and grazing on saplings,then affecting saplingabundance(Campos et al.,2011;Marinho et al.,2016).
The unexpected result was the negative association between abundance of saplings and intensity of habitat use by guanacos.Although guanacos consumeP.flexuosafruits and disperse seeds(Reus et al.,2014;Campos et al.,2008,2020),the input of seeds from guanaco feces was the lowest.The activity of guanacos is strongly associated with areas of rougher rocky substrata and open terrain with sparse plant cover,while exotic ungulates use areas of densest and most productive vegetation,such as the openProsopisforest(Acebes et al.,2010,2012).This could be showing that domestic ungulates are displacing guanacos,such as was observed on other sites (Baldi et al.,2004;Schroeder et al.,2014),and due to this spatial displacement,the intensity of habitat use by guanacos is low in areas with high abundance ofProsopissaplings.
Our results show thatP.flexuosasaplings are benefited from the conditions around adult trees,such as sandy substrates.In addition to this,we found that exotic ungulates in low densities have neutral (i.e.cattle) or positive (i.e.equines) effects on sapling abundance.Notwithstanding,continuous monitoring of abundance of large exotic species would be needed in order to prevent overgrazing and the consequent impact on the protected area.In this way,local people would be able to continue their livestock activities and not lose an economic source.Conservation management plans should consider the functional effect of these species on the dynamics ofProsopissaplings and on forest regeneration.
Funding
This research was supported by“The chica,the retamo,and the algarrobo:umbrella species for the conservation of the Native Forest of the Ischigualasto Provincial Park and nearby zones.Biological interactions,effects of human activities and their mitigation”,Plan for the Conservation of Native Forests Law 26.331.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’contributions
C.F.M.,C.V.E.,B.F.,and C.C.M conceived of the research idea;C.F.M.and C.C.M.collected data;C.F.M.performed statistical analyses;C.F.M.,with contributions from C.V.E.,B.F.,R.L,and C.C.M.wrote the paper;all authors discussed the results and commented on the manuscript.
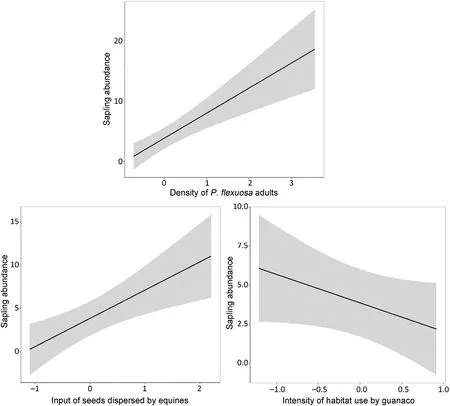
Fig.3.Main effects and 95% credible intervals for biological explanatory variables on abundance of P. flexuosa saplings.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of competing interest
Neither author has competing interests.
Acknowledgements
We thank the staff of Ischigualasto Provincial Park for providing all the necessary facilities during fieldwork.We also thank Nelida Horak for assisting us with the English version and Tomas González for helping us with the imagery analysis.We thank the reviewers for providing constructive and useful comments that improved an earlier version of this article.
List of abbreviations
GDEM Aster Global Digital Model
IPP Ischigualasto Provincial P
LLI Local Insolation Index
BI Brightness Index
WI Wetness Index
TWI Topographic Wetness Index
DBH Diameter at Breast Height
GLM Generalized Linear Models
VIF Variance Inflation Factor
AICcAkaike's information criterion corrected for small sample size;
ΔAICcDelta Akaike's information criterion corrected for small sample size;
wiAkaike's weight
CL Confidence interval limits
sd Standard deviation
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100010.
- Forest Ecosystems的其它文章
- Two-level optimization approach to tree-level forest planning
- No treeline shift despite climate change over the last 70 years
- Variation in water supply leads to different responses of tree growth to warming
- Active forest management accelerates carbon storage in plantation forests in Lishui,southern China
- Patterns and driving factors of leaf C,N,and P stoichiometry in two forest types with different stand ages in a mid-subtropical zone
- Forest height mapping using inventory and multi-source satellite data over Hunan Province in southern China

