Dietary intake of bamboo vinegar and charcoal powder (BVC)enhances resistance of African catf ish Clarias gariepinus to bacterial pathogen*
Kwangjin JU , Moyngsuk KIL , Sanghyok RI , Tongchol KIM , Lining ZHANG ,Maocang YAN ,**, Guangxu LIU
1 College of Animal Sciences, Zhejiang University, Hangzhou 310058, China
2 College of Aquaculture, Wonsan Fisheries University, Wonsan 999093, DPR Korea
3 College of Life Science, Kim Hyong Jik University of Education, Pyongyang 99903, DPR Korea
4 Zhejiang Mariculture Research Institute, Wenzhou 325005, China
Abstract Although the immunomodulatory activity of bamboo vinegar and charcoal powder (BVC) has been increasingly documented in domestic animals, the use of BVC as immunostimulant in f ish aquaculture awaits verif ication. In the present study, the immunostimulatory impacts of dietary BVC intake were investigated in an important aquaculture f ish species, the African catf ish Clarias gariepinus. Results show that the pathogen resistance of African catf ish was signif icantly improved by dietary BVC intake at the tested levels (0.5%-2%), as indicated by higher survival rates (approximately 1.52-1.85 times that without BVC supplementation) upon intraperitoneally injection of bacterial pathogen Aeromonas hydrophila. In addition, both the count and phagocytic activity of blood cells were signif icantly up-regulated by a 45-day dietary supplementation of BVC. Moreover, compared to that of the control, f ish individuals fed BVC containing diets exhibited signif icant higher activities of superoxide dismutase (SOD), myeloperoxidase(MPO), and lysozyme (LYZ). The content of immunoglobulin M (IgM) in serum of African catf ish was also induced by dietary BVC supplementation. Furthermore, the expression levels of interleukin-8 ( IL- 8),toll-like receptor 1 and 5 ( TLR1 and TLR5), myeloid diff erentiation factor 88 ( MyD88), and nuclear factor kappa B ( NFκB) in the head kidney were signif icantly up-regulated. Our f indings indicate BVC could be an eff ective immunostimulant in teleost species, which may enhance f ish immunity through improving hematic parameters, activating bioactive humoral molecules, and up-regulating immune related molecular pathways.
Keyword: bamboo vinegar and charcoal powder (BVC); African catf ish; immunostimulant; pathogen resistance
1 INTRODUCTION
The African catf ishClariasgariepinusis a freshwater aquaculture f ish species, with annual global production of more than 220 000 tons since 2013 according to the estimate of Food and Agriculture Organization of the United Nations (FAO). In recent years, the fast development and wide application of intensive aquaculture provokes disease outbreak risks and poses a great threat to the f ish aquaculture industry(Toranzo et al., 2005; Oliva-Teles, 2012; Dawood and Koshio, 2016). While a great number of studies have demonstrated that administration of some veterinary antibiotics can be eff ective in controlling disease caused by microbial pathogens (Cha and Carlson,2019; Quaik et al., 2020), the practice has been widely criticized for its high cost, various side-eff ects (e.g.growth retardation), environmental as well as food safety concerns (Yonar et al., 2011; Zhou et al., 2020).Therefore, strengthening f ish immunity using immunostimulants is considered to be one of the most promising methods to prevent disease outbreak in aquaculture practice (Awad and Awaad, 2017).
With merits such as processing complex porous structure and having high mineral contents (i.e.potassium, calcium, iron, and sodium), bamboo charcoal is widely used in the production of various industrial and daily products, which is referred as “the black diamond” in Asian countries like Japan and China (Watarai and Tana, 2005; Zhao et al., 2008; Jia et al., 2015; Rattanawut et al., 2017). In addition,bamboo charcoal is not only a legal food additive (i.e.National Standard of China, GB 2760-2014) but is also widely used for medical purposes such as oral antidote in the form of powder (Rattanawut et al.,2017). Bamboo vinegar is a brown transparent liquid collected from the condensed vapor during the pyrolysis process of bamboo charcoal production,which contains rich bioactive chemical components such as organic acids, ketones, aldehydes, and alcohols (Wu et al., 2015). In recent years,immunomodulatory activities including antioxidation,anti-inf lammation, and anti-virus of bamboo vinegar and charcoal powder (BVC) have been increasingly documented (Loo et al., 2007; Marumoto et al., 2012).Although the potential of BVC as immunostimulant has been verif ied in domestic animals such as pigs,ducks, and chickens (Samanya and Yamauchi, 2002;Watarai and Tana, 2005; Wang et al., 2012; Yan et al.,2012; Huo et al., 2016; Rattanawut et al., 2018, 2019;Yu et al., 2018; Schmidt et al., 2019), to the best of our knowledge, it remains elusive in commercial f ish species such as the African catf ish.
Although teleost possess both specif ic (or adaptive)and non-specif ic (or innate) immunities against pathogen infections, non-specif ic immunity through cellular and humoral immune responses is regarded as the main pathway through which natural immunostimulant strengthen the immunity of teleost species (Fierro-Castro et al., 2013; Wang et al., 2016;He et al., 2020). In addition, non-specif ic immunity is thought to be more eff ective against infection due to its rapid and non-specif ic response to a wide spectrum of pathogens (Deng et al., 2020; Yu et al., 2020).Except direct removal of pathogens and/or foreign particles by blood cells via phagocytosis, bioactive molecules such as lysozyme (LYZ), myeloperoxidase(MPO), superoxide dismutase (SOD), and cytokines also play crucial roles in the non-specif ic immune response of teleosts (Dotta et al., 2018; Devi et al.,2019; Yonar, 2019; Li et al., 2020). In addition, it has been well established that immune-related pathways such as the Toll-like receptor and NFκB signaling pathways play important roles during the immune response of teleosts against pathogen challenge(Umasuthan et al., 2017).
Previous studies conducted in terrestrial domestic animals suggest that dietary administration of BVC may enhance immunity of these animals through improving hematic parameters, up-regulating bioactive humoral molecules, and modulating immune related molecular pathways (Yamauchi et al.,2010; Wang et al., 2012; Jia et al., 2015; Huo et al.,2016; Yu et al., 2018). For example, the resistance of pigs to coliform bacteria andSalmonellaspp. infection was signif icantly improved by dietary intake of BVC(0.3%), along with signif icant up-regulated immunoglobulin G (IgG), IgA, MyD88, and IL-10 levels (Chu et al., 2013; Huo et al., 2016; Qin et al.,2018). However, it is still unknown whether BVC can act as eff ective immunostimulant in teleost species through similar eff ects on these immune related parameters.
Therefore, in order to improve our present understanding of the application potential of BVC in aquaculture, the impact of BVC dietary intake on the resistance of African catf ish to pathogen infection was verif ied with a bacterial challenge test in this study. In addition, the eff ects of BVC administration on the count and phagocytic activity of blood cells,the immunoglobulin M (IgM) content, the activities of three immune related enzymes, and the expressions of key genes from Toll-like receptor and NFκB signaling pathway were investigated to reveal potential mechanisms underpinning the enhanced resistance to pathogen infection.
2 MATERIAL AND METHOD
2.1 Animal ethics statement
In this study, all experiments were performed in accordance to the Animal Ethics Committee in the School of Medicine, Zhejiang University (ETHICS CODE Permit NO. ZJU2015-516-15, issued by the Animal Ethics Committee in the School of Medicine,Zhejiang University).
2.2 Animals and feeding experiment
Commercial BVC, produced by mixing bamboo vinegar ref ined liquid and bamboo charcoal at the weight ratio of 3꞉7, followed by low temperature spray drying, was purchased from ZhongLi Bio-tech Co.,Ltd. (Jiangyin, China). According to the manufacturer,bamboo vinegar mainly contains the following bioactive substances, 2.77% phenolic compounds (i.e.phenol, 2, 6-dimethoxy), 9.78% aldehydes (i.e.2-furaldehyde, 5-hydroxymethyl), 0.81% ketones (i.e.4-hydroxydihydro-2(3H)- furanone), 2.26% organic acids (i.e. acetic acid), 3.55% heterocycle compounds(i.e. 2- butyltetrahydrothiophene), and 0.37% alcohols(i.e. furfuryl alcohol).
Five experimental diets were prepared containing 0% (control), 0.5%, 1.0%, 1.5%, and 2.0% commercial BVC, respectively. The basal diet (control) was formulated according to previous publications with minor modif ications (Nya and Austin, 2009), where detailed formulation was provided in Supplementary Table S1. Experimental f ish individuals (weight of 10.25±0.23 g) were purchased from DeRen Agricultural Development Co., Ltd. (Tianjin, China)and acclimated in a 1 000-L tank f illed with 800-L freshwater for 14 days (temperature 28.3±1.2 °C,dissolved oxygen 5.92±0.52 mg/L, and pH 7.05±0.22)before the commencement of experiment. During acclimation, f ish were fed basal diet pellets to satiation three times a day at 09:00 AM, 13:00 PM, and 17:00 PM, respectively. Fish individuals were randomly divided into 5 experimental groups fed diets containing 0%, 0.5%, 1.0%, 1.5%, and 2.0% of BVC,respectively. The feeding experiment lasted for 45 days, during which f ish were fed with corresponding diets three times a day as described above.
2.3 Bacterial challenge test
After corresponding feeding experiment, 150 f ish individuals were collected from each experimental group (3 replicates each contains 50 f ish) and challenged by the bacterial pathogenAeromonashydrophila, following the methods of Thanikachalam et al. (2010). In brief, a virulent strain ofA.hydrophilaobtained from The Query Network for Microbial Species of China (http://www.biobw.org/), was grown in tryptic soy broth (TSB) at 37 °C for 24 h. The culture broth was then centrifuged at 3 000 ×gfor 10 min at 4 °C. Bacterial precipitate collected was resuspended in sterile phosphate buff ered saline(PBS) and used for the challenge test. In this study,f ish individual was challenged by intraperitoneally injection of 0.1-mL PBS containing 1×108CFU/mL liveA.hydrophyla. The survival rate of f ish in each experimental trial was recorded everyday till 7 days post injection and dead f ish were removed three times a day during this period.
2.4 Blood cell count and phagocytic activity analysis
After the feeding experiment and a 24-h fasting, 30 f ish individuals were randomly selected from each experimental group and anesthetized with benzocaine.Following the methods of Xiang et al. (2015) and Rong et al. (2020), approximately 100-μL blood sample was collected individually from the caudal arch of African catf ish using a 32-gauge needle and a plastic 2-mL syringe. Blood collected from 10 f ish was pooled as one replicate for each experimental group (n=3). After immediately mixing with anticoagulant edathamil (v꞉v=7꞉3), blood samples were then used to estimate the counts of red blood cell(RBC) and white blood cell (WBC) using a veterinary hematology analyzer (BC-5000Vet, Mindray, China),following the manufacturer’s procedure and those reported previously (Rong et al., 2020).
Following the methods of Su et al. (2018) and Tang et al. (2020), blood samples collected from three catf ish (n=3) as described above were used to estimate the phagocytosis for each experimental group. In brief, approximately 100-μL blood sample collected individually from each catf ish was mixed with equal amount of Alsever’s solution, followed by a 30-min incubation at 25 °C with pre-prepared yeast suspension(Instant dry yeast dissolved in Alsever’s solution) at a yeast꞉haemocyte ratio of 10꞉1. A volume of 100-μL 2.5% glutaraldehyde was thereafter used to terminate the phagocytosis process. Blood smears were subsequently prepared with the blood sample and stained with Wright-Giemsa stain (G1020, Solarbio Life Sciences Co., Ltd., Beijing, China). Stained blood smears were then examined under a Nikon eclipse E600 light microscope at the magnif ication of 400× and the counts of blood cells with and without engulfed yeast cells were scored and used to calculate the phagocytic activity of each sample (Du et al.,2020; Han et al., 2021).
2.5 SOD, MPO, and LYZ activity assays
Blood samples collected with syringes as described above from 10 f ish individuals were pooled as one replicate (3 replicates in total,n=3) for each experimental group. The blood samples obtained were allowed to clot overnight at 4 °C and then were centrifuged at 600×gfor 15 min (4 °C). Serum(supernatant) of each sample was subsequently used to estimate the activities of SOD, MPO, and LYZ.Following the protocols provided by manufacturer,SOD activity was measured with total superoxide dismutase assay kit with tetranitroblue tetrazolium chloride (NBT) (S0109, Beyotime Biotechnology Co., Ltd., Shanghai, China). Brief ly, 20 μL of serum obtained was mixed with 160 μL of NBT solution and 20-μL reaction solution in a 96-well plate. After incubation at room temperature for 30 min, the absorption value of each sample was determined at the wavelength of 560 nm with a microplate reader(Thermo MultiskanGo, USA). The SOD activity of the sample was subsequently estimated by referring the absorption value obtained to the standard curve.The total protein concentration of each sample was measured with a BCA protein assay kit (P0012,Beyotime Biotechnology Co., Ltd., Shanghai, China)using the Bradford method. Then, the SOD activity of each sample was calibrated and expressed as USOD per mg protein.
The activities of MPO and LYZ were determined using commercial MPO and LYZ activity ELISA kits(69-22426, MSKBIO Technology Co., Ltd., Wuhan,China and FK-97441, FKBIO Biotechnology Co.,Ltd., Shanghai, China), respectively. Following manufacturer’s instruction and those reported previously (Borgia et al., 2018; Su et al., 2018; Rong et al., 2020), 10-μL serum collected from each replicate was mixed with 40-μL diluent and then incubated at 37 °C for 30 min. After a wash with detergent, 50-μL enzyme-labeling reagent was added to each sample, followed by another 30-min incubation at 37 °C. Chromogenic reagents A and B(50 μL each) were added to each sample after washing and subsequently incubated in dark for 15 min. The chromogenic reaction was then terminated with 50-μL terminating solution. The absorption value of each sample was determined with a microplate reader(Thermo Multiskan Go, United States) at 450-nm wavelength. The activities of MPO and LYZ were then calculated by referring the absorption values obtained to corresponding standard curves and calibrated with the volume of serum.
2.6 Determination of IgM content
After corresponding treatment, approximately 100-μL blood sample was collected from the caudal arch of each catf ish individual using a plastic syringe. Blood samples collected from 10 f ish were pooled as one replicate for each experimental group (n=3) and then allowed to clot overnight at 4 °C. Serum was obtained by collecting supernatant after centrifugation at 600×gfor 15 min (4 °C) and subsequently used to estimate the content of IgM following the method reported(Siwicki et al., 1994). Brief ly, serum obtained from each sample was divided into two aliquots, one of which was used to determine the total protein content with a BCA protein assay kit (P0012, Beyotime Biotechnology Co., Ltd., Shanghai, China) using the Bradford method. The other aliquot of serum was mixed with equal volume of 12% (w/v) solution of polyethylene glycol (Sigma) followed by incubation with constant agitation for 2 h. After centrifugation at 3 000×gfor 15 min, total protein content in supernatant was measured as described above. The IgM content was subsequently estimated as the change in total protein content before and after polyethylene glycol treatment (Roberts and Jones, 2008).
2.7 Expression analysis of immune-related genes
After the feeding experiment, 9 f ish collected from each experimental group were pooled as one replicate(3 replicates in total,n=3) and used to analyze the expression levels of immune-related genes following our previously reported methods with slight modif ication (Shi et al., 2019, 2021; Zha et al., 2019).In total, f ive genes encoding Interleukin-8 (IL-8),myeloid diff erentiation factor 88 (MyD88), toll-like receptor 1 (TLR1), toll-like receptor 5 (TLR5), and nuclear factor kappa B (NFκB) were analyzed in the present study. Brief ly, head kidney was collected from anesthetized f ish and used for total RNA extraction using EASY spin RNA extraction kit (RN2802,Aidlab Co., Ltd., Beijing, China). The cDNA template was synthesized with the RNA obtained using the PrimeScript RT (RR037A, TaKaRa, Dalian, China)and then used for the PCR amplif ication with a StepOnePlus Real-Time PCR System (Thermo Scientif ic, USA). In this study, real-time quantitative PCR was conducted in triplicates using the following amplif ication protocol: 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Melting curve analysis was performed to ensure amplif ication specif icity and the 2-ΔΔCtmethod was used to calculate the relative expression levels of genes tested. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the expression of which is constant under diff erent conditions, was used as an internal reference in this study. All primers (Table 1)were designed according to the sequence data(accession No. PRJNA487132, NCBI Sequence Read Archive database) reported for African catf ish (Li et al., 2019).
2.8 Statistical analysis
One-way ANOVAs followed by Tukey’s post hoc tests were conducted to detect signif icant diff erence among experimental groups. For all analyses,Levene’s test and Shapiro-Wilk’s test were used to verify homogeneity of variances and normality of data distribution, respectively. In cases where these assumptions were not satisf ied by the raw data, arcsine square root transformation was performed prior to analysis. The Duncan’s multiple range test was used to compare expression level of genes investigated. All analyses were carried out with the Origin-Pro 8.0 software package and aPvalue less than 0.05 was accepted as a statistically signif icance.
3 RESULT
3.1 Survivorship upon bacterial challenge
The survival rate of African catf ish uponA.hydrophilachallenge was signif icantly improved by the dietary administration of BVC (Fig.1,P<0.05),which were approximately 1.52, 1.77, 1.84, and 1.85 times of that of the control after 7 days of pathogen injection for f ish fed diet containing 0.5%,1.0%, 1.5%, and 2.0% BVC, respectively. In addition,African catf ish fed diets containing 1.0%-2.0% BVC exhibited a signif icant higher survival rate than that administrated with 0.5% BVC.
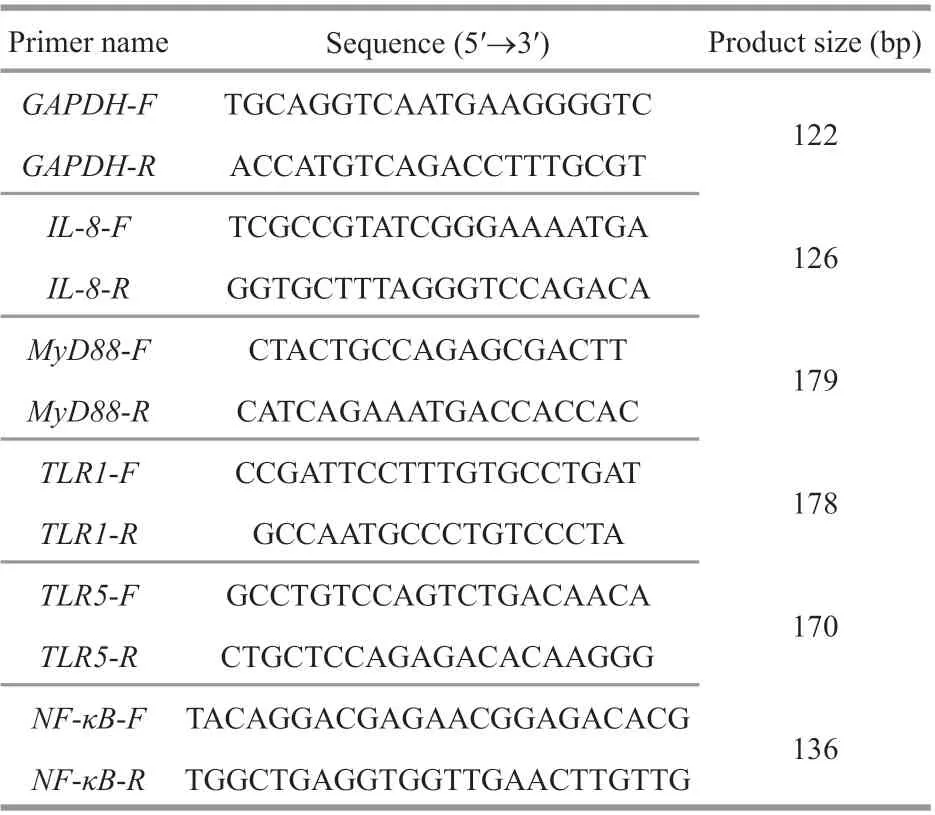
Table 1 Sequence information of the primers used for gene expression analysis in this study
3.2 Impacts of BVC dietary intake on the count and phagocytic activity of blood cells
Data obtained demonstrated that both the count and the phagocytic activity of blood cells were signif icantly up-regulated by the 45-day dietary intake of BVC (Table 2;P<0.05). Specif ically, RBC signif icantly increased by 14% for f ish fed diet containing 0.5% BVC and further heightened to approximately 1.9-2.1 times of the control for the rest of experimental groups supplemented with higher amount of BVC. Similarly, WBC signif icantly increased to 1.10, 1.16, 1.33, and 1.34 times of that of the control for f ish fed diets containing 0.5%,1.0%, 1.5%, and 2.0% BVC, respectively. In addition, compared to that of the control, signif icant higher phagocytic rates increased by 18.9%, 60.4%,81.2%, and 82.9% were detected for f ish fed diets containing 0.5%, 1.0%, 1.5%, and 2.0% BVC,respectively.

Fig.1 Daily survival rates of African catf ish fed diets containing 0% (control), 0.5%, 1.0%, 1.5%, and 2.0% BVC on A. hydrophyla challenge
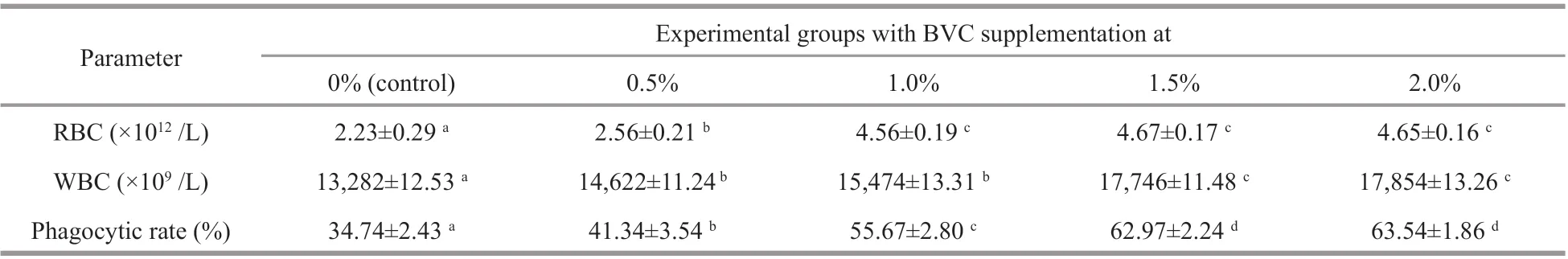
Table 2 The count and phagocytic activity of blood cells of African catf ish after 45 days feeding with diets containing 0%(control), 0.5%, 1.0%, 1.5%, and 2.0% BVC, respectively
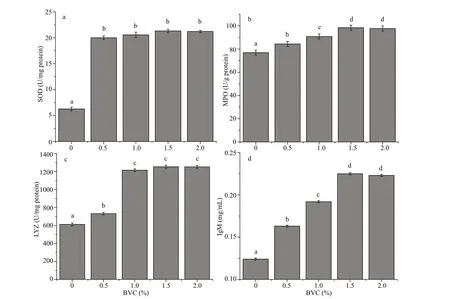
Fig.2 Enzyme activities and IgM content in serum of African catf ish fed diets containing 0% (control), 0.5%, 1.0%, 1.5%,and 2.0% BVC, respectively
3.3 Impacts of BVC dietary intake on enzyme activities and IgM content
The activities of SOD, MPO, and LYZ in serum of African catf ish were signif icantly up-regulated by the 45-day dietary intake of BVC (Fig.2;P<0.05), which were approximately 3.19-3.38 (Fig.2a), 1.13-1.26(Fig.2b), and 1.19-2.04 (Fig.2c) times that of the control for SOD, MPO, and LYZ, respectively. Fish fed diets containing higher amount of BVC presented signif icantly higher IgM content, which were approximately 1.31 (0.5% BVC group), 1.54 (1.0%BVC group), 1.71 (1.5% BVC group), and 1.70 (2.0%BVC group) times of the control, respectively(Fig.2d).
3.4 Impact of dietary intake of BVC on the expression of immune-related genes
Compared to that of the control, except for the expression ofNFκBin f ish fed the lowest BVC dose(0.5%), the expression levels of all immune-related genes investigated were signif icantly upregulated by the 45-day dietary intake of BVC (Fig.3;P<0.05),which increased to approximately 2.96-17.59, 2.34-5.04, 5.71-11.86, 3.82-11.92, and 4.68-14.46 times of the control forMyD88,NFκB,TLR1,TLR5, andIL-8, respectively.
4 DISCUSSION
Data obtained in this study show that dietary administration of BVC signif icantly enhanced the resistance of African catf ish to the challenge of bacterial pathogenA.hydrophila, and validated the immunostimulatory function of BVC in teleost species for the f irst time. More importantly, we further revealed the candidature pathways targeted by BVC.
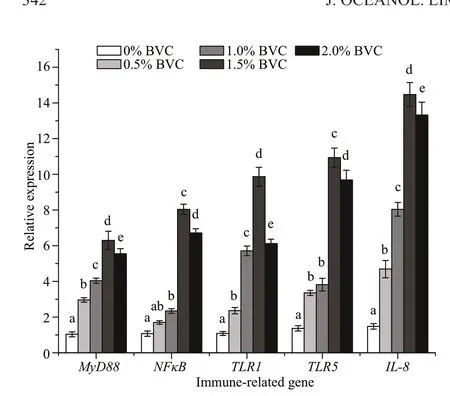
Fig.3 The relative expression levels of immune-related genes in the head kidney of African catf ish after 45-day dietary intake of 0% (control), 0.5%, 1.0%,1.5%, and 2.0% BVC, respectively
First, it is well known that non-specif ic innate immunity through phagocytosis of blood cells is the f irst line of defense against pathogen infection (Van Doan et al., 2019). Therefore, hematic parameters such as blood cell count and phagocytic activity are widely used indicators for f ish immunity status(Harikrishnan et al., 2003; Rong et al., 2020). In this study, it was shown that both parameters were signif icantly improved by the dietary intake of BVC.Since larger numbers of more active blood cells are apparently more eff ective in removing pathogen particles via phagocytosis, the improvement in the hematic parameters may partly contribute to the enhanced pathogen resistance upon BVC administration detected in this study.
Secondly, it has been well established that SOD and MPO play crucial roles in oxygen-dependent degradation of pathogen, whereas LYZ is the key enzyme destroying pathogen particles through the oxygen-independent degradation pathway during non-specif ic immune responses of animals (Sahu et al., 2007; Harikrishnan et al., 2009; Nya and Austin,2009; Talpur and Ikhwanuddin, 2013). Furthermore,it has been demonstrated that elevation in LYZ activity may lead to increases in leucocyte number and/or antibody titre (Jha et al., 2007; Saurabh and Sahoo, 2008). In this study, it was found that the activities of SOD, MPO, and LYZ were signif icantly up-regulated by the dietary intake of BVC. Thus, upregulation of these important bioactive molecules will confer individual an enhanced ability to evacuate pathogen particles more eff ectively achieving enhanced pathogen resistance.
Thirdly, the immunoglobulin M (IgM) characterized by μ heavy chain and the large size, plays important roles in both innate and adaptive immune responses(Wiegertjes et al., 1996; Bunnoy et al., 2020). Unlike other immunoglobulins, IgM is called “natural antibody” because it can quickly recognize and initiate an immune response by directly neutralizing pathogens without prior immunization (Zou et al.,2020). Data obtained in this study showed that IgM level was signif icantly up-regulated by the dietary intake of BVC, which could be another reason behind the enhanced pathogen resistance of African catf ish.In addition, IL-8, the best known member of cytokines,can induce a full pattern of immune responses such as attracting and activating leukocytes, releasing stored immune-related enzymes, and inducing the production of reactive oxygen metabolites (Gonzalez-Aparicio and Alfaro, 2020). Although the level of IL-8 in serum was not measured directly in this study, data obtained showed the expression ofIL-8was signif icantly upregulated by the dietary BVC administration, which may also contribute to the improved pathogen resistance detected.
At last, immune responses are known to be a series of cascade reactions mediated by molecular pathways such as the Toll-like receptor and NFκB signaling pathways (Li et al., 2019). Upon pathogen infection,the pattern recognition receptor (PRRs) mediated recognition is essential to initiate immune responses through activating downstream pathways (Akira et al., 2006). TLRs are transmembrane proteins serving as major PRRs to detect pathogen-associated molecular patterns (PAMPs) such as the lipopolysaccharides, peptidoglycan, and β-glucans(Wang et al., 2013; Ji et al., 2020). As main members of TLR family, TLR1 can recognize bacterial peptidoglycan and lipoproteins in concert with TLR2,whereas TLR5 is able to sense bacterial f lagellin expressed by f lagellated bacteria (Hayashi et al.,2001; Waugh and Wilson, 2008). Once activated by corresponding PAMPs, TLRs activate the NFκB signaling pathway through MyD88 to promote a proinf lammatory response and modulate both innate and adaptive immune responses (Gewirtz et al., 2001).Since the expression levels ofTLR1,TLR5,MyD88,andNFκBwere all signif icantly up-regulated by the 45-day dietary administration of BVC, the improvement in pathogen resistance may partly result from activation of these immune related pathways.
By the way, due to the existence of complicated regulatory network among the physiological and/or biochemical parameters investigated, the alterations observed in some parameters upon BVC supplementation may partially result from changes in other ones. For instance, it has been well known that NFκB signaling pathway play an essential role in regulating phagocytosis (Ren et al., 2017). Therefore,the upregulation in phagocytic activity of blood cell detected upon BVC supplementation may partially due to the activation of this molecular pathway(indicated by downregulation of genes from NFκB signaling pathway). In addition, NFκB is also an important regulator for hematopoiesis (González-Murillo et al., 2015). Hence, the upregulation ofNFκBupon BVC supplementation may be one of the direct causes for the increases in the counts of RBC and WBC observed.
5 CONCLUSION
Although the elaborated mechanisms underpinning the immunostimulatory impacts of BVC (i.e. which the specif ic component is responsible for these eff ects)await further investigation, the results of this study suggest that dietary BVC administration at the levels of 1%-2% could be an eff ective method to strengthen the immunity of African catf ish, off ering promising application potency in aquaculture practice.
6 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- Spatial diff erence in net growth rate of Yesso scallop Patinopecten yessoensis revealed by an aquaculture ecosystem model*
- Bacterial community composition in gut content and ambient sediment of two tropical wild sea cucumbers ( Holothuria atra and H. leucospilota)*
- Comparative transcriptomic analysis of Macrobrachium nippon ense in response to Aerom onas veronii or Staphylococcus au reus infection*
- Cloning of catalase gene and antioxidant genes in Scophthalmus maximus response to metalloprotease of Vibrio anguillarum stress*
- Taxonomy and regeneration of a newly recorded Polychaete Capitella teleta (Annelida, Capitellidae) in the coastal water of Shandong, China*
- Five new records of Xanthidae (Crustacea: Brachyura) from Hainan Island, China*
