Cloning of catalase gene and antioxidant genes in Scophthalmus maximus response to metalloprotease of Vibrio anguillarum stress*
Hai REN , Jian LI , Ping LIU , Xianyun REN , Tao SONG , Guisheng GAO ,Duwen LI , Shuaiting LIU
1 Hebei Key Laboratory of Preventive Veterinary Medicine, Hebei Normal University of Science and Technology, Qinhuangdao 066004, China
2 Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
Abstract Metalloproteases represent a class of extracellular proteases found in Vibrio anguillarum that can generate toxic and pathogenic eff ects in turbot ( Scophthalmus maximus). The toxicological eff ect partly results from oxidative damage due to the production of excessive reactive oxygen species (ROS). Catalase(CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) are major antioxidant enzymes induced by various oxidative stresses and can scavenge peroxides generated in cells. To evaluate the eff ects of metalloprotease-induced ROS on the antioxidation defense mechanism of S. maximus head kidney cells,the cDNA of CAT gene (designated as SmCAT) was cloned and characterized. SmCAT comprises a 1 584-bp coding sequence that encodes a protein containing 527 amino acids with a poly(A) tail. Bioinformatics analysis revealed an active site signature sequence, a heme-ligand signature sequence, and three catalytic amino acid residues. The deduced SmCAT amino acid sequence shares a sequence similarity of 66.1%-92.4% with those of other species. Phylogenetic analysis revealed that SmCAT is classif ied with CAT of other f ishes. Quantitative real-time PCR analysis showed that SmCAT was extensively expressed in all tested tissues, especially in blood. The expression of SmCAT, SmMnSOD, and SmGPx were inhibited signif icantly in head kidney cells treated with metalloprotease from 12 to 24 h. In 6 to 24 h metalloprotease-treated groups compared to that of the untreated group, it was found that the production of ROS was markedly increased,and the mitochondrial membrane potential was decreased considerably. Hoechst 33342 staining revealed the presence of apoptotic bodies when the cells were incubated with 8.0 or 40.0 μg/mL metalloprotease for 12 and 24 h. Hence, the toxic eff ects of metalloprotease are associated with the down-regulation of antioxidant enzyme expression and increased ROS levels, which trigger the activation of apoptosis in the head kidney cells of turbot. Our f indings provide a better understanding on the mechanism of metalloprotease-induced apoptosis in f ish.
Keyword: gene cloning; expression; reactive oxygen species; metalloprotease; head kidney cells
1 INTRODUCTION
Reactive oxygen species (ROS) are commonly formed in all aerobic biological systems, but the overproduction of ROS in organisms can induce oxidative injury, lipid peroxidation, cell membrane destruction, apoptosis, and cell death (Nordberg and Arnér, 2001; An et al., 2008; Nwani et al., 2013; Kim et al., 2019). To antagonize the detrimental eff ects due to excessive ROS, aerobic organisms, through evolution, have developed complex antioxidant defense systems using antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD),glutathione peroxidase (GPx) and peroxidases(Afonso et al., 2007; Yu et al., 2017). SOD catalyzes the dismutation of superoxides into oxygen (O2) and hydrogen peroxide (H2O2), which is then alleviated by CAT and GPx (Zhang et al., 2018).
CAT, a major antioxidative oxidoreductase that exists virtually in all aerobic organisms is involved in several cellular processes, such as apoptosis,mutagenesis, and inf lammation (Bandyopadhyay et al., 1999; Klotz and Loewen, 2003). To date, numerous catalase genes isolated from diff erent organisms have been cloned and studied (Gerhard et al., 2000;Yamamoto et al., 2005; Li et al., 2008).CATisolated from several species of f ish, includingDaniorerio(Ken et al., 2000),Sebastesschlegelii(Elvitigala et al., 2015),Lizahaematocheila(Qi et al., 2015),Oplegnathusfasciatus(Elvitigala et al., 2013),MegalobramaamblycephalaYih (Sun et al., 2014),andLarimichthyscrocea(Yan et al., 2017) have been cloned and expressed. To study the roles and molecular evolution of f ishCAT, characterization ofCATfrom a diverse range of species is required.
Turbot (Scophthalmusmaximus), a large-sized demersal f ish naturally distributed in the coasts of Europe at Northeast Atlantic is known for its rapid growing speed and low-temperature resistance (Qin et al., 2008). Turbot has been bred with an increasing scale since its introduction to China in 1992. However,vibriosis, especially the infection caused byVibrioanguillarumhas caused tremendous economic loss to the turbot breeding industry. A study has conf irmed that the pathogenicity ofV.anguillarumis related to various internal virulence factors, such as extracellular protease, lipopolysaccharide, f lagella, and hemolysin(Ge et al., 2014). Among these factors, extracellular protease, which is a zinc-containing metalloprotease has been shown to be associated with strong pathogenicity (Denkin and Nelson, 2004; Rock and Nelson, 2006). Extracellular metalloprotease has been considered as a pivotal virulence factor involved in the pathogenic mechanism ofV.anguillarum. Chen et al. (2009) have found that extracellular metalloprotease purif ied fromV.amguillarumcan induce tissue damage and death of the f ish. Chi (2006)has conf irmed that extracellular metalloprotease ofV.anguillarumis obviously toxic to f lounder gill cells by inducing apoptotic corpuscles and cell disaggregation. Extracellular metalloprotease isolated and purif ied fromV.anguillarumhas been found to be lethal to mice (Inamura et al., 1985). The above studies imply that extracellular metalloprotease of pathogens can generate toxicity and pathogenicity to organisms. The protease may attack the defense system of the host organism to induce tissue damage and create conditions that facilitate infection of the pathogen (Wei et al., 2002; Chen, 2003). To date,there is no data about the eff ects of metalloprotease on the antioxidative status and ROS index, as well as on apoptosis in the head kidney cells of turbot.
The aim of this study was to clone full-length cDNA encodingCATfrom the liver ofS.maximusand compare its sequence with other knownCATs.Then we wished to: clarify the expression prof ile ofS.maximuscatalase (SmCAT) in various tissues fromS.maximus, analyze the expression prof iles of manganese superoxide dismutase(MnSOD),CAT,andGPxin head kidney cells ofS.maximusafter metalloprotease treatment, measure ROS and mitochondrial membrane potential from head kidney cells, and f inally evaluate apoptosis in these cells by f luorescent microscopy. These results provide a systematic method to understand the mechanism of metalloprotease-induced toxicity, especially apoptosis,in the head kidney cells ofS.maximus.
2 MATERIAL AND METHOD
2.1 Animals and isolation of head kidney cells
Turbots weighing about 45 g were obtained from a local farm in Changli, China. The turbots, ten per 160-L breeding tank, were raised in aerated clean seawater with 19 salinity and >6.0-mg/L dissolved O2at 16±1 °C.
Turbot primary head kidney cells were cultured as previously described (Wang et al., 2010; Bain and Schuller, 2012) but with slight modif ication. Brief ly,the f ish were anesthetized with clove oil and surfacedisinfected with 75% ethanol, following which the head kidney tissues were collected and transferred to a clean bench. The kidney tissues were rinsed several times with phosphate buff er solution (pH 7.4), and added with 400-U/mL penicillin and 400-μg/mL streptomycin before being cut with scissors. The tissues were then placed in 15-mL centrifugal tubes where 10 volumes of 0.25% parenzyme were added for digestion for 30 min at room temperature.Trypsinization was terminated using 2 mL of L-15 complete medium (200-U/mL penicillin, 200-μg/mL streptomycin, 20% fetal bovine serum, 100-ng/mL beta f ibroblast growth factor). After f iltration using a50-mesh f ilter screen, the resulting cell suspension was transferred into 15-mL centrifuge tubes for centrifugation at 1 600 r/min for 5 min. After discarding the supernatant, the cells were adjusted to a concentration of 5×105cells/mL before being inoculated on cell culture plates and cultured in a CO2incubator at 25 °C. The L-15 complete medium of the cell culture was semi-exchanged at an interval of 2-3 d.
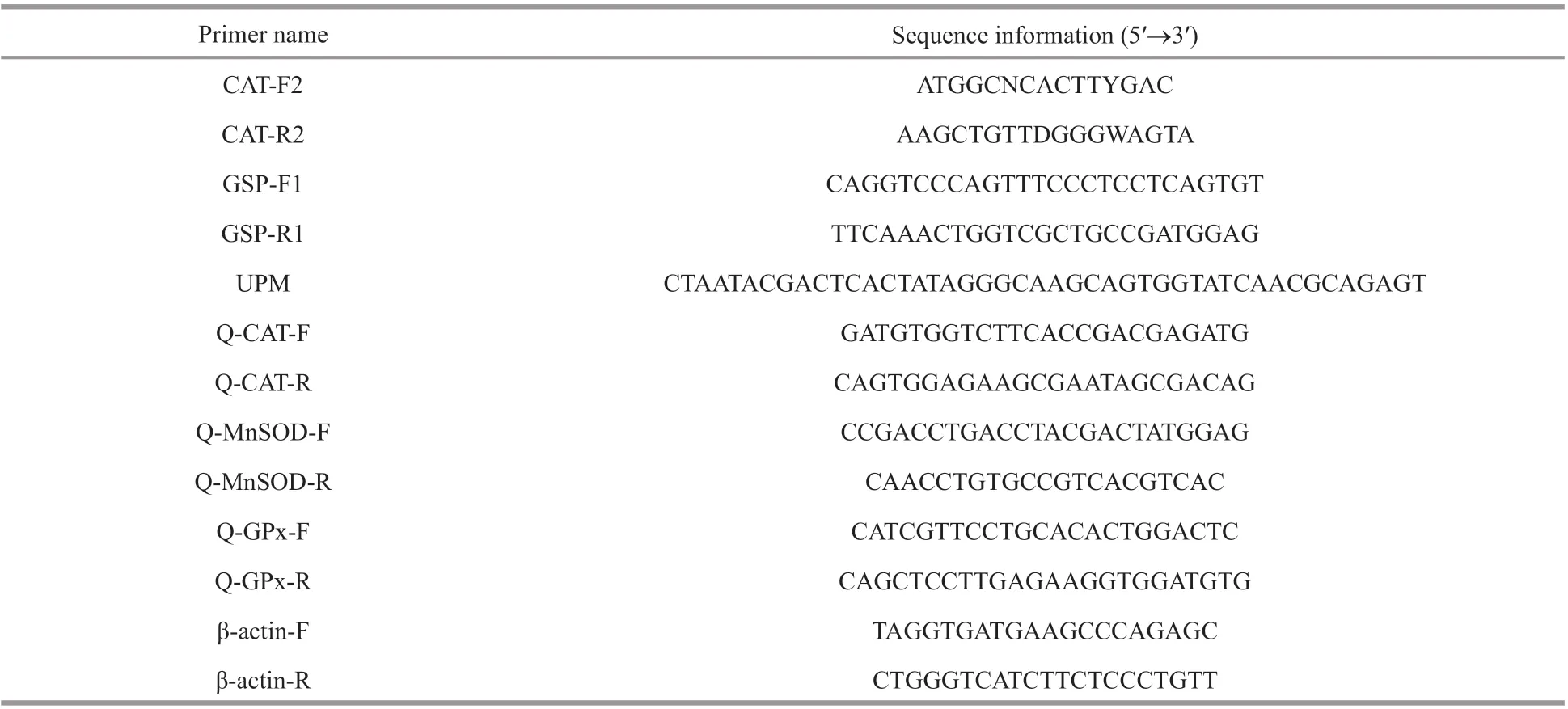
Table 1 Primers used in this study
2.2 Total RNA isolation and cDNA preparation
Total liver RNA was immediately isolated from freshly harvested cells using an RNAiso Plus device(TaKaRa, Japan). The quantity and quality of isolated RNA were evaluated using a ND-1000UV spectrophotometer (NanoDrop, USA) and 1.0%agarose electrophoresis, respectively. The OD260/OD280 ratio of isolated RNA varied from 1.8 to 2.0.First-strand cDNA was synthesized using M-MLV reverse transcriptase (TaKaRa). The 5′ and 3′ rapid amplif ication of cDNA ends (RACE) cDNA templates were obtained using a SMARTTMcDNA kit (TaKaRa).All procedures were conducted as recommended by the manufacturers.
2.3 Cloning of S. maximus CAT
Full-length cDNA ofSmCATwas obtained by reverse-transcription polymerase chain reaction (RTPCR) and RACE. Conserved regions in the central fragment ofSmCATcDNA sequence were detected with two degenerate primers CAT-F2 and CAT-R2(Table 1) and by sequence alignment with the corresponding genes ofO.fasciatus,Rachycentroncanadum,S.schlegelii,Sinipercachuatsi,Sparusaurata, andTotoabamacdonaldi. The following thermal cycling condition was used: initial denaturation at 94 °C for 5 min, followed by 33 cycles of denaturation at 94 °C for 35 s, annealing at 57 °C for 35 s and elongation at 72 °C for 70 s, and f inal extension at 72 °C for 8 min before conservation at 4 °C. Finally,CATcDNA with a length of about 1 046 bp was amplif ied.
Based on the partial sequence ofCAT, the 5′ and 3′ends of the above fragment sequence ofSmCATwere acquired using SMARTTMRACE cDNA kit(Clontech). The amplif ication reactions for both 5′and 3′ ends involved the use of RACE cDNA templates from liver RNA, GSP-R1, and universal anchor primer (UPM, Table 1). The PCR was conducted as recommended by Clontech.
Amplif ied products were analyzed on 1% agarose gel, in which the DNA fragment of interest was excised and purif ied using gel isolation kit (TaKaRa).Purif ied DNA was then subcloned into pMD18-T cloning vector as per manufacturer’s introduction.The resulting DNA construct was used forEscherichiacoliDH5α transformation. Positive clones containing the DNA insert were screened by PCR before being verif ied by DNA sequencing (Sangon Biotech.Co. Ltd., Beijing, China).
2.4 Sequence analyses
Comparative investigation of nucleotide and estimated amino acid sequences ofSmCATcDNA were analyzed using BLAST (www.blast.ncbi.nlm.nih.gov/Blast). Translation and protein analysis were performed using ExPASy tools (//us.expasy.org/tools/). Prediction of signal peptide was carried out using Signal P-4.1 (www.cbs.dtu.dk/services/SignalP/). Multi-alignment of turbot CAT was executed using DNAMAN. Phylogenetic tree of CAT was conducted by neighbor-joining on MEGA 4.0.Statistical support for reliability was tested using 1 000 bootstrap replications (Tamura et al., 2007).
2.5 Diff erential expression prof iles of S mCAT by qRT-PCR
To detect the tissue distributions ofSmCATexpression in healthy turbots, total RNA from healthyS.maximustissues (blood, gill, kidney, liver, stomach,muscle, heart and intestine) was reverse transcribed into cDNA using gDNA Eraser (TaKaRa, China) in accordance to the manufacturer’s recommended protocol. The expression levels ofCATmRNA transcript in each tissue were evaluated by quantitative RT-PCR (qRT-PCR). The PCR primers ofSmCAT(Q-CAT-F and Q-CAT-R) were chosen based on analysis by Primer Premier 5.0. The expression of β-actin was used as internal housekeeping control(GenBank accession number: AY008305). The primer sequences of β-actin-F and -R (Table 1) were based on a previously published study (Yun et al., 2012).
2.6 Expression of antioxidant genes after metalloprotease challenge
Extracellular metalloprotease ofV.anguillarumwas obtained as previously described (Yang et al.,2007). The protein concentration was quantif ied using Bradford method with bovine serum albumin as the reference.
Cells collected from cultivation f lasks were subjected for qRT-PCR analysis using SYBR premix Ex Taq™ (TaKaRa, China) with SYBR green I in a CFX96 device (Bio-Rad, US).S.maximushead kidney cells, after being cultured for 3, 6, 12, or 24 h,were sampled using 0-, 1.6-, 8.0-, or 40.0-μg/mL metalloprotease, following which the expression levels ofMnSOD,CAT, andGPxwere examined. The sequence-pecif ic primers forSmMnSOD(Q-MnSOD-F and Q-MnSOD-R),SmCAT, andSmGpx(Q-GPx-F and Q-GPx-R) used in qRT-PCR were based on analysis using Primer Premier 5.0 (Table 1).
Each qRT-PCR reaction system (10 μL), performed in triplicates, comprised SYBR Premix Ex TaqTMII(TaKaRa; 5 μL), RT solution (1 μL), each primer(10 μmol/L, 0.2 μL) and ultrapure water (3.6 μL). The thermal cycling conditions were performed as per recommended by the manufacturer. The threshold cycle value (Ct) of each PCR thermal prof ile estimated on CFX96™ real-time PCR 1.0 was used to calculate ΔCtof each sample. The relative expression ofSmMnSOD,SmCAT, andSmGPxwere determined by 2-ΔΔCt(Livak and Schmittgen, 2001).
2.7 Detection of ROS levels
The levels of ROS were detected using 2′,7′-dichlorof luorescein-diacetate (DCFH-DA,Beyotime, China) using the manufacture’s recommended protocol. Brief ly, head kidney cells cultivated in 96-well plates (5×105cells/well) were treated with 40-μg/mL metalloprotease. At diff erent time points after metalloprotease treatment, the cells were cultivated with 10-mmol/L DCFH-DA at 25 °C for 20 min in darkness, and then washed 2 times with PBS and f iltrated through a 200-mesh nylon mesh.The f luorescent signal intensity of DCF was analyzed by a f low cytometer (Becton Dickinson, US).
2.8 Measurement of mitochondrial membrane potential (Δ ψ m)
Variations in Δψmwere assessed using JC-1 kit(Beyotime, China) in accordance to the protocol recommended by the manufacturer. Brief ly, cells cultured in 96-well plates were treated with metalloprotease. After diff erent time points, the Δψmof metalloprotease-treated cells was measured in triplicates. Prior to Δψmmeasurement, cells were rinsed with PBS, collected and incubated with the JC-1 staining solution (1×) for 20 min at 25 °C. Cells were then rinsed with JC-1 buff er two times before being analyzed by f low cytometry. The Δψmof each test group was computed as the ratio of red (aggregates)to green (monomers) f luorescence signals as previously described (Cui et al., 2011).
2.9 Hoechst 33342 assay
To distinguish normal cells from apoptotic cells at 12 and 24 h, cells from the normal and metalloproteasetreated groups (8.0 and 40.0 μg/mL) were stained with 5.0-μg/mL Hoechst 33342 (Beyotime) for 20 min at 25 °C according to the protocol recommended by the manufacturer. Cells were then washed in PBS twice. The morphological changes in the cell nucleus were observed under a f luorescence microscope (Nikon, Japan) to distinguish between the condensed chromatin of apoptotic cells and the looser chromatin of living cells.
2.10 Statistical analyses

Fig.1 Nucleotide sequence and deduced amino acid sequence of SmCAT cDNA
All experimental data are shown as mean ± standard deviation (n=3). The data of qRT-PCR were analyzed by one-way analysis of variance (ANOVA). Diff erent treatments were compared using multiple-comparison(Tukey) on SPSS 20.0.P<0.05 implies statistical signif icance.
3 RESULT
3.1 Characterization and bioinformatic analysis of SmCAT sequence
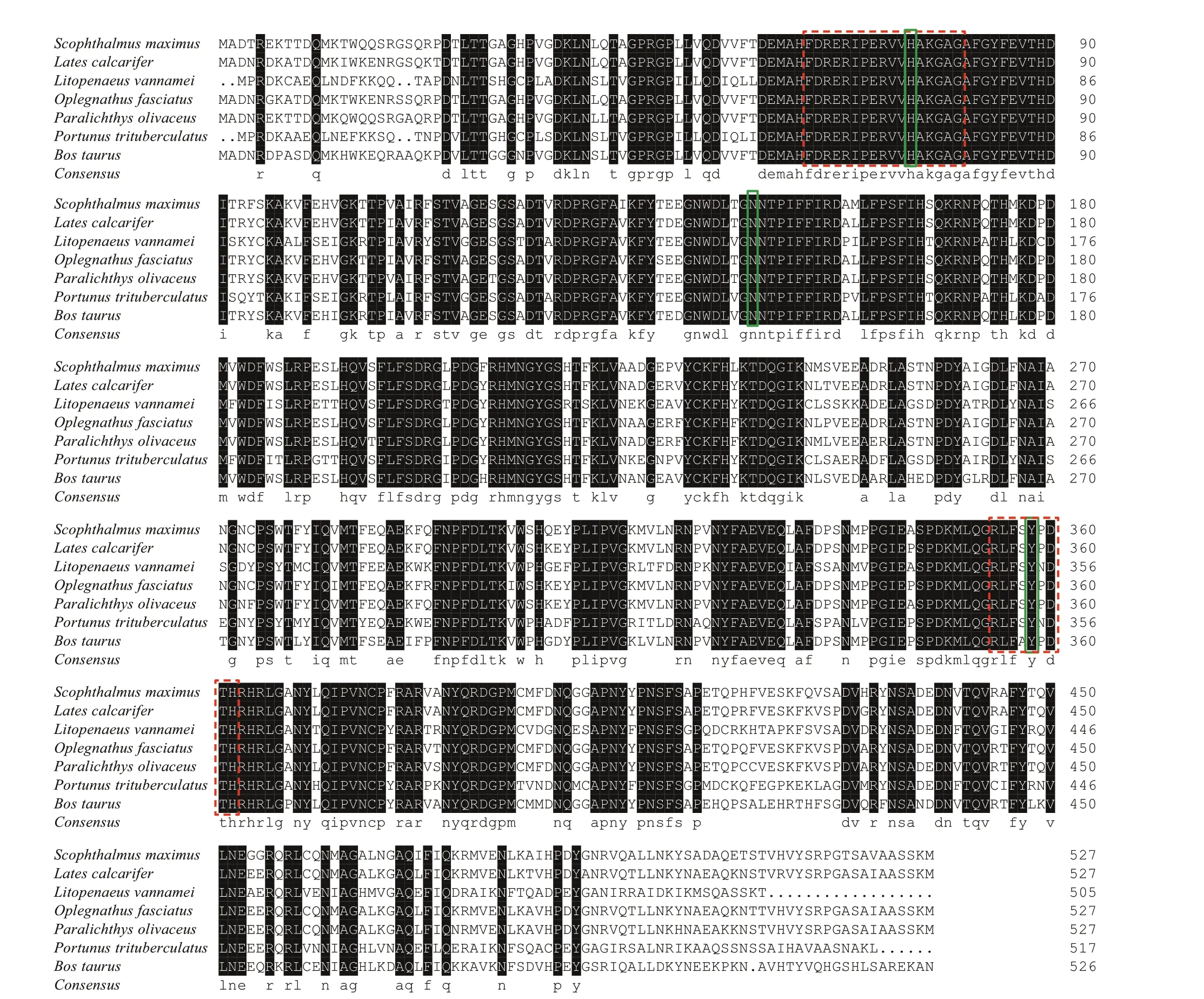
Fig.2 Multiple alignment of deduced amino acid sequence of SmCAT and other known CATs: L. calcarifer (XP018531423),L. vannamei (AAR99908), O. fasciatus (AAU44617), P. olivaceus (BAJ79013), P. trituberculatus (ACI13850), and B.taurus (DAA21837)
The full-lengthSmCATcDNA obtained by RACE (Fig.1)was submitted to GenBank database (No. MG253621).The 3 174-bpSmCATcDNA with a poly(A) end contains a 1 584-bp open reading frame (ORF) that encodes a 527-amino-acid protein (Fig.1) with a molecular mass of 59.44 kDa and a theoretical isoelectric point of 6.83. Analysis by PROSITE revealed that theSmCATamino acid sequence contains a proximal active-site signature sequence(64FDRERIPERVVHAKGAG80), a proximal hemeligand signature sequence (354RLFSYPDTH362), and three conserved catalytic amino acid residues (His75,Asn148, and Tyr358).
3.2 Homology and phylogeny analyses of SmCAT
Multiple sequence alignment showed that the deduced sequence ofSmCATwas highly similar with CAT ofParalichthysolivaceus(92.4%),Latescalcarifer(91.8%),O.fasciatus(91.3%),Rachycentroncanadum(91.3%),Serioladumerili(92.0%),Bostaurus(77.8%),Portunustrituberculatus(67.6%), andLitopenaeus vannamei(66.9%). The signature sequences(64FDRERIPERVVHAKGAG80and354RLFSYPDTH362)and catalytic residues (His75, Asn148, and Tyr358) were found to be largely conserved in diverse species (Fig.2).
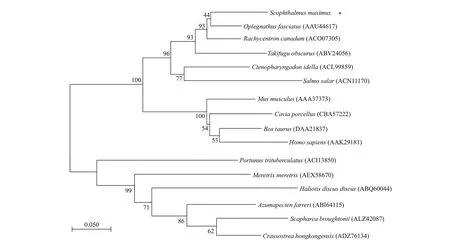
Fig.3 Phylogenetic tree of amino acid sequences of CAT from diff erent species
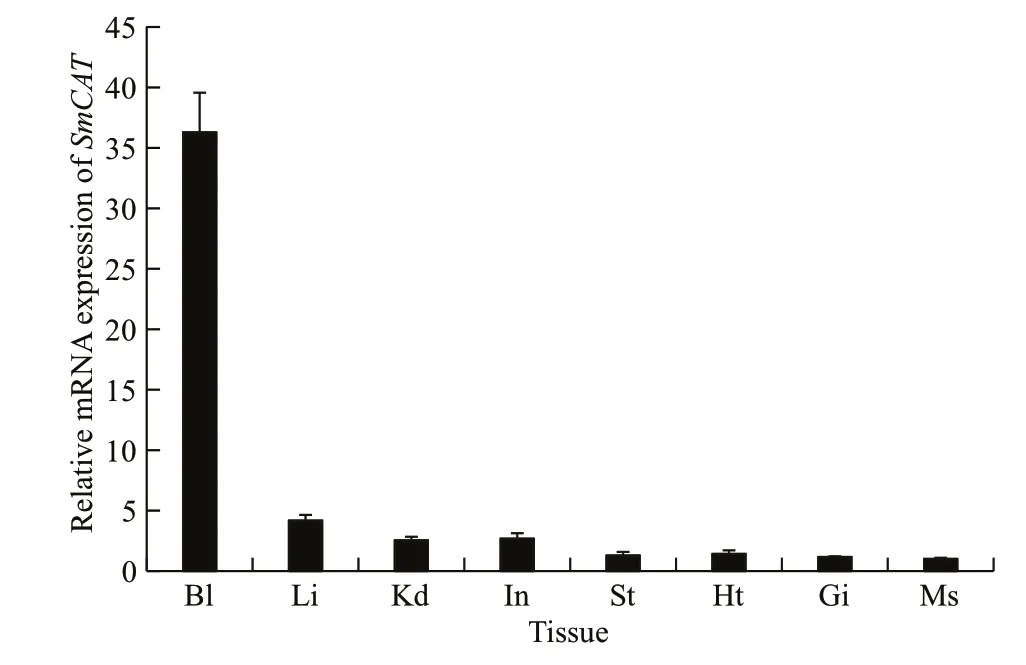
Fig.4 Relative SmCAT mRNA transcript levels in eight tissue types of healthy turbot determined by qRT-PCR
The identity and phylogeny of SmCAT were further validated using phylogenetic tree built on MEGA 4.0 with selected CAT sequences (Fig.3). TheCATgene family can be broadly separated into invertebrates and vertebrates. SmCAT was found to be highly related to the CATs of some f ish species, but less related to the CATs ofHomosapiens,Caviaporcellus,B.taurus, andMusmusculus.
3.3 Basal tissue expression of SmCAT mRNA
The mRNA expression ofSmCATin eight tissue types of healthy turbot, including blood, liver, kidney,intestine, stomach, heart, gill, and muscle was evaluated (Fig.4). Our results show thatCATwas ubiquitously expressed in all tested tissues. The dissociation curves ofSmCATand β- actin only showed one peak, indicating that the amplif ications were specif ic.SmCATwas found to have the lowest expression level in muscle tissues and the highest expression level in blood. The expression ofSmCATwas observed to be moderate in other tissue types.
3.4 Eff ects of metalloprotease on the expression of antioxidant genes
Temporal expression of antioxidant genes(SmMnSOD,SmCAT, andSmGPx) in head kidney cells at designated time points after metalloprotease treatment are displayed in Fig.5. Whilst the expression ofSmMnSODin diff erent concentration groups were unchanged at 3 h after treatment(P>0.05); the expressions ofSmCATandSmGPxwere dramatically higher (P<0.05) than that of the control group. The gene expression ofSmMnSOD,SmCAT, andSmGPxin cells treated with metalloprotease at all tested concentrations from 12 to 24 h were found to be dramatically downregulation. Hence, metalloprotease induces the expression ofSmMnSOD,SmCAT, andSmGPxin head kidney cells at diff erent levels within the 24-h exposure.
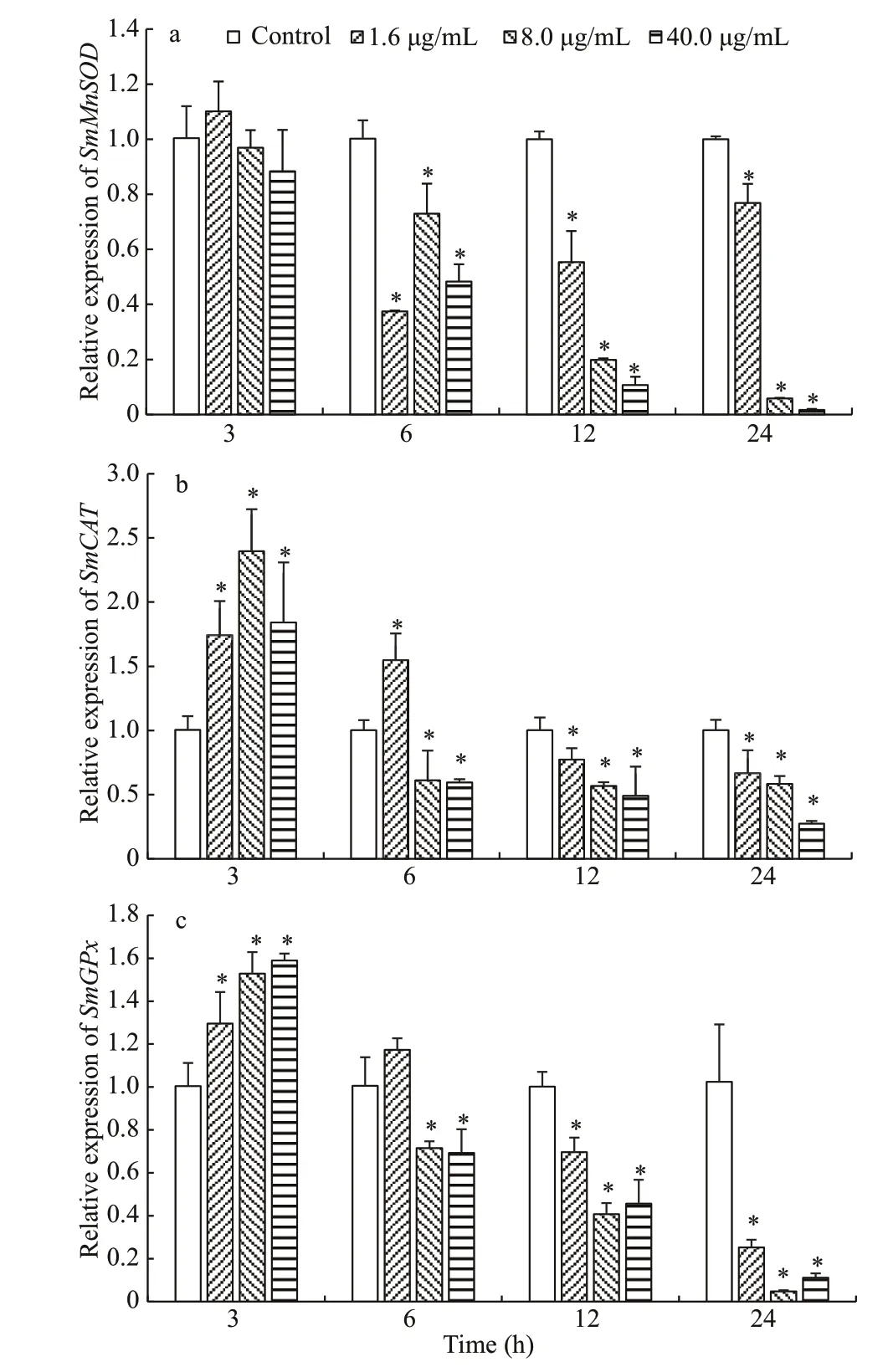
Fig.5 Expression of SmMnSOD (a), SmCAT (b), and SmGPx(c) after metalloprotease treatment, determined by qRT-PCR
3.5 Eff ects of metalloprotease exposure on ROS level
ROS production in head kidney cells after treatment with metalloprotease is shown in Fig.6. Flow cytometry indicated that the accumulation of ROS is higher in metalloprotease-treated cells relative compared to that of untreated cells (Figs.6-7). ROS production in head kidney cells, compared to that of the control group, was found to be increased signif icantly in all treatment groups (P<0.05), except for the 3-h treatment group.
3.6 Eff ects of metalloprotease exposure on mitochondrial membrane potential (Δ ψ m)
To explore the participation of mitochondriamodulated pathway in metalloprotease-induced apoptosis, the Δψmin head kidney cells was measured through f low cytometry and JC-1 staining. Our results showed that metalloprotease dose-dependently caused the loss of Δψm, which dropped considerably from 6 to 24 h compared to that of the control group(Fig.8)
3.7 Eff ects of metalloprotease treatment on cell morphology
Next, the morphology of metalloprotease-induced head kidney cells was visualized by f luorescent microscopy. As shown in Fig.9a & d, untreated cells were stained uniformly with blue f luorescence,suggesting that the chromatin was evenly distributed in the nucleolus, implying a normal healthy shape.Conversely, as shown in Fig.9b, c, e, & f, cells after incubation with 8.0- or 40.0-μg/mL metalloprotease for 12 and 24 h displayed condensed chromatin and shrunken nucleus to some extent. The number of Hoechst-positive cells was increased, depending on metalloprotease treatment time and dosage, compared to that of untreated cells.
4 DISCUSSION
As a major prevalent bacterial pathogen,V.anguillarumhas been reported as the cause of severe infections and high mortality in many marine and freshwater f ishes (Planas et al., 2005; Toranzo et al., 2005). Extracellular metalloprotease, a critical virulence index ofV.anguillarumhas been shown to be lethal to a number of f ish species (Varina et al.,2008; Han et al., 2011). To our knowledge, there is currently no report about the eff ects ofV.anguillarumextracellular metalloprotease on the expression of antioxidant genes and on cell apoptosis in turbots.To better understand the antioxidative resistance of turbots in response to the virulence factor, we cloned the cDNA ofSmCAT, and then investigated the expression of antioxidant enzymes (SmMnSOD,SmCAT, andSmGPx), ROS level, Δψm, and apoptosis in the head kidney cells ofS.maximusunder metalloprotease stress.
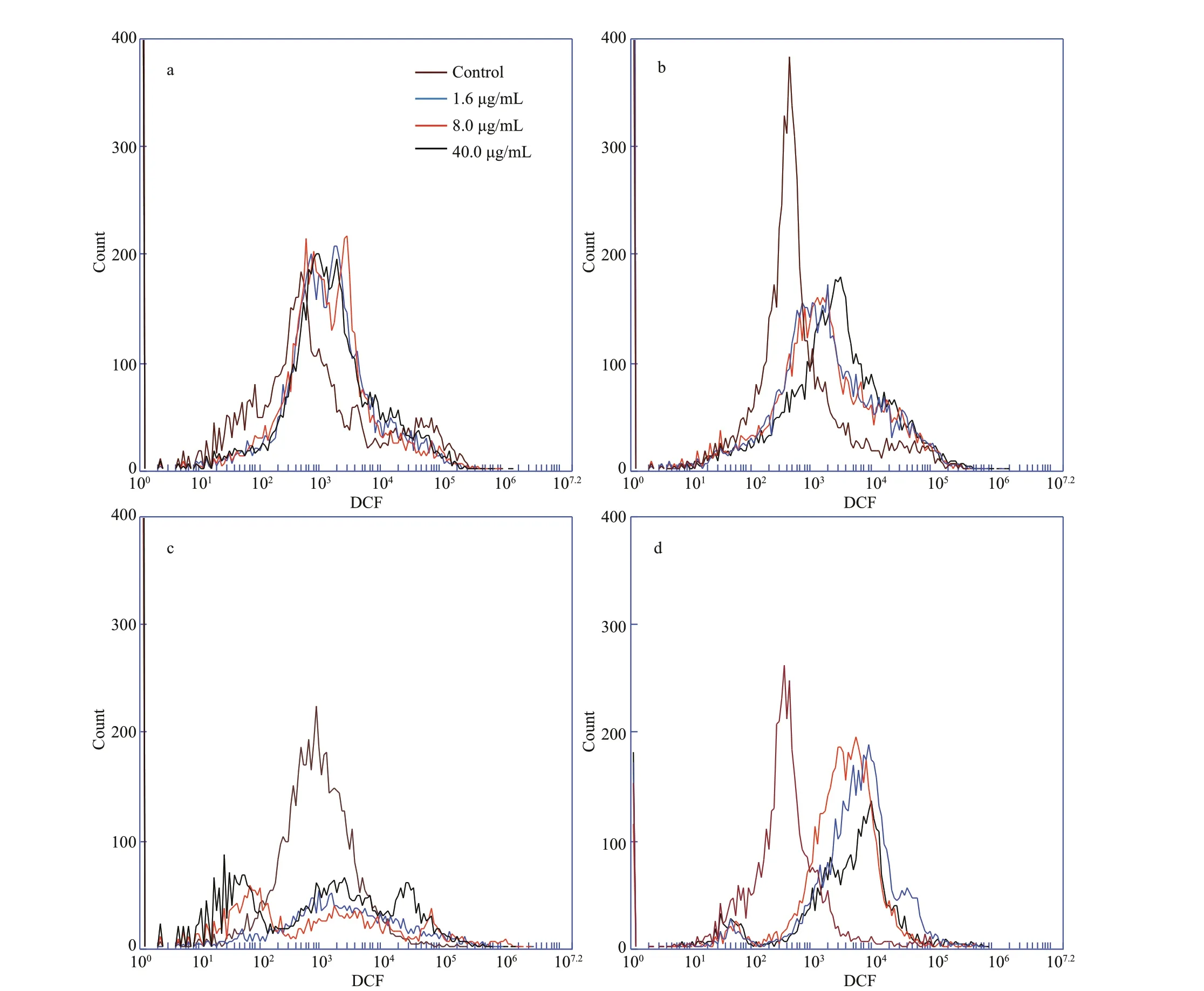
Fig.6 Metalloprotease treatment causes ROS accumulation in head kidney cells
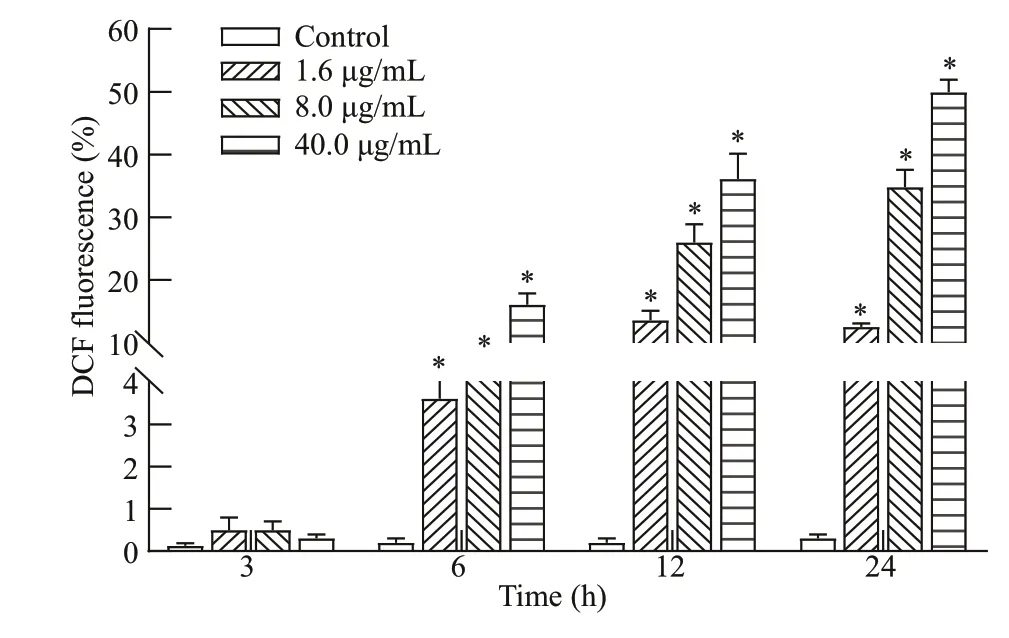
Fig.7 Fluorescence intensities of DCF in metalloproteasetreated or untreated cells.
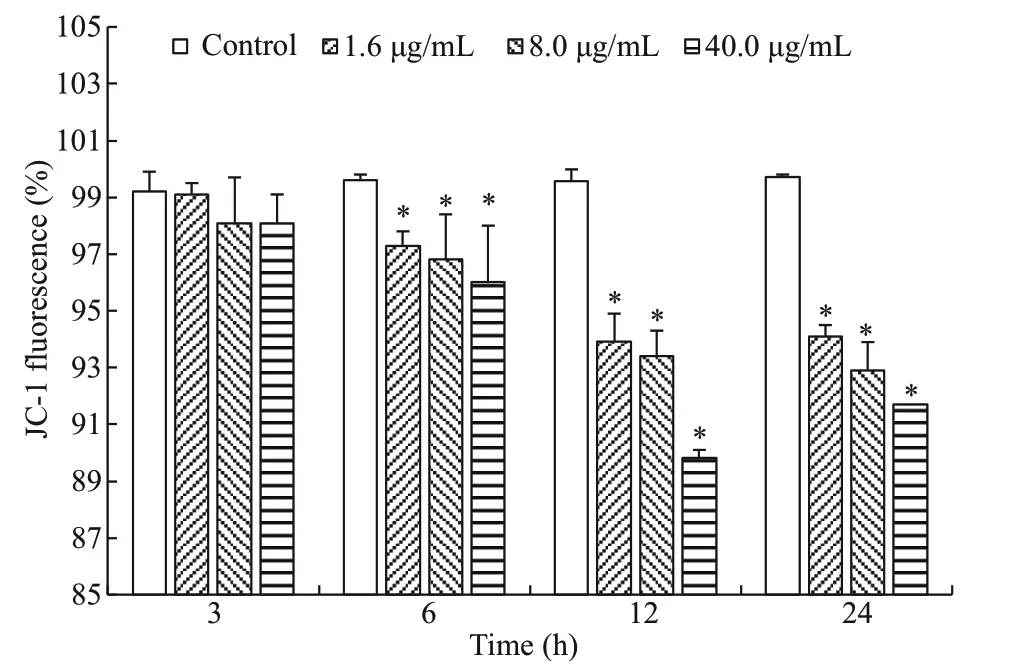
Fig.8 Metalloprotease causes the loss of Δ ψ m, measured by f low cytometry
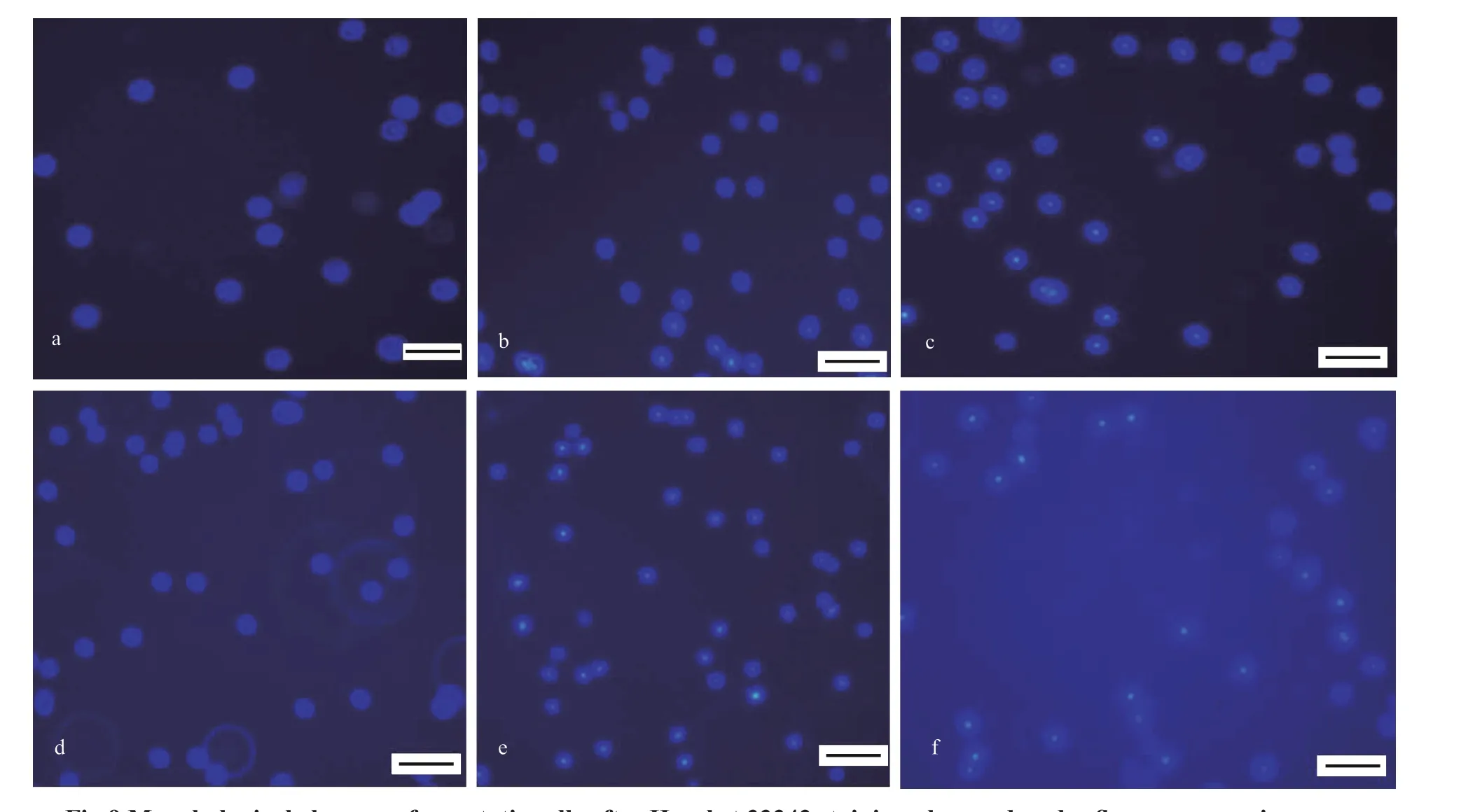
Fig.9 Morphological changes of apoptotic cells after Hoechst 33342 staining observed under f luorescence microscopy
In this study, we found that the SmCAT amino acid sequence contained a proximal heme ligand-binding site (354RLFSYPDTH362), an active-site signature sequence (64FDRERIPERVVHAKGAG80) and three conserved catalytic amino acid residues (His75, Asn148,and Tyr358). The catalytic and peroxidase activities of CATs may rely on a charge-relay network to stabilize the reactive intermediates in order to repair the resulting damage by enhancing the peroxide cleavage(Sellaththurai et al., 2019). Residues His75, Asn148, and Tyr358that are pivotal in the charge-relay network have been reported as the basic residues required to complete the enzymatic activity (Heck et al., 2010).Our phylogenetic analysis further illustrates the existence of two extensive categories of CATs(vertebrates and invertebrates) and SmCAT that was found to be clustered into the vertebrate category was most closely related to the enzyme fromO.fasciatus.The observed relationships within CAT homologues indicate that SmCAT may be evolutionally distant from CATs of invertebrates.
The evaluation of tissue expression distribution showed thatSmCATis expressed in all tissue types examined. The basal expression level ofSmCATwas found the highest in blood followed by liver, mainly due to the diff erent metabolic activities associated with ROS production among these tissues(Ekanayake et al., 2008). Blood cells and phagocytes are critical in removing pathogen invasion through oxygen consumption to form ROS (Lee and Söderhäll, 2002). The liver is also a site where various oxidative reactions occur (Gül et al., 2004;Avci et al., 2005), hence, liver cells are prone to oxidative stress due to the high metabolic activity(Kamata et al., 2005). It has been suggested that blood and liver tissues are important for modulating redox homeostasis in turbots, and thatCATmay play an essential role in detoxifying oxygen-free radicals and avoiding their overproduction in organisms(Tavares-Sánchez et al., 2004). Additionally,diff erentially expressedSmCATmRNA transcripts among various turbot tissues may be associated with the oxidative levels of those tissues (Sellaththurai et al., 2019).
Antioxidative resistance systems are critical in preventing oxidative damage due to excess ROS(Kim et al., 2012). As the major constituents of antioxidative defense mechanism,SOD,CAT, andGPxcan orchestrate cell defense against oxidative stress-induced cell injury, prevent peroxidation and balance cellular redox status (Lortz et al., 2000). In this study, the expression ofSmCATandSmGPxin cells at 3 h after exposure to diff erent concentrations of metalloprotease dramatically surpassed that of untreated cells, indicating that the expression of antioxidant enzymes can be induced by metalloprotease. The sharp increase in the mRNA transcript levels ofSmCATandSmGPxat 3 h may be an adaptive response known as hormesis by Stebbing(1982), that is induced to counteract ROS toxicity and production (Woo et al., 2009). Similar observations have been reported inL.vannamei(Qian et al., 2014)andCrassostreagigas(Jo et al., 2008). With prolonged metalloprotease exposure time, the gene expression ofSmMnSOD,SmCAT, andSmGPxin cells were found to be dramatically declined from 12 to 24 h. A possible interpretation is that the formation of excessive radicals may have surpassed the neutralizing ability of antioxidant enzymes, therefore causing oxidative destruction that damages normal cell functions (Jo et al., 2008; Ren et al., 2015). The induction or inhibition of antioxidant enzyme expression is also associated with the degree of metalloprotease-induced oxidative injuries in head kidney cells. However, to better understand the role of antioxidant enzymes in the prevention of free radical production, or reactive species production initiated by metalloprotease, future studies looking at the mRNA and protein levels of MnSOD, CAT, and GPx in order to off set metalloprotease activity are required.
In f ish tissues, although the antioxidation defense mechanisms are capable of modulating the production and removal of cellular ROS, but the dynamic balance of ROS can be altered by a number of stress factors. In this study, the ROS levels in head kidney cells were found to be signif icantly increased in all metalloprotease treatment groups from 6 to 24 h, indicating that excessive ROS can be induced through metalloprotease exposure. This suggests a possible mechanism for metalloproteaseinduced toxicity via mitochondria dysfunction that causes increased ROS levels. Additionally, the expression of antioxidant enzymes (MnSOD,CAT,andGPx) were shown to be completely inhibited at 12 to 24 h, probably due to the generation of excessive metalloproteinase-induced ROS.
Mitochondria have been suggested as the key organelle that modulate apoptosis (Desagher and Martinou, 2000). ROS, as a byproduct of cell metabolism, is mainly produced in the mitochondria,causing damages to mitochondrial components(Martindale and Holbrook, 2002). Mitochondria are highly sensitive to oxidative stress, and can become dysfunctional under excessive ROS in many cell types (Lee et al., 2009; Guo et al., 2013). Changes in Δψmis typically observed in apoptotic cells under many stimuli. In this study, a signif icant drop in Δψmwas found in all metalloprotease-treated groups from 6 to 24 h, indicating that metalloprotease can decrease Δψmto initiate mitochondrial injuries in head kidney cells, probably caused by excessive ROS generation.Previous studies have indicated that arsenic trioxide and enrof loxacin can promote the alteration of Δψmand elevate the production of ROS in f ish cells(Selvaraj et al., 2013; Cui, 2014).
Extracellular metalloprotease is a critical virulent factor of pathogens that can cause death in turbot and f lounder when injected intraperitoneally (Mo et al., 2002; Varina et al., 2008; Han et al., 2011). A previous study has conf irmed that the metalloproteinase ofV.anguillarumis toxic and can cause apoptosis in gill cells (Chi, 2006). Staining using Hoechst 33342 has been extensively applied in genotoxicity test in vitro to evaluate nuclear fragmentation and condensation due to diff erent stress factors (Speit and Rothfuss, 2012). In this study, Hoechst 33342 f luorescent staining of head kidney cells treated with 8.0- or 40.0-μg/mL metalloprotease for 12 or 24 h showed that the cells were apoptotic to diff erent extents after diff erent treatments. Moreover, our results from the evaluation of cell apoptosis rate and Caspase 3 gene expression demonstrated that cells undergo apoptosis at diff erent levels under the tested metalloprotease concentrations and exposure time (data not shown). Taken together,these f indings suggest that extracellular metalloproteases may inhibit expression of antioxidant genes in vivo, induce oxidative damage,and ultimately cell apoptosis.
5 CONCLUSION
The full-length cDNA ofSmCATthat belongs to the conserved CAT family was cloned the f irst time.With the highest level of expression in blood,SmCATwas found to be extensively expressed in all tested tissue types. The expression ofSmCAT,SmMnSOD,andSmGPxin head kidney cells were observed to be signif icantly inhibited in response to metalloprotease treatment from 12 to 24 h. Metalloprotease can cause mitochondrial damage in head kidney cells by lowering the Δψmand by increasing the production of ROS. Hoechst 33342 staining showed metalloprotease can cause apoptosis in head kidney cells. To our knowledge, this is the f irst study that demonstrates the toxic eff ects of metalloprotease in the inhibition of antioxidant enzyme expression and in the initiation of apoptosis and oxidative stress in living cells.Moreover, we show that the increase of metalloprotease-induced intracellular ROS levels is an essential component that triggers the apoptosis of head kidney cells by inhibiting the expression of antioxidant enzymes.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- Spatial diff erence in net growth rate of Yesso scallop Patinopecten yessoensis revealed by an aquaculture ecosystem model*
- Bacterial community composition in gut content and ambient sediment of two tropical wild sea cucumbers ( Holothuria atra and H. leucospilota)*
- Comparative transcriptomic analysis of Macrobrachium nippon ense in response to Aerom onas veronii or Staphylococcus au reus infection*
- Dietary intake of bamboo vinegar and charcoal powder (BVC)enhances resistance of African catf ish Clarias gariepinus to bacterial pathogen*
- Taxonomy and regeneration of a newly recorded Polychaete Capitella teleta (Annelida, Capitellidae) in the coastal water of Shandong, China*
- Five new records of Xanthidae (Crustacea: Brachyura) from Hainan Island, China*
