Five new records of Xanthidae (Crustacea: Brachyura) from Hainan Island, China*
Ziming YUAN , Zhongli SHA , Wei JIANG
1 Laboratory of Marine Organism Taxonomy and Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract Five new records of the family Xanthidae are reported from China: Hypocolpus haanii Rathbun,1909, Neoliomera variolosa (A. Milne-Edwards, 1873), Leptodius australis Ward, 1936, L. davaoensis Ward, 1941, and Xanthias joanneae Mendoza, 2013. The specimens were collected from Hainan Island and deposited in the Marine Biological Museum, Chinese Academy of Sciences. Diagnosis based on observation,photographs, line drawings, and some taxonomic discussions are provided.
Keyword: morphology; Xanthidae; new records; South China Sea; taxonomy
1 INTRODUCTION
Xanthidae MacLeay, 1838, is one of the most diverse families in the Brachyura and currently includes 15 subfamilies, 124 genera, and 639 species(Ng et al., 2008; Lai et al., 2011; Mendoza and Guinot,2011; Mendoza and Manuel-Santos, 2012; Mendoza et al., 2012). They are common shallow water fauna worldwide especially in tropical reef habitats of Indo-West Pacif ic region, and are abundant in areas with rock, shell, or coral rubble substrates (Serène, 1984).Some members of this family are dominant taxa and may play a signif icant ecological role in reef food webs, such as Chlorodiellinae crabs, which were 37%of the crustaceans found in f ive reef-associated f ish species guts in Moorea (Leray et al., 2012; Lasley et al., 2015). Recent systematic studies showed that Xanthidae is polyphyletic and most of the 15 subfamilies are also not monophyletic. The family clearly requires further systematic revision, and future studies will require greater taxon sampling and area coverage (Lai et al., 2011).
There are few systematic studies on the Xanthidae of Chinese waters, although they are frequently referred in surveys and studies of brachyuran species.Yang et al. (2008) provided a checklist of Brachyura from China seas, including 161 species of Xanthidae.Ng et al. (2017) provided a checklist of Brachyura from Taiwan Island, which contained 132 species of Xanthidae. To date 180 species from 61 genera and 11 subfamilies have been recorded from Chinese waters(Yang et al., 2008; Ng et al., 2017).
More systematic studies are necessary for Xanthidae from Chinese waters. In a recent collection,f ive new records of xanthid species were reported from Hainan Island, South China Sea. In this paper,their detailed diagnosis, photographs, line drawings,and taxonomic discussions are provided.
2 MATERIAL AND METHOD
All specimens were preserved in 70% Ethanol and deposited in the Marine Biological Museum, Chinese Academy of Sciences (MBMCAS) in Qingdao,China.
Specimens were observed by ZEISS Stemi 2000-c and ZEISS Stemi SV 11 Apo stereo microscope.Morphologies of G1 were observed by Nikon Eclipse Ci-L microscope. Photographs were taken by Canon EOS 6D camera with Canon EF 100-mm and Canon MP-E 65-mm lens.
Terminologies used for adult morphology follows that def ined by Dana (1852) and Serène (1984). The following abbreviations were used in the text: CW(maximum carapace width); CL (median carapace length); G1 (f irst gonopod of male); st1-8 (thoracic sternites 1-8); P1-5 (pereopods 1-5); 1-2F (frontal regions 1-2); 1-4M (medial regions 1-4); 1-6L(antero-lateral regions 1-6); 1-3R (postero-lateral regions 1-3); 1-2P (posterior regions 1-2).
3 TAXONOMY
Family Xanthidae MacLeay, 1838
Subfamily Euxanthinae Alcock, 1898
GenusHypocolpusRathbun, 1897
Hypocolpus haaniiRathbun, 1909
Cancer(Xantho)granulatusHaan, 1837, 65, Pl.18,Fig.3.
HypocolpushaaniiRathbun, 1909: 114; Mendoza and Ng, 2010: 62, Figs.2A, 3C, D.
HypocolpushaaniSakai, 1939: 457, 458, Fig.27,Pl. 89, Fig.1.
HypocolpusgranulatusGuinot-Dumortier, 1960:187, Pl.1, Fig.5, Pl.2, Fig.12, Pl.3, Fig.18, Pl.6,Fig.33, Pl.9, Figs.52, 53; Sakai, 1976: 417, Textf ig.220b, Pl.150, Fig.4; Serène, 1984: 76, 79, Fig.35.
Material examined
South China Sea: MBM286755, 1♂, Lingao Cape,Hainan, 15-30 m, Yunhao PAN coll., 20 August 2018;CW 45.27 mm, CL 34.23 mm.
Diagnosis: Carapace (Fig.1a) transversely oval,breadth about 1.3 times the length; dorsal surface regions distinctly def ined by wide, deep and smooth grooves; regions convex, covered with granules and interspersed with short setae, brush-like setae presenting on 1M, outer lobules of 2M, 3-5L, and 1P.Front produced, def lexed, divided into 2 lobes which touching each other on tips. Dorsal inner orbit raised,ventral inner orbital angle projecting. Subhepatic cavity (Figs.1b, c, & 2a) reniform, inner brim blunt and outer brim sharp, inner surface smooth.Anterolateral border divided into 3 lobes, the f irst lobe forming the dorsal brim of the subhepatic cavity,the second lobe broad, and the third lobe triangular.Posterolateral border shorter than anterolateral border,distinctly concave. Lateral surface of carapace clothed with setae. Antennule situated transversely; orbital hiatus f illed by basal antennal segment, f lagellum small. Third maxilliped (Figs.1c & 2b) completely covering buccal orif ice, covered with granules; merus subquadrate, anterolateral angle slightly produced;ischium subrectangular; ischium and merus with a smooth groove respectively. Thoracic sternites(Fig.1d) covered with dense and prominent granules;the groove between st1-2 and 3 deep, middle slightly convex backwards, st3 and st4 divided by a deep groove, the median line of st4 short and deep.
Chelipeds (Figs.1e, f, & 2c) stout and symmetrical,subhepatic cavity exposed completely when they are folded, interspersed with short setae; merus with long setae on edges; carpus very stout, with granules and tubercles on the dorsal surface, inner angle armed with a prominent tooth pointing to the dorsal; palm with large warty granules on outer surface, interspersed with clavate setae; dorsal surface smooth, inner edge cristate; f ingers def lexed slightly, dactylus with 2 rows longitudinal dorsal spiny granules, cutting edges of f ingers with 5 teeth respectively; f inger tips sharp, the tip of f ixed f inger inside when close.
Ambulatory legs (Fig.1a) short; merus with setae on dorsal surface and edges, granules on anterior edge and posterior dorsal surface; carpus and propodus with spiny granules; dactylus with curving setae, tip claw-shaped.
Pleonal somites (Fig.1d) 3-5 completely fused in male. G1 (Fig.2d & e) slender, tip blunt, with little spines, armed with 7 subdistal setae: 5 long plumose setae, 1 shorter and slender non-plumose seta, and a stick-like truncated seta, may be a broken plumose seta.
Distribution: Hainan Island; Philippine Islands(Mendoza and Ng, 2010), Gulf of Siam (Rathbun,1909), southeast coast of Japan (de Haan, 1833-1850;Sakai, 1939, 1976).
Type locality: Japan.
Remark:Hypocolpushaaniican be identif ied by its smooth, fusiform subhepatic cavity that does not have any depression, crests, or granules. The specimen from Hainan Island examined f its with previous descriptions (de Haan, 1833-1850; Sakai, 1939;Guinot-Dumortier, 1960), except there are 5 plumose setae in Guinot-Dumortier’s line drawing (Guinot-Dumortier, 1960), while 7 intact or broken setae in our specimen, one of which is shorter and nonplumose.H.haaniiis most related toH.rugosus(Henderson, 1893), on they have similar carapace regions (Guinot-Dumortier, 1960; Serène, 1984).H.rugosuscan be identif ied by the ovoid subhepatic cavity with a marked depression at the exterior of emarginate edge, and eroded thorax and abdomen with hollow and depressions (Serène, 1984). The only species ofHypocolpusrecorded from China Sea previously isHypocolpusabbotti(Rathbun, 1894),which has a more setose carapace and a subhepatic cavity with 2 longitudinal crests.
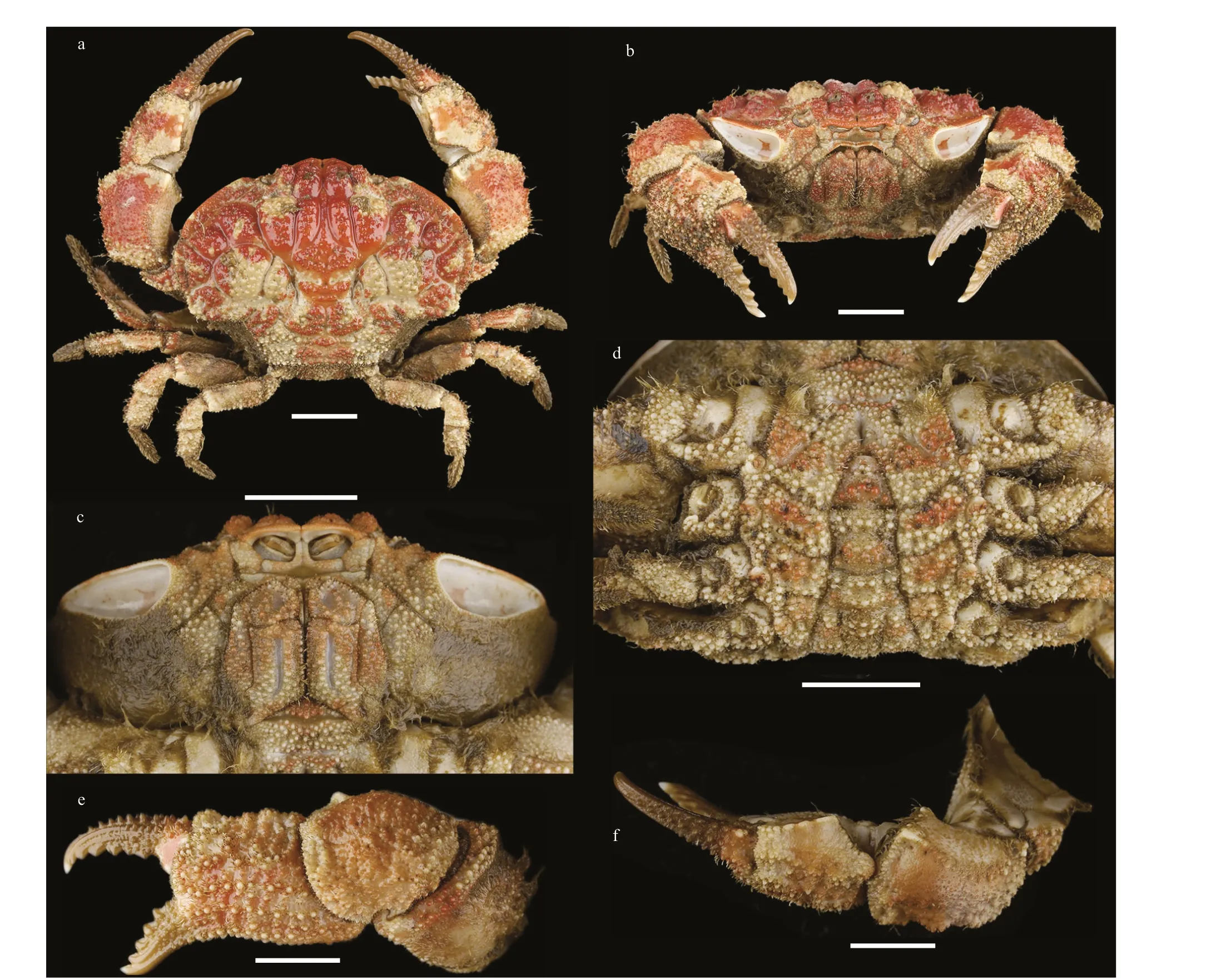
Fig.1 Hypocolpus haanii Rathbun, 1909, male, 45.27 mm×34.23 mm (MBM286755)
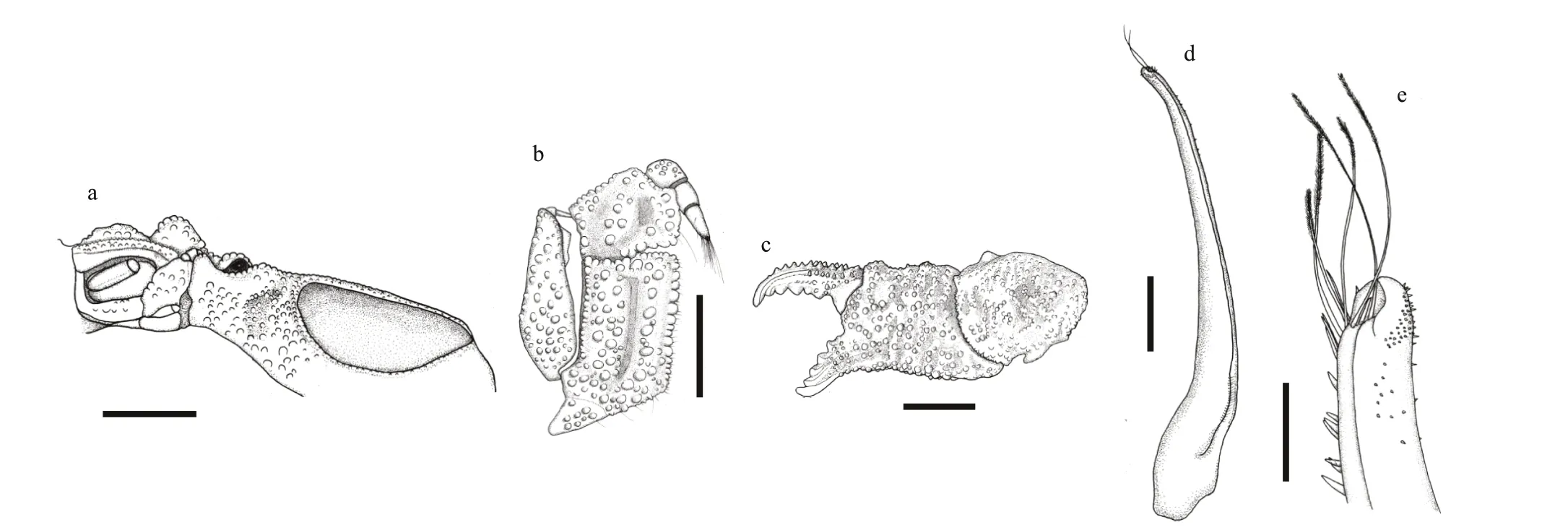
Fig.2 Hypocolpus haanii Rathbun, 1909, male, 45.27 mm×34.23 mm (MBM286755)
Subfamily Liomerinae Sakai, 1976
GenusNeoliomeraOdhner, 1925
Neoliomera variolosa(Milne-Edwards, 1873)
LiomeravariolosaA. Milne-Edwards, 1873: 255,Pl.1, Fig.5.
Neoliomeravariolosa Odhner, 1925: 30, Pl.2,Fig.10; Tweedie, 1949: 89; Poupin, 1996: 41.
Material examined
South China Sea: MBM286756, 1♂, Moon Bay,Wenchang, Hainan, 15-30 m, Yunhao PAN coll., 4 December 2018; CW 27.75 mm, CL 17.85 mm.
Diagnosis: Carapace (Figs.3a & 4a) transversely oval, breadth about 1.6 times the length; regions welldef ined anteriorly while vague posteriorly; dorsal surface covered with rounded granules, granules on sides larger then middle, interspersed with short setae and long yellow setae. Anterolateral border (Figs.3e& 4a) divided into 4 blunt lobes by deep grooves, the f irst lobe connecting outer orbit, the second lobe forming a small tubercle, hepatic region protruding forward and separated from the f irst two anterior lobes by a very deep and distinct groove; front def lexed, divided into 2 lobes. Posterolateral border about equal to anterolateral border. Antennule situated transversely; orbital hiatus f illed by antennal f lagellum. Third maxilliped (Fig.3c) completely covering buccal orif ice; merus subquadrate,anterolateral angle slightly produced; ischium subrectangular, with a smooth groove. Thoracic sternites (Fig.3d) smooth, interspersed with pits; the groove between st1-2 and 3 straight, the median line of st4 short and shallow.
Chelipeds (Figs.3f & 4b) symmetrical; merus covered with f lat granules; dorsal surface of carpus,dorsal and outer surface of palm covered with granules and setae similar to carapace; palm stout and short,outer surface covered with large granules, inner and ventral surface smooth; f ingers short and broad,dactylus with 2 longitudinal dorsal grooves, cutting surface of f ingers with 2 rows of f lat teeth; f inger tips blunt and concave.
Ambulatory legs (Figs.3a, b, & 4c) short and strong, clothed with granules, long yellow setae on edges of ambulatory legs. Dactylus with yellow setae,tip claw-shaped.
Pleonal somites (Fig.3d) 3-5 completely fused in male. G1 (Fig.4d & e) slender, with about 15 long plumose subdistal setae, 7 stick-like setae on the margin of the distal tip, should be residual proximal parts of truncated long plumose setae; apical lobe acute, slightly upturned.
Distribution: Hainan Island; Aur Island (Tweedie,1949), Upolu Island (Milne-Edwards, 1873), Moorea Island (Poupin, 1996).
Type locality: Upolu Island.
Remark:N.variolosawas originally described by Milne-Edwards (1873) from Upolu Island with a brief description and line drawing. Odhner (1925) provided a more detailed description of carapace and a more accurate drawing for this species. In addition to the type location, Poupin (1996) listed this species in French Polynesian fauna, distributed in Moorea Island, and Tweedie (1949) reported it from Aur Island based on 4 males and 2 females collected. The specimen from Hainan Island examined f it well with previous descriptions and line drawings (Milne-Edwards, 1873; Odhner, 1925). Color photograph and a description of G1 are provided here for the f irst time.
Neoliomeravariolosais most related toN.sabaea(Nobili, 1906) morphologically on both of them have a red and setose appearance (Nobili, 1906; Forest and Guinot, 1961; Galil and Vannini, 1990).N.variolosacan be identif ied by the deep and distinct groove in front of hepatic region, shallower and narrower carapace grooves, and the G1 with an upturned apical lobe, which is straight inN.sabaea(Forest and Guinot, 1961).
Subfamily Xanthinae MacLeay, 1838
GenusLeptodiusMilne-Edwards, 1863
Leptodius davaoensisWard, 1941
LeptodiusdavaoensisWard, 1941: 10, Figs.13, 14;Lee, 2012: 245-250, Figs.173-175.
Material examined
South China Sea: MBM286757, 1♂, Fengjiawan,Wenchang, Hainan, Junlong ZHANG coll., 15 November 2016; MBM286758, 2♀, Xiaodonghai,Hainan, Wei JIANG coll., 23 March 2008;MBM286759, 1♂, Xiaodonghai, Hainan, Wei JIANG coll., 25 December 2007; MBM282433, HBRC-3293, 3♂3♀, Xiaodonghai, Hainan, 24 December 2007; in MBM163925, 90C-887, Linchang, Hainan,1 December 1990; in MBM163922, 90C-472, 3♂,Luhuitou, Hainan, 21 November 1990; MBM286760,92C-460, 5♂4♀, Luhuitou, Sanya, Hainan, 17 March 1992; MBM286765, 1♂, Luhuitou, Sanya, Hainan,Yunhao PAN and Fei MENG coll., 6 July 2020; CW 9.91-19.34 mm, CL 6.56-12.54 mm.

Fig.3 Neoliomera variolosa (Milne-Edwards, 1873), male, 27.75 mm×17.85 mm (MBM286756)
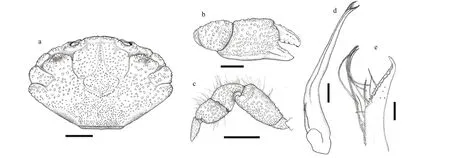
Fig.4 Neoliomera variolosa (Milne-Edwards, 1873), male, 27.75 mm×17.85 mm (MBM286756)

Fig.5 Leptodius davaoensis Ward, 1941, male, 19.34 mm×12.54 mm (MBM286760)
Diagnosis: Carapace (Figs.5a & 6a) hexagonal,breadth about 1.5 times the length; anterior 2/3 of dorsal surface well-def ined; regions granulated,covered with f ine granules, sporadic long setae on anterior margin of regions. Front (Figs.5d & 6a)def lexed and produced, divided into 2 lobes by a narrow Y-shape notch. Dorsal orbital edge with double cracks. Anterolateral border (Figs.5d & 6a)armed with 4 triangular teeth except outer orbital angle, f irst small, with an appendant little tooth beneath, second and third large, last tooth small,following by a crest or a bump, this bump producing a sharp f ifth tooth in some large male individuals.Posterolateral border shorter than anterolateral border,somewhat concave; lateral surface of carapace with bushy setae that visible from dorsal. Antennule situated transversely; orbital hiatus f illed by antennal f lagellum. Third maxilliped (Fig.5b) completely covering buccal orif ice; merus subquadrate,granulated, anterolateral angle produced; ischium subrectangular, with a smooth groove. Thoracic sternites (Fig.5c) smooth; the groove between st1-2 and 3 straight, the median line of st4 long and shallow.
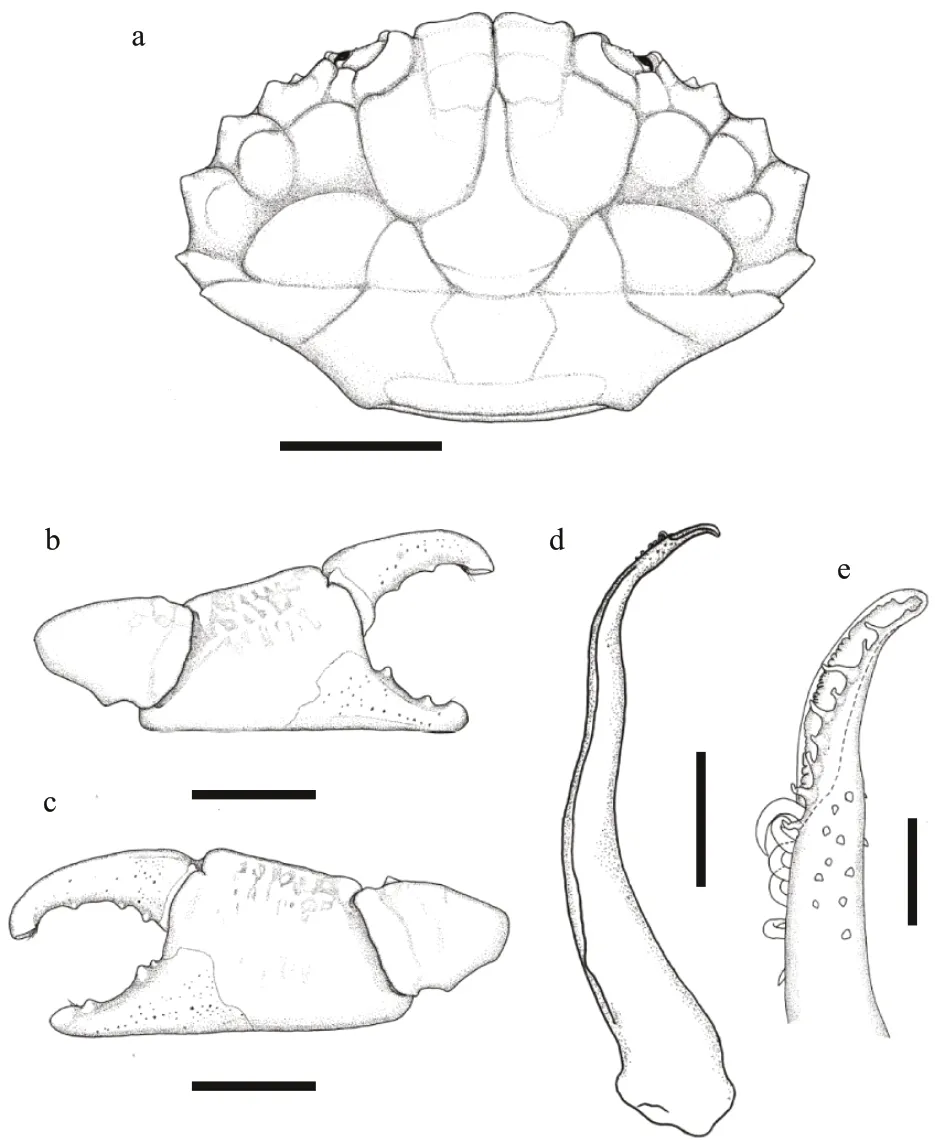
Fig.6 Leptodius davaoensis Ward, 1941, male, 19.34 mm×12.54 mm (MBM286760)
Chelipeds (Figs.5e, f, 6b, & c) unequal; merus with bushy setae on edges; carpus armed with a tooth on inner angle, dorsal surface granulated with tubercles,inner surface with long setae; palm strong, prolonged and granulated, dorso-external surface rugose;dactylus with 2 longitudinal dorsal grooves, cutting edges with 2-3 triangular teeth, dactylus meeting f ixed f inger only at tips when closed; f inger tips spoon-shaped with a tuft of setae.
Ambulatory legs (Fig.5a) granular; edges with long setae; dactylus with little spines and setae, tip claw-shaped.
Pleonal somites (Fig.5c) 3-5 completely fused in male. G1 (Fig.6d & e) slender and curving, subdistal portion with 6 strong spines curving to dorsal, apical lobe prolonged, ventral edge armed with 9 tongueshaped extensions.
Living color is shown in Fig.7.
Distribution: Hainan Island; Singapore: Tioman Island, Singapore Strait, Malaysia: Pisang Island,Indonesia: West Lombok Island, Micronesia: Kosrae Island, Philippines: Gulf of Davao (Ward, 1941; Lee,2012).
Type locality: Gulf of Davao.

Fig.7 Leptodius davaoensis Ward, 1941, male, 17.25 mm×11.36 mm (MBM286765)
Remark:L.davaoensiswas originally described by Ward (1941) from Gulf of Davao. Lee (2012) gave a redescription based on specimens collected from Indo-West Pacif ic. The present specimens generally f it with the previous descriptions (Ward, 1941; Lee,2012), but slightly diff er from the f igures (Ward,1941: Figs.13, 14; Lee, 2012: Fig.174) by the following characters: 1) the front more produced in present specimens, while only slightly produced and with more distinct granules in the holotype; 2) the anterolateral tooth broader in present specimens,while it more produced and narrower in the holotype.Moreover, the number of tongue-shaped extensions of G1 is 9 in the largest male specimen, while the number is 10-12 in Lee’s description.
Leptodiusdavaoensisis most related toL.australis,and the diff erences are discussed under the remarks ofL.australis.L.davaoensisis also similar withL.sanguineus(Milne Edwards, 1834) for having a produced bump following the last anterolateral tooth and forming a f ifth tooth on large specimens (Fig.5d).However, based on present observation,L.sanguineusdiff ers fromL.davaoensisby having a less produced front, wider carapace grooves, and the G1 with a much shorter apical lobe (Serène, 1984: 180, Fig.108,Pl.26, Fig.B).
Takeda (1980) suggested that the holotype ofL.davaoensisis conspecif ic withL.leptodonForest and Guinot, 1961. Ng et al. (2008) listedL.leptodonas a junior synonym ofL.davaoensis. Lee (2012)suggestedL.davaoensisas a distinct species fromL.leptodon. In fact, the present specimens are distinct from the description ofL.leptodonby Forest and Guinot (1961) in the following characters: 1) the apical lobe of G1 curving to outside and with 9 tongue-shaped extensions, whileL.leptodonhavingmore slender apical lobe of the G1 curving to inside and with 14 tongue-shaped extensions; 2) the anterolateral teeth of carapace triangular, while the f irst anterolateral teeth rounded and the second truncated inL.leptodon.

Fig.8 Leptodius australis Ward, 1936, male, 17.84 mm×10.80 mm (MBM286761)
Undoubtedly, the taxonomy ofLeptodiusis not satisfactorily resolved presently (Forest and Guinot,1961; Serène, 1984; Lee, 2012; Lee et al., 2013). G1 is probably a better morphological character for the diagnosis. A revision ofLeptodiusis needed.
Leptodius australisWard, 1936
LeptodiusaustralisWard, 1936: 6, Pl.2, Figs.7-9;Lee, 2012: 240-244, Figs.170-172.
Material examined
South China Sea: MBM286762, 3♂, Xiaodonghai,Hainan, Wei JIANG coll., 23 March 2008;MBM286763, 2♂1♀, Xiaodonghai, Hainan, Wei JIANG coll., 25 December 2007; MBM282511, 2♂,Wei JIANG coll., 23 March 2008; in MBM163879,92C-521, 3♂3♀, Ximao Island, Sanya, Hainan, 20 March 1992; in MBM163881, 90C-197, 1♀,Xiaodonghai, Hainan, 11 November 1990; in MBM163872, 90C-405, 1♂1♀, Luhuitou, Sanya,Hainan, 13 November 1990; MBM286761, 92C-460,6♂2♀, Luhuitou, Sanya, Hainan, 17 March 1992;CW 5.51-17.84 mm, CL 3.67-10.95 mm.
Diagnosis: Carapace (Figs.8a & 9a) transversely oval, breadth about 1.6 times the length; anterior 2/3 of dorsal surface well-def ined; regions covered with f ine f lat granules; partition groove with sporadic long setae. Front (Figs.8d & 9a) def lexed, not very produced, divided into 2 lobes by a wide V-shapenotch. Dorsal orbital edge with double cracks.Anterolateral border (Figs.8d & 9a) armed with 4 teeth except outer orbital angle, f irst tooth small, with an appendant little tooth beneath, second and third tooth broad, last tooth small and distinct. Posterolateral border about equal to anterolateral border, somewhat concave. Lateral surface of carapace with bushy setae that visible from dorsal. Antennule situated transversely; orbital hiatus f illed by antennal f lagellum. Third maxilliped (Fig.8b) completely covering buccal orif ice; merus subquadrate,granulated, anterolateral angle produced; ischium subrectangula. Thoracic sternites (Fig.8c) smooth; the groove between st1-2 and 3 straight, the median line of st4 long and shallow.
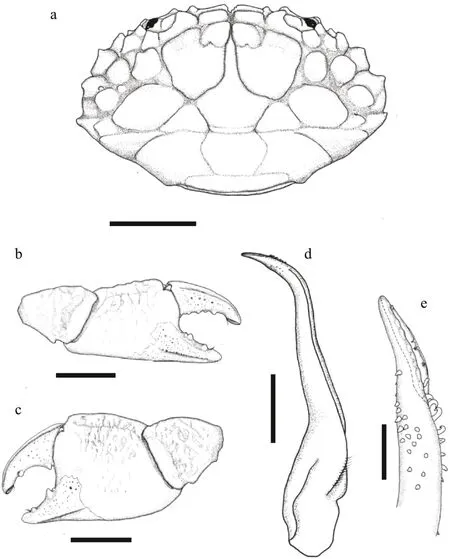
Fig.9 Leptodius australis Ward, 1936, male, 17.84 mm×10.80 mm (MBM286761)
Chelipeds (Figs.8e, f, 9b, & c) unequal; merus with bushy setae on edges; carpus armed with a tooth on inner angle, dorsal surface with irregular areoles,inner surface with long setae; palm stout and granular,dorso-external surface rugose; dactylus with 2 longitudinal dorsal grooves, cutting edges with 2-4 triangular teeth, dactylus meeting f ixed f inger only at tips when closed; f inger tips spoon-shaped with a tuft of setae.

Fig.10 Leptodius australis Ward, 1936, from Hainan Island
Ambulatory legs (Fig.8a) granular, edges with long setae; dactylus with little spines and setae, tip clawshaped.
Pleonal somites (Fig.8c) 3-5 completely fused in male. G1 (Fig.9d & e) slender and curving, subdistal portion with 8 strong spines curving to dorsal, apical lobe prolonged, ventral edge armed with 8 tongueshaped extensions.
Living color is shown in Fig.10.
Distribution: Hainan Island; Philippine Islands(Lee, 2012), Australia (Ward, 1936).
Type locality: Queensland.
Remark: The present specimens f it with the previous descriptions (Ward, 1936; Lee, 2012).L.australisis most similar toL.davaoensisfor the hirsute appearance and having the triangular anterolateral border teeth. Based on the present material,L.australisdiff ers fromL.davaoensisby:the not produced front and having a wider V-shape middle notch; the apical lobe of G1 with 8 extensions,and shorter and more straight than that inL.davaoensis.
GenusXanthiasRathbun, 1897
Xanthias joanneaeMendoza, 2013
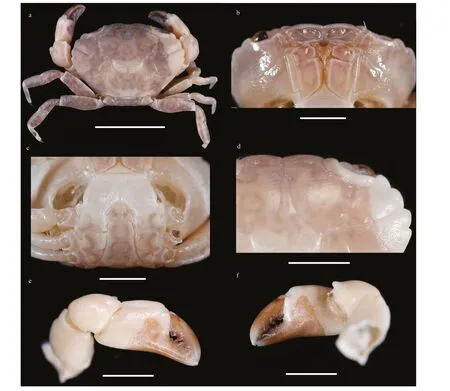
Fig.11 Xanthias joanneae Mendoza, 2013, male, 8.34 mm×5.48 mm (MBM286764)
Xanthias joanneaeMendoza, 2013: 375, Figs.1-4.
Material examined
South China Sea: MBM286764, 1♂, Hainan,9-10 m, Junlong ZHANG coll., 21 November 2016.CW 8.34 mm, CL 5.48 mm, front width 2.65 mm, P5 merus width 1.13/1.12 mm; MBM163770, 1♀,Yalong Bay, Hainan, 20 November 1990. CW 7.45 mm, CL 4.72 mm, P5 merus width 0.92/0.95 mm.
Diagno sis: Carapace (Figs.11a & 12a) transversely oval, breadth about 1.5 times the length, with 30-32 ocelli arranged symmetrical; dorsal surface welldef ined by narrow but distinct grooves, regions smooth. Front (Figs.11d & 12a) double-lobes,rounded and broad. Orbital region broad, dorsal edge with 2 f issures. Anterolateral border (Figs.11d & 12a)armed with 4 rounded tooth include outer orbital angle, separated from each other by V-shape notches.Posterolateral border straight, longer than anterolateral border. Antennule situated transversely; orbital hiatus f illed by antennal f lagellum. Third maxilliped(Fig.11b) completely covering buccal orif ice; merus subquadrate; ischium subrectangular; merus and ischium with a large ocellus respectively. Thoracic sternites (Fig.11c) smooth; the groove between st1-2 and 3 nearly straight, the median line of st4 long and shallow; st5-st7 with a pair of ocelli on margin respectively.
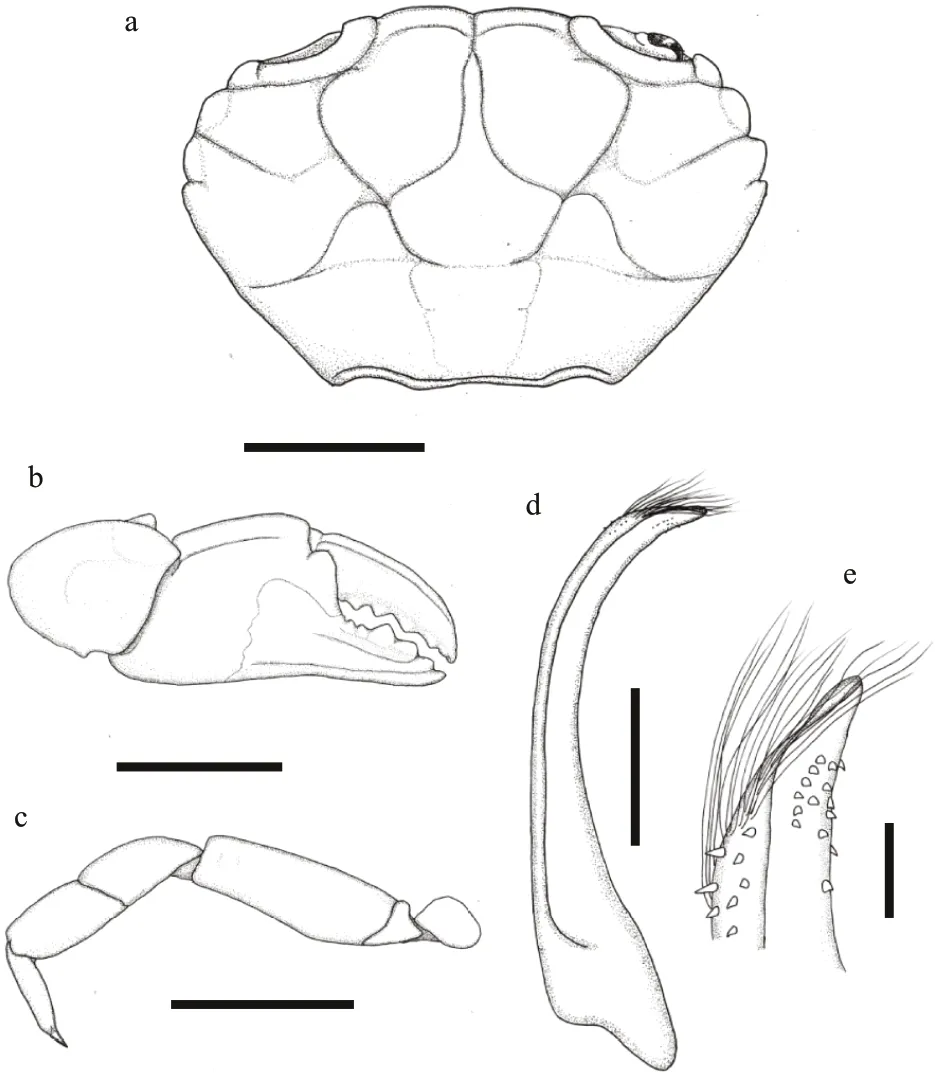
Fig.12 Xanthias joanneae Mendoza, 2013, male, 8.34 mm×5.48 mm (MBM286764)
Chelipeds (Figs.11e, f, & 12b) equal and f inely granular; merus with setae on edges; carpus armed with a blunt tooth on inner angle; palm smooth except a shallow longitudinal groove on dorso-external surface; f ingers black brown, the color of f ixed f inger extending to palm inner and outer in male; cutting edges with 5 triangular teeth, staggered when close,only little gap proximal; f ixed f inger with 2 carinae on outer surface; f inger tips sharp, concave.
Ambulatory legs (Figs.11a & 12c) smooth with ocelli on dorsal surface, merus f lattened with a sharp anterior edge; dactylus with setae, tip claw-shaped.
Pleonal somites (Fig.11c) 3-5 completely fused in male; ab1-3 with broken ocelli marginal and central,ab4-6 with a intact ocelli respectively, telson proximal broaden, with an incomplete ocellus. G1 (Fig.12d &e) stout, with long plumose subdistal setae, apical lobe acute.
Distribution: Hainan Island; Philippines: Bohol Sea (Mendoza, 2013).
Type locality: Bohol Sea.
Remark: Up to now, there are 2 ocellatedXanthiasspecies:XanthiasmaculatusT. Sakai, 1961 andXanthiasjoanneaeMendoza, 2013. Sakai (1961)describedX.maculatusfrom Japan originally. Serène(1984) reported 4 related specimens from Kenya with similar color and ocelli, but they still have noticeable diff erence morphologically (Serène, 1984; Mendoza,2013). He attributed those specimens and a specimen from Vietnam toXanthiasaff .maculatusand suggest these variations may be intraspecif ic depending the locality. Mendoza (2013) def ined related specimens of Philippines to a new speciesX.joanneae. Diagnosis ofX.joanneaeandX.maculatushas been discussed(Mendoza, 2013). Later, Poupin et al. (2018) reported 5 specimens from Mayotte, which have intermediate characters betweenX.maculatusandX.joanneae,and he attributed those specimens toXanthiasmaculatussensu lato and suggested that a revision is needed to f igure out whether it is a single species,with regional variations or there are more than 2 species must be considered in the ‘ocellated’Xanthias.
Specimens from Hainan Island examined here also have intermediate characters betweenX.maculatusandX.joanneae, it’s morphology f it with the description ofX.joanneaeby Mendoza (2013),except: 1) although the specimens has faded, the number of ocelli on dorsal surface of carapace can still be judged to be around 30 depending on the residual pigment (at least 40 inX.joanneaeand about 14 inX.maculatus); 2) the ratio of the width of the P5 merus to carapace length=0.205 in the male specimen,0.195-0.201 in the female specimen (0.22 inX.joanneaeand 0.17 inX.maculatus); 3) carapace regions well def ined, especially 2M, 3M (not well def ined inX.joanneaeand well def ined inX.maculatus); 4) anterolateral borders tooth separated by narrow, V-shaped notches, f irst two tooth rounded while last two tooth with acute apices. (anterolateral tooth with more rounded apices, closer together,separated by narrow, V-shaped notches inX.joanneaeand more acute apices, farther apart, separated by wide troughs inX.maculatus).
4 CONCLUSION
Five species of Xanthidae,H.haanii,N.variolosa,L.australis,L.davaoensis, andX.joanneae, have been reported from China for the f irst time. Based on observation and comparing, morphological characters ofH.haanii,N.variolosa, andL.australisfrom Hainan Island are consistent with previous descriptions. Specimens ofX.joanneaehave intermediate characters betweenX.maculatusandX.joanneae. Its morphology is similar to the description of Mendoza (2013) excepting for some slight diff erences. Specimens ofL.davaoensisare distinct from previous descriptions ofL.leptodon, which once thought to be a junior synonym of the former. It is also slightly diff erent with f igures of the holotype ofL.davaoensis, mainly on the morphology of carapace.Further comparison may help to clarify whether these diff erences are intraspecif ic or interspecif ic.
5 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon reasonable request.
6 ACKNOWLEDGMENT
The authors are thankful to Junlong ZHANG,Yunhao PAN, and Fei MENG for their signif icant help in collecting materials. We also thank Xu ZHANG and Fei MENG for providing color photographs of living crabs used in this paper.ReferencesDana J D. 1852. Crustacea. Part I. United States Exploring Expedition. During the years 1838, 1839, 1840, 1841,1842. Under the command of Charles Wilkes, U.S.N. Vol.13. Sherman C, Philadelphia. 685p, https://doi.org/10.5962/bhl.title.69333.de Haan W, 1833-1850. Crustacea.In: von Siebold P F ed.,Fauna Japonica, sive, Descriptio Animalium, Quae in Itinere per Japoniam, Jussu et Auspiciis, Superiorum, qui Summum in India Batava Imperium Tenent, Suscepto,Annis 1823-1830. Collegit, Noitis, Observationibus et Adumbrationibus Illustravit. Lugduni Batavorum, Leiden.p. 1-17, 1-31, 1-243, pls. 1-55, A-Q, 2, https://doi.org/10.5962/bhl.title.124951.Forest J, Guinot D. 1961. Crustacés Décapodes Brachyoures de Tahiti et des Tuamotu.In: Expédition Français sur les Récifs Coralliens de la Nouvelle-Calédonie. Paris: A.Lahure. pp.1-195, https://decapoda.nhm.org/references/referenceinfo.html?ref id=11061. Accessed on 2021-01-12.Galil B, Vannini M. 1990. Research on the coast of Somalia.Xanthidae Trapeziidae Carpiliidae Menippidae (Crustacea Brachyura).TropicalZoology, 3(1): 21-56, https://doi.org/10.1080/03946975.1990.10539447.Guinot-Dumortier D. 1960. Révision des genres Euxanthus Dana etHypocolpusRathbun (Crust. Decap. Brach.).Mémoires du Muséum national d’Histoire naturelle.NouvelleSérie.SérieA,Zoologie, 20(2): 153-218.Henderson J R, 1893, A contribution to Indian carcinology.TransactionsoftheLinneanSocietyofLondon(2)Zoology, 5: 325-458, pls. 36-40, https://doi.org/10.1111/j.1096-3642.1893.tb00653.x.Lai J C Y, Mendoza J C E, Guinot D, Clark P F, Ng P K L.2011. Xanthidae Macleay, 1838 (Decapoda: Brachyura:Xanthoidea) systematics: a multi-gene approach with support from adult and zoeal morphology.Zoologischer Anzeiger, 250(4): 407-448, https://doi.org/10.1016/j.jcz.2011.07.002.
Lasley R M Jr, Klaus S, Ng P K L. 2015. Phylogenetic relationships of the ubiquitous coral reef crab subfamily Chlorodiellinae (Decapoda, Brachyura, Xanthidae).ZoologicaScripta, 44(2): 165-178, https://doi.org/10.1111/zsc.12094.
Lee S K. 2012. Systematic study on the Korean pilumnoid and xanthoids (Crustacea: Decapoda: Brachyura) based on morphology and molecular data. Laboratory of Systemtics and Molecular evolution, School of Biological Sciences,The Graduate School, Seoul National Universit, Seoul.343p.
Lee S K, Mendoza J C E, Ng P K L, Kim W. 2013. On the identity of the indo-west pacif ic littoral xanthid crab,Leptodiusexaratus(H. Milne Edwards, 1834) (Crustacea:Decapoda: Brachyura: Xanthidae).TheRaffl esBulletinof Zoology, 61(1): 189-204.
Leray M, Boehm J T, Mills S C, Meyer C P. 2012. Moorea BIOCODE barcode library as a tool for understanding predator-prey interactions: insights into the diet of common predatory coral reef f ishes.CoralReefs, 31: 383-388, https://doi.org/10.1007/s00338-011-0845-0.
Mendoza J C E. 2013. A new species of ocellatedXanthiasRathbun, 1897 (Crustacea: Decapoda: Brachyura: Xanthidae)from the Bohol Sea, Philippines.Zootaxa, 3636(2): 374-384,https://doi.org/10.11646/zootaxa.3636.2.8.
Mendoza J C E, Clark P F, Ng P K L. 2012. The identity ofPilumnoplaxacanthomerusrathbun, 1911 (Crustacea:Decapoda: Brachyura: Xanthidae), with new records from the central and western Pacif ic.Zootaxa, 3367(1): 211-221, https://doi.org/10.11646/zootaxa.3367.1.20.
Mendoza J C E, Guinot D. 2011. Revision of the genusGlyptoxanthusA. Milne-Edwards, 1879, and establishment of Glyptoxanthinae nov. subfam. (Crustacea: Decapoda:Brachyura: Xanthidae).Zootaxa, 3015(1): 29-51, https://doi.org/10.11646/zootaxa.3015.1.4.
Mendoza J C E, Manuel-Santos M R. 2012. Revision ofGarthiellaTitgen, 1986 (Crustacea: Decapoda: Brachyura:Xanthidae), with description of a new subfamily and a new species from the central Philippines.Zootaxa, 3446(1): 32-48, https://doi.org/10.11646/zootaxa.3446.1.2.
Mendoza J C E, Ng P K L. 2010. The euxanthine crabs(Crustacea: Brachyura: Xanthidae) of the Philippines.The Raffl esBulletinofZoology, 58(1): 57-74.
Milne-Edwards A. 1873. Description de quelques crustacés nouveaux ou peu connus provenant du Musée de M.C.Godeff roy.JournaldesMuseumGodeff roy, 1: 253-264,https://doi.org/10.5962/bhl.title.10644.
Milne Edwards H. 1834-1840. Histoire naturelle des Crustacés,comprenant l’ anatomie, la physiologie et la classif ication de ces animaux. Vol. 1-3. Paris: Librairie Encyclopédique de Roret. (1)468, (2)532, (3)638p, Atlas: 1-32, Plates:I-XLII, https://doi.org/10.5962/bhl.title.16170.
Ng P K L, Guinot D, Davie P J F. 2008. Systema Brachyurorum:Part I. An annotated checklist of extant brachyuran crabs of the world.TheRaffl esBulletinofZoology, 17: 1-286.
Ng P K L, Shih H T, Ho P H, Wang C H. 2017. An updated annotated checklist of brachyuran crabs from Taiwan(Crustacea: Decapoda).JournaloftheNationalTaiwan Museum, 70(3-4): 1-185, https://doi.org/10.6532/JNTM.201712_70(3;4).01.
Nobili G. 1906. Diagnoses préliminaires de 34 espèces et variétés nouvelles, et de 2 genres nouveaux de Décapodes de la Mer Rouge.BulletinduMuséumd′HistoireNaturelle,6: 393-411.
Odhner T. 1925. Monographierte Gattungen der Krabbenfamilie Xanthidae. l.GöteborgsKungligaVetenskaps-och Vitterhets-SamhällesHandlingar, 29(1): 3-92.
Poupin J. 1996. Crustacea Decapoda of French Polynesia(Astacidea, Palinuridea, Anomura, Brachyura).Atoll ResearchBulletin, 442: 1-114.
Poupin J, Cleva R, Bouchard J M, Dinhut V, Dumas J. 2018.The crabs from Mayotte Island (Crustacea, Decapoda,Brachyura).AtollResearchBulletin, 617: 1-109, https://doi.org/10.5479/si.0077-5630.617.
Rathbun M J. 1894. Descriptions of two new species from the Western Indian Ocean, presented to the National Museum by Dr. W. L. Abbott.ProceedingsoftheUnitedStates NationalMuseum, 17(979): 21-24, https://doi.org/10.5479/si.00963801.979.21.
Rathbun M J. 1909. New crabs from the Gulf of Siam.ProceedingsoftheBiologicalSocietyofWashington, 22:107-114.
Sakai T. 1939. Studies on the Crabs of Japan IV. Brachygnatha,Brachyrhyncha. Vol. 3. Yokendo Co., Ltd., Tokyo. p.365-741.
Sakai T. 1961. New species of Japanese crabs from the collection of His Majesty the Emperor of Japan.Crustaceana, 3(2): 131-150, https://doi.org/10.1163/156854061X00635.
Sakai T. 1976. Crabs of Japan and the Adjacent Seas. Kodansha Ltd., Tokyo. 773p.
Serène R. 1984. Crustacés Décapodes Brachyoures de l’Océan Indien occidental et de la Mer Rouge. Xanthoidea:Xanthidae et Trapeziidae. Avec un addendum par Crosnier,A.: Carpiliidae et Menippidae.FauneTropicale, 24:1-349.
Takeda M. 1980.PilumnusplanusEdmondson andLeptodius leptodonForest & Guinot as synonyms ofForestia depressa(White) andLeptodiusdavaoensisWard(Decapoda, Brachyura).Crustaceana, 39(3): 318-320,https://doi.org/10.1163/156854080X00797.
Tweedie M W F. 1949. A collection of crabs from Aor Island,South China Sea.BulletinoftheRaffl esMuseum, 21: 83-96.
Ward M. 1936. Crustacea Brachyura from the coasts of Queensland.MemoirsoftheQueenslandMuseum, 11(1):1-13.
Ward M. 1941. New Brachyura from the Gulf of Davao,Mindanao, Philippine Islands.AmericanMuseum Novitates, 1104: 1-15.
Yang S L, Chen H L, Jiang W. 2008. Family Xanthidae MacLeay, 1838.In: Liu R Y ed. Checklist of Marine Biota of China Seas. Science Press, Beijing, China. p.792-799.(in Chinese with English abstract)
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
