In-situ experiments reveal mineralization details of porphyry copper deposits
Weidong SUN , , , Xiuqi SHANG , ,3
1 Center of Deep Sea Research, Institute of Oceanology, Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Laboratory for Marine Geology, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
Abstract In-situ hydrothermal experiments using diamond-anvil cell show that sulfate and sulf ide are the dominant sulfur species under P-T conditions similar to those of porphyry magmas. No sulf ite was identif ied using in-situ Raman spectrometer. This supports that porphyry copper mineralization is controlled by sulfate reduction, rather than sulf ite disproportionation.
Keyword: diamond cell; sulfate; sulf ide; porphyry deposits; magnetite crisis
Porphyry copper-gold deposits host more than 70% of the world’s total copper reserves (Sillitoe,2010). They are exclusively related to oxidized magmas, with abundant coexisting sulfate and sulf ide(Ballard et al., 2002; Liang et al., 2006; Sillitoe, 2010;Sun et al., 2015, 2017; Zhang et al., 2017). This was either attributed to sulfate reduction (Sun et al., 2004,2013), or sulf ite disproportionation (Richards, 2015).Recent experiments using diamond-anvil cell equipped with in-situ Raman spectrometer f ind that sulfate and sulf ide are dominant species, with considerable amount of S3ˉ and S2ˉ radical ions under conditions before porphyry mineralization started. No sulf ite is detected(Fig.1). This strongly supports that sulfate reduction,rather than sulf ite disproportionation, is the process that controls porphyry mineralization (Sun et al., 2013).
The experiment was carried out through in-situ Raman spectroscopic analyses of samples in a highpressure optical cell. Four diff erent silica glasses with diff erent alkalinity ([(K+Na)/Al]=0.6, 1.1, 1.3, and 3.0) and aqueous thiosulfate solutions (either K2S2O3or Na2S2O3) were used (Colin et al., 2020). Sulfur redox balance was imposed through stoichiometric breakdown of thiosulfate and enable oxygen fugacity and acidity buff ering via the dominant reactions:
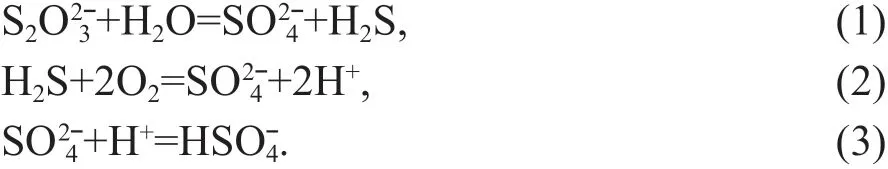
The experimental conditions are dramatically diff erent from that of porphyry and other hydrothermal deposits. These experiments were carried out at 700 °C, 0.3-1.5 GPa, and oxygen fugacity in the vicinity of the sulf ide-sulfate transition (near the nickel-nickel oxide (NNO) buff er) (Colin et al.,2020). All these conditions are diff erent from those of porphyry Cu deposits.
The temperature of the experiments (700 °C) is higher than that of porphyry mineralization (mostly<500 °C, mostly less than 400 °C), but is similar to that of primitive porphyry magmas. The pressures of the experiments (0.3-1.5 GPa) correspond to depths of 9-45 km. Note, under such high pressures, no hydrothermal deposit forms because aqueous f luids are usually dissolved in magmas (Sun et al., 2007).Porphyry Cu deposits usually form at depths of 2-4 km (Sillitoe, 2010). Therefore, the experimental conditions are dramatically diff erent from that of porphyry and other hydrothermal deposits.Nevertheless, most porphyry deposits are connected to underlying composite plutons at depths of 5-15 km(Sillitoe, 2010), with pressures overlap with that of the experiments (Colin et al., 2020), supporting that sulfate and sulf ide are the dominant sulfur species in the porphyry magmas.
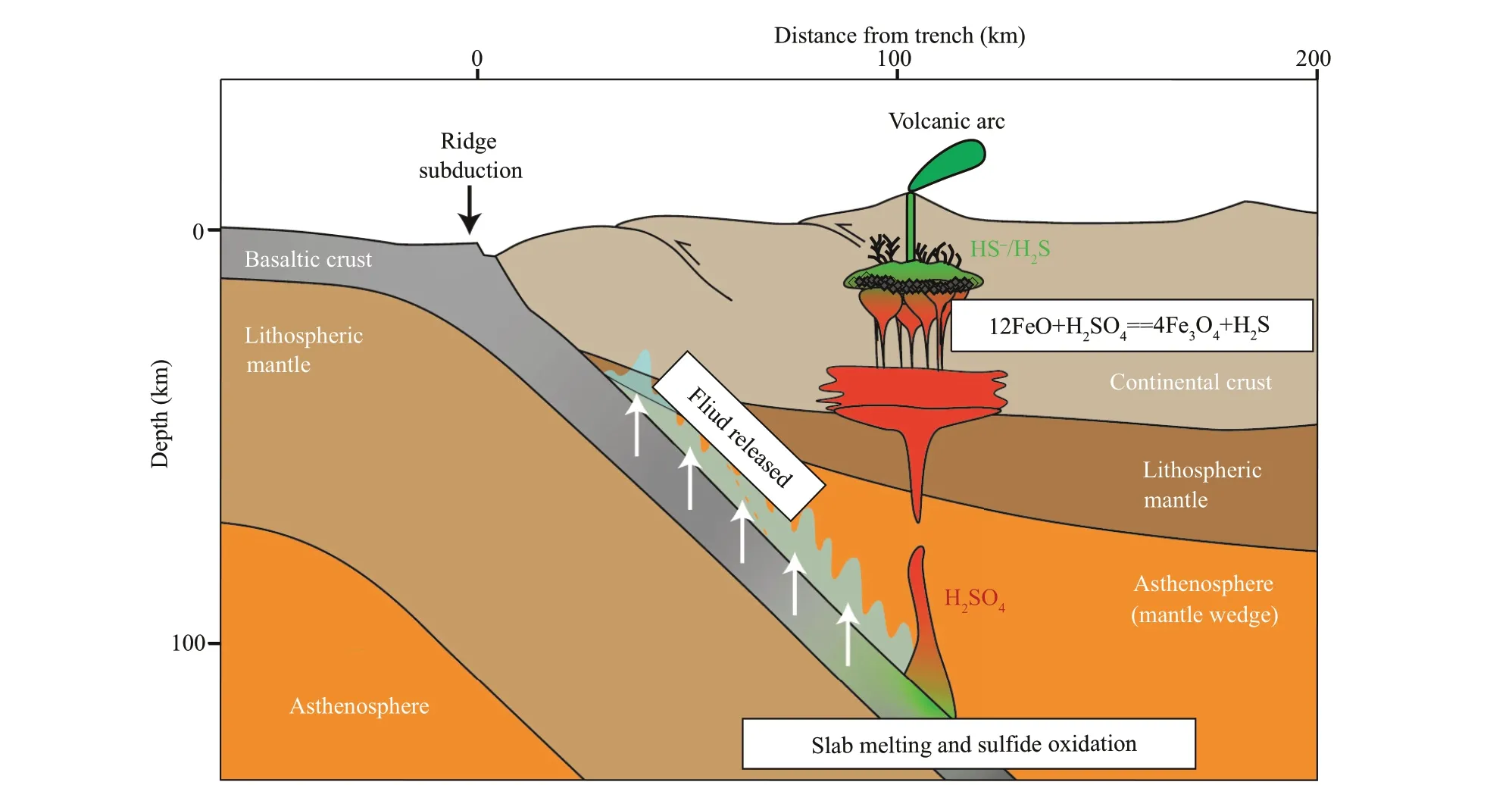
Fig.1 The magnetite crisis and sulfate reduction model of porphyry copper deposits
The oxygen fugacity of the porphyry magmas( Δ FMQ+(1.5-2.0)) (Sun et al., 2017; Zhang et al.,2017) is marginally higher than that of the experiment(~ Δ FMQ+1, near the NNO buff er). Given that the higher the oxygen fugacity, the more stable the sulfate is, whereas sulf ide is far more soluble in hydrous f luids than sulfate (Huang and Keppler, 2015),porphyry magmas should have higher sulfate/sulf ide ratios than the experiments.
Previous authors once proposed that the dominant sulfur species in porphyry magmas is sulf ite, which forms the coexistence of sulfate and sulf ide through disproportionation during mineralization (Richards,2015). This is not supported by the experiment results of Colin et al. (2020), which clearly show that sulfate and sulf ide, rather than sulf ite, are the stable sulfur species in porphyry magmas. Considering that the detection limits of laser Raman is quite low (Zhang et al., 2020), the absence of sulf ite in the experiments indicates that sulf ite is not responsible to porphyry mineralization nor other hydrothermal deposits.
Oxygen fugacity is a key factor that controls sulfur species, and consequently, porphyry mineralization(Sun et al., 2015; Liu et al., 2020), although high oxygen fugacity alone cannot form porphyry deposits(Lee et al., 2012). Previous studies suggest that sulfate is the dominant sulfur species in porphyry magmas before the onset of mineralization (Zhang et al.,2017). It is subsequently reduced to sulf ide during magnetite crystallization at the late stage of magmas evolution (Sun et al., 2004; Liang et al., 2009). This process is called the “magnetite crisis” (Jenner et al.,2012). Given that sulf ide is highly soluble in hydrous f luids (Huang and Keppler, 2015), the newly formed sulf ide is scavenged into the ore forming hydrothermal f luids that prop up porphyry mineralization.
In addition to sulf ide (H2S and HS-) and sulfate(HSO4ˉ and SO42ˉ), S3ˉ, and S2ˉ radical ions were also discovered in the experiments (Colin et al., 2020),which may favor the mobility of metals (Au, Cu, and Mo) in geological f luids (Sun et al., 2013; Colin et al.,2020). Nevertheless, the experiments used aqueous thiosulfate solutions as the starting materials, which may artif icially increase the proportion of S3ˉ and S2ˉ radical ions in the run products.
In-situ Raman spectroscopic analyses makes hydrothermal diamond-anvil cell and other highpressure optical cells, e.g., fused silica capillary, more practical in hydrothermal experiments. More attentions should be paid on systems close to natural processes, e.g., porphyry copper deposits are usually associated with intermediate adakitic rocks. Future experiments should focus on sulfate reduction, rather than decomposition of aqueous thiosulfate. In addition, hydrothermal mineralization mostly occurs at pressures lower than 0.2 GPa and temperatures lower than 500 ° C. Such experiments may better be carried out using fused silica capillary.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
- Characterization of dissolved organic matter in submarine groundwater from a salt marsh in Chongming Island, China*
