Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
Keli YANG , Yaoling ZHANG , Yaping DONG , Wu LI
1 Key Laboratory of Comprehensive and Highly Effi cient Utilization of Salt Lake Resources, Qinghai Institute of Salt Lakes,Chinese Academy of Sciences, Xining 810008, China
2 Qinghai Technology Research and Development Center of Comprehensive Utilization of Salt Lakes Resources, Xining 810008,China
3 Qinghai Provincial Key Laboratory of Resources and Chemistry of Salt Lakes, Xining 810008, China
Abstract Dissolved organic matter (DOM) plays a vital role in promoting carbon and nutrient cycling.It is a food source for organisms and controls the migration and transformation of trace metals and other contaminants in aquatic systems. The contributions of aquatic DOM to the environment and ecology of a system are closely related to its abundance and chemical structure. In this study, the chemical composition and binding properties of DOM in a hypersaline lake watershed were investigated for the f irst time using dissolved organic carbon (DOC) analysis, absorption spectroscopy, Fourier transform infrared spectroscopy,pyrolysis-GC-MS (Py-GC-MS), and f luorescence parallel factor (PARAFAC) analysis combined with Pb(II)titration techniques. The results showed that DOM from the tributaries that f lowed into the lake had a lower DOC content, higher molecular weight, and higher specif ic UV absorbance than the DOM in lake water.Protein-like f luorophores were mainly found in tributary and lake surface water DOM (LSDOM) and humiclike substances were abundant in lake groundwater DOM (LGDOM). Using this multi-methodological approach, we found that the DOM from the hypersaline lake watershed was mainly from microbial origins,and consisted of aromatics, carbohydrates, and aliphatics. The results from quantitative analysis showed that DOM from the inf lowing tributaries contained more aromatics, lower carbohydrates, and lower aliphatics than DOM in the lake. Monocyclic aromatic hydrocarbons and carbohydrates were more abundant in LSDOM than LGDOM. The results from the Pb(II) titration technique coupled with PARAFAC analysis suggested that PARAFAC-derived components had relatively low condition stability constants (log K M <2).Of the two types of lake DOM, the LGDOM had a higher Pb(II) binding potential than the LSDOM. From this study we have improved our understanding of how DOM within a hypersaline lake watershed varies in its composition and potential to bind with metals.
Keyword: dissolved organic matter; hypersaline lake watershed; composition; parallel factor analysis;Pb(II) titration
1 INTRODUCTION
Dissolved organic matter (DOM) is ubiquitous in aquatic systems, and is one of the largest dynamic reservoirs of organic carbon on earth.Biogeochemically active, DOM plays a critical role in the natural environment as a nutrient source for aquatic organisms and photosensitizer for anthropogenic compounds. It also inf luences biogeochemical cycling and the mobility of pollutants,such as trace heavy metals and persistent organic pollutants (Li and Minor, 2015; Jiang et al., 2017; Jin et al., 2019). Researchers have already reported information about the chemical composition of DOM in diff erent aquatic systems, including rivers (Blanchet et al., 2017; Chon et al., 2017; Xu and Guo, 2017;Wang et al., 2019), freshwater lakes (Ilina et al., 2014;Sugiyama et al., 2014; Zigah et al., 2014; Li and Minor, 2015; Bravo et al., 2017), and oceans (Dittmar et al., 2006; Sleighter and Hatcher, 2008; Andrew et al., 2013; Green et al., 2014; Lechtenfeld et al., 2014).Molecular information about DOM has also been reported in individual or linked aquatic systems, such as coastal marine environments (Liu et al., 2012;Perminova et al., 2014; Gomez-Saez et al., 2015;Zhang et al., 2016) and large lake watersheds (Minor and Stephens, 2008; Minor et al., 2012; Zigah et al.,2014; Xu et al., 2019a). Despite the numerous studies,few have studied the DOM composition within saline lake watersheds in highlands, which account for 20%of total lake surfaces worldwide and include transient saline wetlands, playa lakes, and deep permanent meromictic lakes (Osburn et al., 2011). These lakes have an important role in global carbon cycling, and,by studying them, we can gain an improved understanding of biological and/or abiotic processes in relatively isolated and frail ecosystems. These lakes are usually rich in valuable elements such as potassium (K), boron (B), and lithium (Li) (Bian et al., 2017; Peng et al., 2017a), which means that DOM has a strong inf luence on the extraction processes and the color and luster of the lake (Zhao, 2010; Shalev et al., 2018). In recent years, researchers have realized that the molecular features of DOM in saline lakes can be exploited to achieve high DOM removals and obtained high purity products (Yang et al., 2017a, b).
Various analysis technologies such as f luorescence or UV-Visible (UV-VIS) light absorption (Stedmon et al., 2003; Minor et al., 2012), Fourier transformed infrared spectroscopy (FTIR) (Wang et al., 2012), and1H/13C nuclear magnetic resonance (NMR) (Hertkorn et al., 2013; Zhou et al., 2015), have been used to characterize and understand the chemical composition,sources, and transformations of DOM from rivers(Perminova et al., 2014; Chon et al., 2017), lakes (Li and Minor, 2015; Li et al., 2017) and ocean water(Green et al., 2014; Lechtenfeld et al., 2014; Wozniak et al., 2015) origins. Advances in mass spectrometry(MS) techniques have also facilitated investigations of the molecular-level composition of DOM isolated from the aquatic environment (Li and Minor, 2015;Waska et al., 2015; Jiang et al., 2017; Kaal et al.,2017). With only a single method, it is diffi cult to gain a comprehensive understanding of the general chemical structures of DOM and how these structures vary under diff erent environmental conditions because of the wide heterogeneity of structures and molecular weights within diff erent aquatic systems(Minor et al., 2014; Sandron et al., 2015). We therefore need to combine a number of proxies to quantify how the chemical compounds and structure of DOM might diff er between a river and a saline lake fed by the river, and how the chemical structure of DOM changes over time in saline lakes, as this could aff ect how it interacts with other elements in the lake.
When studying DOM in saline water using FTIR or MS analysis, suffi cient amounts of representative material need to be isolated and desalted to ensure we gain comprehensive information about its structure.In a previous study, we showed that modif ied styrene divinyl benzene polymer type sorbent (Varian PPL)performed well and facilitated a high recovery of dissolved organic carbon (DOC) from lake water,from which representative DOM was subsequently isolated in solid-phase extraction (SPE) (Yang et al.,2017a). This approach has also been used to isolate DOM from freshwater and seawater, with the ultimate goal of DOM purif ication (Dittmar et al., 2008; Li et al., 2016).
Da Qaidam Lake is located at the northeastern edge of the Qaidam Basin in China’s northwestern desert region. This lake contains several valuable elements, including Li, B, and K at concentrations that either meet or exceed the Chinese standards for industrial exploitation (Bian et al., 2017; Peng et al.,2017b; Yang et al., 2017a). The lake has three main tributaries, namely Datou Yang River, Bali River, and Hotspring River, which are to the northeast of Da Qaidam Lake. Besides the importance of DOM for the environment and ecology of the watershed, there are also economic concerns, including the impacts of DOM on the quality of the lake brine for mineral extraction, through smell, color, and luster. At present,there is very little information available about the abundance and composition of DOM, and how DOM interacts with trace metals in the lake and the lake basin.
In this study, samples of lake and river water were collected from the watershed of Da Qaidam Lake.The DOM content of these samples was characterized using DOC analysis, UV-VIS, f luorescence, FTIR spectroscopy, and pyrolysis-gas chromatographymass spectrometry (Py-GC-MS) techniques. There are lead deposits throughout the area, so we chose Pb(II) as a representative heavy metal, and investigated its binding properties with the DOM from the lake and its tributaries using f luorescence quenching titration combined with parallel factor (PARAFAC)analysis. The objectives of this study were to (1)isolate DOM from the river and lake water and identify the molecular signatures of the DOM, (2)determine the links between the riverine and lacustrine DOM by comparing their chemical compositions, and(3) investigate the complexation of Pb(II) and lake and river DOM.
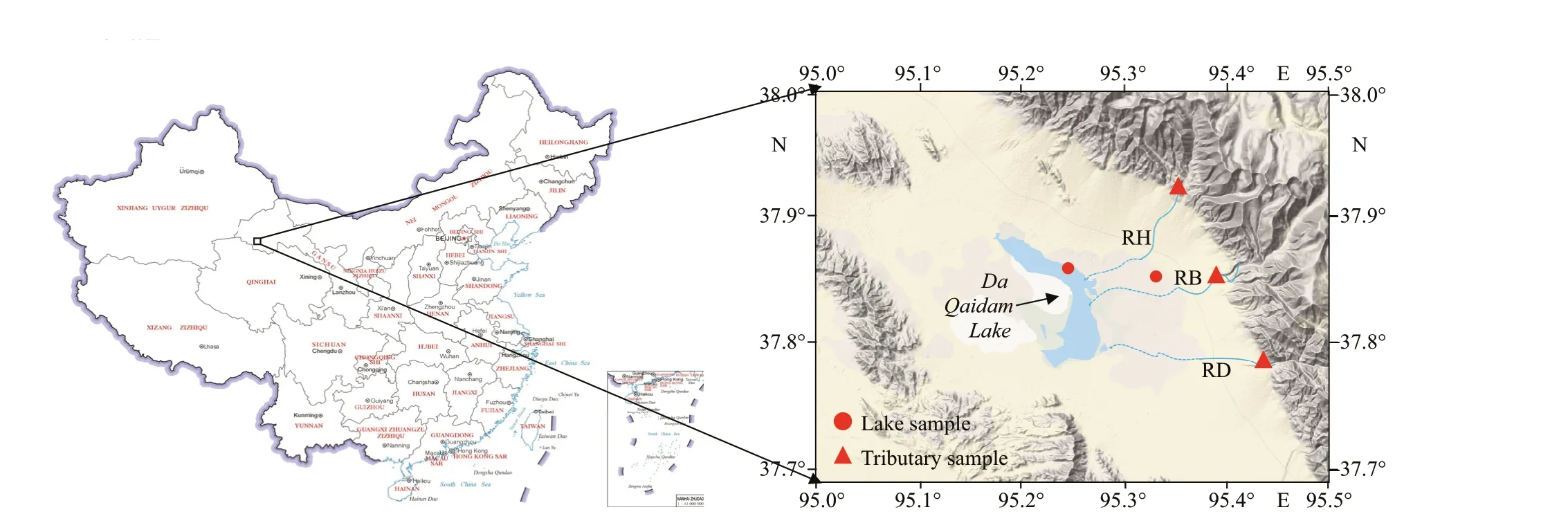
Fig.1 The sampling site
2 MATERIAL AND METHOD
2.1 Lake and tributary sampling
Da Qaidam Lake (Fig.1) is in the northeastern part of the Qaidam Basin, Qinghai Province, China. It covers an area of 240 km2and, at between 0.2- and 0.7-m deep, it is a shallow lake. It has a salinity of up to 200 g/L, which is dominated by sodium, magnesium,chloride, and sulfate. The three main tributaries in the basin discharge into the eastern side of the lake.
Samples of river water were obtained in late July and early August 2017 from three sites on the main tributaries to the lake, specif ically from the northern branch of Da Qaidam Lake, i.e., Datou Yang River(RD, 37°47′13″N, 95°27′30.1″E), Bali River (RB,37°50′46″N, 95°22′54.1″E), and Hotspring River(RH, 37°55′56.7″N, 95°22′34.9″E). These are ephemeral streams that f low mainly in July and August. The sampling sites were at the headwaters of the rivers, and were in remote, sparsely populated areas that were expected to be free from anthropogenic inputs. We also collected two samples of lake water,one of lake surface water (LS: 37°51′10.8″N,95°15′17.8″E), and one of lake groundwater (LG:37°50′50.2″N, 95°14′42.5″E). On collection, the f ive samples were immediately f iltered through glass f iber f ilters with a pore size of 0.7 μm (Whatman GF/F,combusted at 450 ℃ for 5 h before use), and then were kept in the dark and cooled on ice during transport to the laboratory.
2.2 DOC and optical measurements
The DOC and total nitrogen (TN) contents of the samples were determined with a total organic carbon(TOC) analyzer (Analytikjena 3100, Germany) using a high temperature catalytic oxidation method. The TOC analyzer was calibrated using potassium hydrogen phthalate (KHP). Because of the hypersaline nature of the brine sample, the f iltered sample was diluted (1꞉10) with Milli-Q water and then acidif ied to pH 2 with HCl before analysis. The samples were analyzed in triplicate and the results are reported as the mean values of the triplicate accepted injections.
The UV-VIS data of lake and tributaries DOM were obtained at 190-900 nm using a UV-VIS spectrophotometer (Puxi, China). The following parameters were used to investigate the optical properties of each sample: the absorption coeffi cientαλ(m-1) was calculated asαλ=2.303Aλ/L, whereAλis the absorbance at wavelengthλandLis the path length in m; the specif ic UV-VIS absorbance (SUVA)measured at 254 nm was calculated as SUVA254=αλ/cDOC,wherecDOCis the DOC concentration to characterize the abundance of aromatics; the ratio of the UV-VIS absorption coeffi cients at 250 nm to those at 365 nm(E2꞉E3) was calculated as follows:E2꞉E3=α250꞉α365, which had been reported that this ratio was negatively correlated with the DOM molecular size (Helms et al.,2008; Macdonald and Minor, 2013; Yang et al., 2019).
Fluorescence emission-excitation matrix (EEM)spectra of lake and tributaries DOM were gathered with a f luorescence spectrophotometer (Hitachi F-7000; Japan) to investigate the f luorescence substances, which were measured at emission (Em)wavelengths between 250 and 550 nm at 2-nm increments and excitation (Ex) wavelengths between 200 and 500 nm at 10-nm increments. The Raman and Rayleigh scatter peaks were eliminated accordingly(Ohno et al., 2008). Three f luorescence indices (i.e.HIX, BIX, and FIX) were also calculated from the EEM data, which can provide information on the source or degree of humif ication of DOM. FIX is the ratio of the Em intensity between 450 and 500 nm at Ex 370 nm, BIX is the ratio of the Em intensity at 380 nm divided by the maximum of the Em intensity between 420 and 435 nm when the Ex is 310 nm, and HIX is the ratio of the area between Em at 435-480 nm and the Em at 300-345 nm and 435-480 nm when the Ex is 254 nm. All values were determined in duplicate and the mean values are reported.
The PARAFAC model can decompose the EEM data into individual components and give the relative concentrations of diff erent f luorophores. The PARAFAC analysis was carried out using MATLAB R2018b with the DOMFluor toolbox (Stedmon and Bro, 2008). The f irst and second order scatter peaks were removed and the PARAFAC models were computed with between two and seven components,and the number of components was corrected on the basis of split half analysis, residual analysis, and visual inspection.
2.3 Quenching titration and complexing model
The f ive f iltrates that had been passed through the 0.7-μm f ilter papers were adjusted so that the pH was 6, and then divided into a series of vials that each contained 20 mL of f iltrate, to which we added 0.1 or 0.01-mol/L Pb(Ⅱ) (GR, >99.999%) stock solutions.The trace metal concentrations in the f inal solutions ranged from 0 to 100 μmol/L by adding >20 μL of the titrant. All the solutions were shaken immediately and then kept at room temperature in the dark for 24 h to ensure the complexation reached equilibrium before f luorescence scanning.
The complexing of the metals was quantif ied with PARAFAC-derived components using the modif ied quenching model described by Hur and Lee (2011), as follows:
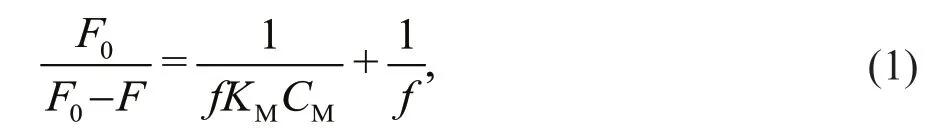
whereF0andFare the reference f luorescence intensities of the sample without and with metal additions,frepresents the fraction of the ligand꞉metal complex when the binding capacity has been reached,KMis the binding constant, andCMis the metal concentration. The main assumption of the model is that only 1꞉1 metal/ligand complexes are formed. The values offandKMhave been calculated for single pairs of the excitation and emission wavelengths for an individual f luorescent component (Hur and Lee, 2011).
2.4 Solid phase extraction (SPE) of DOM
The solid phase extraction was performed using PPL (Bond Elut, Agilent) as described in our previous study (Yang et al., 2017a). The lake and tributaries f iltrates (<0.7 μm) were acidif ied to pH 2 by adding ACS-certif ied HCl before the extraction process, and aliquots of the acidif ied samples were loaded onto the PPL cartridge with a peristaltic pump, rinsed with Milli-Q water with a pH of 2, and eluted with MeOH.The eluted retentate was concentrated in a vacuum at 30 ℃ and dried with a freeze dryer. The freeze-dried samples were ground and stored in a desiccator for further analysis.
2.5 FTIR analysis
The FTIR spectra of the f ive SPE-DOM samples were measured on a Thermo Nicolet NEXUS 670 instrument at wavelengths between 4 000 to 400 cm-1.The pellets for this analysis were prepared from a mixture that contained exactly 1.0 mg of the freezedried DOM sample and 100 mg of spectrographic grade KBr (>99.5%, Aladdin), and then were ground and homogenized to reduce light scatter (Abdulla et al., 2010a). The main bands were identif ied from the average and standard deviation spectra, and the relative proportions of the absorption peaks were calculated from the absorbance intensity.
2.6 Pyrolysis-gas chromatography-mass spectrometry
Pyrolysis-gas chromatography-mass spectrometry(Py-GC-MS) analysis was performed on a gas chromatograph-mass spectrometer (GCMS-QP2010,Shimadzu Corporation, Kyoto, Japan) with a pyrolyzer (Pyroprobe 2000, CDS Analytical Inc.,USA). The samples were loaded into glass woolcontaining f ire-polished quartz tubes and pyrolyzed at temperatures from 250 to 610 °C (heating rate 50 °C/ms), and then held at 610 °C for 10 s. The GC was equipped with a HP-5MS capillary column that was 30-m long and had an internal diameter of 0.25 mm and a f ilm thickness of 0.25 μm. Helium was used as the carrier gas (constant gas f low,1 mL/min). The GC oven was programmed to 40 ℃for 2 min, then increased to 310 ℃ at a rate of8 ℃/min, and maintained at 310 ℃ for 10 min. The ion source of the MS operated in electron impact mode (70 eV), and measured fragments in them/z45-650 range. The relative proportions of the pyrolysis products were calculated as the percentage of the total quantif ied peak area. The pyrolysate was identif ied from the National Institute of Standards and Technology (NIST) library, GC retention times, and other publications (Otto et al., 2016; Kaal et al., 2017;Yang et al., 2017b).
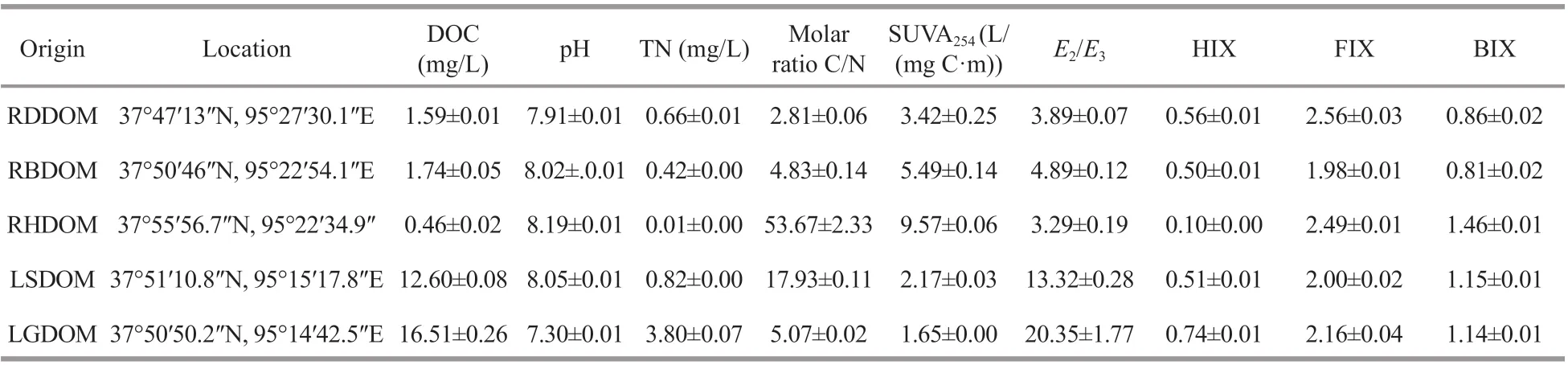
Table 1 Information of characterized f ive DOM samples
3 RESULT AND DISCUSSION
3.1 Abundance of DOM and UV-VIS spectra absorbance properties of DOM from the hypersaline Da Qaidam Lake and its tributaries
Information about the properties of the DOM is summarized in Table 1. The concentrations of DOC in the lake samples, at 12.60 and 16.51 mg/L for lake surface water DOM (LSDOM) and lake groundwater DOM (LGDOM), respectively, were similar and were relatively high. The high DOC concentrations in the lake ref lect the long residence time of water in the saline lake. This is the opposite of what is usually observed in lakes in humid regions, where DOC concentrations tend to decrease as the water residence time increases (Curtis and Adams, 1995). The DOC concentrations in samples RDDOM, RBDOM, and RHDOM from the tributaries, were 1.59, 1.74, and 0.46 mg/L, respectively. These concentrations were signif icantly lower than the average DOC value(7.92 mg/L) reported in tributaries on the south shore of Lake Superior (Urban et al., 2005), and lower than the average river DOC content of around 5.76 mg/L reported by Meybeck (1982) in 1982. The low values in this study indicate the lack of terrestrial plants and organic-rich soil in the surrounding landscape. The DOC contents of RDDOM and RBDOM were comparable but were obviously higher than those in RHDOM, and the diff erence may ref lect the volatilization or thermal degradation of bacterial biomass because of high water temperatures in RH.The DOC and TN contents (Table 1) were positively correlated (R=0.83), which suggests that the majority of the N is organic. The molar C꞉N value of LSDOM(17.93) was greater than that of LGDOM (5.07), and may indicate more N-containing compounds in the lake groundwater than in the lake surface water. The C꞉N ratio of the tributaries was highest in RHDOM,which suggests that there was little bacterial biomass in RH. To give insights into the spectral properties of the DOM from the lake and the tributaries, the parameters of pristine UV-VIS spectra were calculated, i.e., SUVA254,E2/E3, and the results are presented in Table 1. The results show that the SUVA254data for DOM in the lake were lower than those of the tributary DOM, which indicates that allochthonous DOM in tributaries had a higher aromaticity than those in the lake, which is consistent with the results reported by Zigah et al. (2014), who found that there were more aromatic protons in Amity Creek than in the lake it discharged into (Zigah et al.,2014). Further analysis showed that the SUVA254value of RHDOM was clearly higher than the values of RDDOM and RBDOM, which corresponds well with the relatively lowE2/E3value observed in RHDOM. TheE2/E3values were higher in the lake DOM than in the tributary DOM herein, suggesting that the DOM in the tributaries had a high molecular size, which was consistent with the results reported by Minor and Stephens (2008) from their study in the watershed of Lake Superior.
3.2 Fluorescence EEM spectra of DOM in Da Qaidam Lake and its tributaries
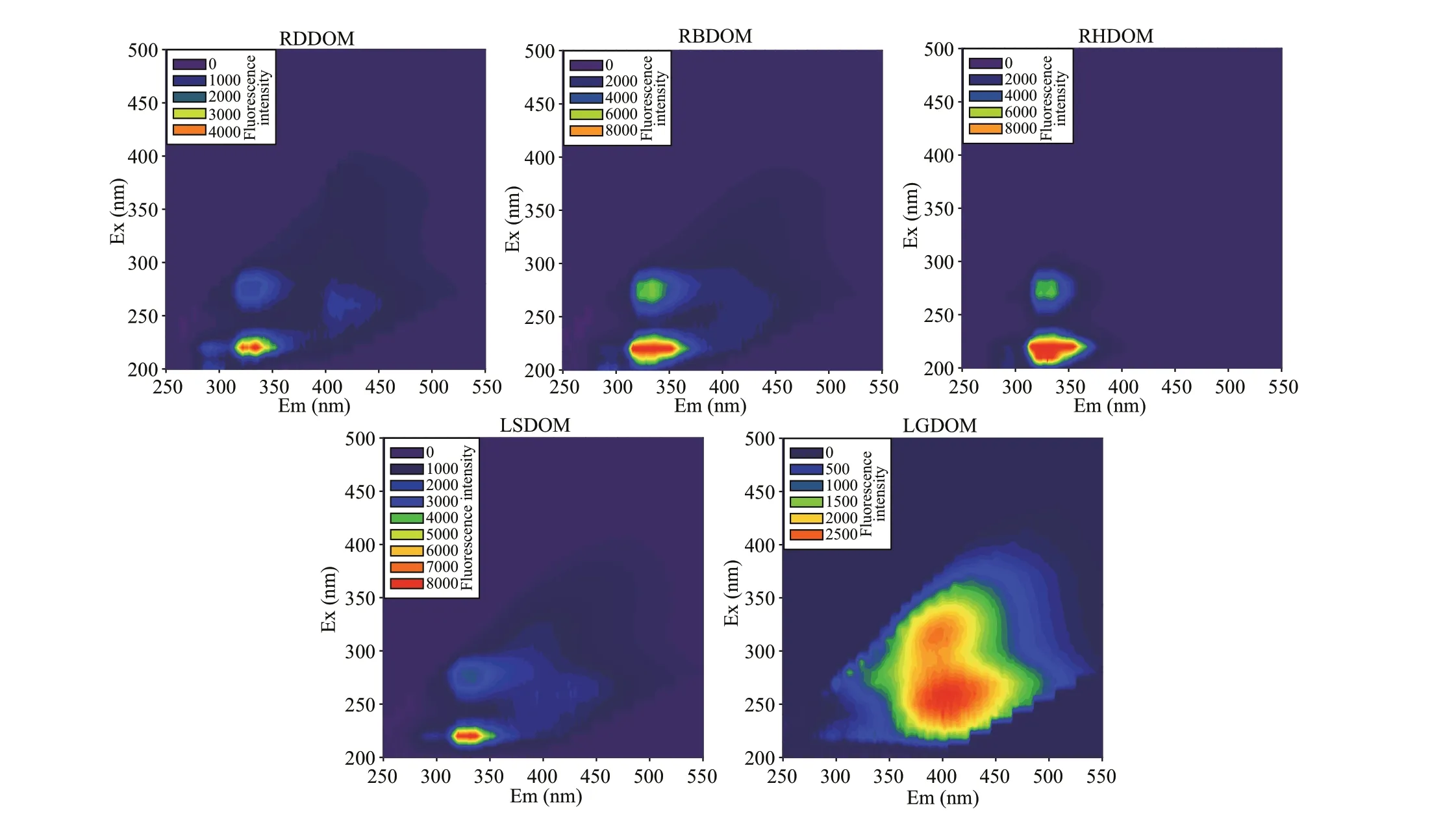
Fig.2 Fluorescence EEM spectra of DOMs from rivers and lake
We investigated the chemical structure of the f luorescent DOM from the results of f luorescence EEM analysis. As shown in Fig.2, the DOM from both the tributaries and the lake comprised four f luorophores, namely peak B (Ex/Em: 220 nm/330 nm), peak T (Ex/Em: 275 nm/330 nm), peak A1(Ex/Em: 260 nm/410 nm), and peak A2 (Ex/Em:320 nm/410 nm). Peaks B and T are ubiquitous protein-like peaks, which were also observed in the Jiulong River estuary (Yi et al., 2014), and in the Salt and Gila Rivers (Chen et al., 2013), and result from protein aromatic- and tryptophan-like f luorophores,respectively. Peaks A1 and A2 resemble humic acidand fulvic acid-like f luorophores, respectively, and were found in sediment DOM by Xu et al. (2019a). In the DOM from the tributaries, peaks B and T, fulvic acid-like f luorophores, were signif icantly higher than peaks A1 and A2, humic-like f luorophores, which shows that there were more bacterial soluble microbial products or bacteria in DOM from the tributaries compared to lake water. The humic-like f luorophore was almost absent from the f luorescence EEM spectrum, suggesting that RHDOM lacked humic substances. There were abundant protein-like peaks in the lake LSDOM, similar to the f luorescence EEM spectra of RDDOM and RBDOM, while LGDOM was abundant in the humic-like component. These results indicate that LSDOM was inf luenced by DOM from the tributaries and that LGDOM was dominated by humif ied (humic acid- or fulvic acid-like) material.
We calculated f luorescence indices, i.e., FIX, HIX,and BIX from the EEM data (Table 1), to identify the main sources of DOM. The HIX indicates the humif ication degree of DOM. The HIX values were very low (<1), which shows that the DOM from the lake or tributaries was dominated by non-humif ied DOM of biological or aquatic bacterial origin. These values were obviously lower than the values (>12)observed by Yang et al. (2015) in the less-polluted Longquanwan subterranean estuary. The value of HIX was signif icantly higher in LGDOM than in RHDOM, and corresponded well to the spectra discussed above. The FIX is an indicator of the sources of DOM with higher (~1.8) and lower (~1.2)values related to DOM released as extracellular material or leachate from bacteria/algae and terrestrial DOM sources, respectively (McKnight et al., 2001;DeVilbiss et al., 2016). The values of FIX were high in all the samples (>1.8), suggesting that the DOM was mainly from microbial sources. The BIX indicates the contribution of autochthonous sources or freshly produced DOM. The values of BIX were high for DOM in the lake than in both RDDOM and RBDOM,which shows that DOM in the lake was mainly derived from in situ products. The value of BIX was slightly higher in the LSDOM than in the LGDOM,which shows that the DOM in the lake surface water was more freshly produced than that in the groundwater.
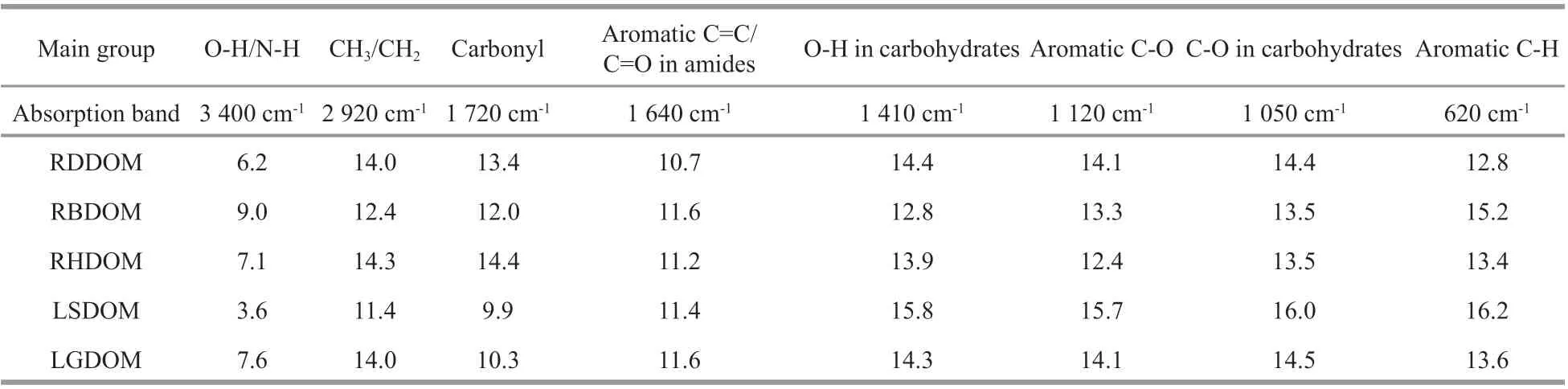
Table 2 A relative content in percentage (%) of FTIR regions of the river and lake DOM sample
3.3 Infrared spectroscopy of SPE-DOM
The major absorption bands in the FTIR spectra(Table 2) were located at 3 400 cm-1(O-H in carbohydrate/carboxyl or N-H groups in amide),2 920 cm-1(CH3/CH2in aliphatic), 1 720 cm-1(C=O stretching of carbonyl), 1 640 cm-1(C=C of aromatic and C=O in amides), 1 410 cm-1(O-H in-plane bending of carboxyl/carbohydrates), 1 120 cm-1(C-O in phenols), 1 050 cm-1(C-O of carbohydrates), and 620 cm-1(C-H in aromatics) (Abdulla et al., 2010b;Jiang et al., 2017). From further quantitative analysis(Table 2), it was clear that DOM from the tributaries(RD, RB, and RH) was richer in carbonyl groups(1 720 cm-1, mainly carboxylic acids) than DOM in the lake. This may be related to the complexation of carboxylic groups with inorganic components in saline lake water (Curtis and Adams, 1995; Xu et al.,2018a), which then causes the contents of the carbonyl groups in lake water DOM to decrease. The trends for oxygen-containing aromatics (1 120 cm-1)and carbohydrates (1 410 and 1 050 cm-1) were the opposite to those of the carbonyl group, but there is no clear explanation for this. It may be associated with the origin of the DOM or DOM photooxidation over time, which would have, given the long residence time of water in the lake, increased the oxygen-containing compounds in lake water DOM(Waiser and Robarts, 2000; Abdulla et al., 2010a).There were signif icantly more carbohydrates and aromatics (1 120 and 620 cm-1) in the LSDOM than in the LGDOM, which agreed well with the results from the carbohydrates and the SUVA254analysis.This suggests that the high molecular weight DOM aggregated and precipitated in groundwater, because of the high ionic strength (Xu et al., 2018b). There were more bands at 3 400 cm-1in LGDOM than in LSDOM and these bands probably could be assigned to N-containing compounds, in line with the information about TN.
3.4 Py-GC-MS analysis
The total ion chromatograms (TICs) of the pyrolysis products obtained from the lake- and tributary DOM are presented in Supplementary Fig.S1. Information about the peaks present is summarized in Supplementary Table S1. The compounds may be classif ied into monocyclic aromatic hydrocarbons,carbohydrates, phenols, polycyclic aromatic hydrocarbons, aliphatics, nitrogen-containing compounds, products of lignin and lignin-like phenolics, fatty acids, halogen organic compounds,and contaminants. The relative proportions of the compound groups were based on the total quantif ied peak area and the reference samples data. This analysis was carried out in duplicate, so the results in Table 3 are the average values.
Monocyclic aromatic hydrocarbons (MAHs)ranged from 20.79% to 43.33%, which was in the range of the values from the reference samples. As in numerous other studies (Jiang et al., 2017; Yang et al.,2017b; Zhang et al., 2018), the most abundant products from pyrolysis of DOM included toluene and alkylbenzenes with saturated alkyl chains (C1-C5). With no special origins, these compounds can be traced to many biomolecular sources (Saiz-Jimenez,1994; Li et al., 2017) and can also be produced artif icially during f lash pyrolysis (Zhao et al., 2009;Song and Peng, 2010). These groups were more abundant in DOM from the tributaries than from the lake. This result is comparable to the values for reference samples of DOM in stream water and water from an oilf ield and was also consistent with the UVVIS and FTIR data. The trend for aliphatics was the opposite, which suggests that MAHs may be photochemically oxidized, leaving the more aliphatic portions behind, as also reported by Donahue et al.(1998).
Carbohydrates, accounting for 24.37%-28.32%,were the second most abundant product from pyrolysis of DOM. These proportions were notably lower than those reported for stream DOM by Kaal et al. (2017),but similar to the values found in DOM in TGR soil(FL) from the Three Gorges Region (Jiang et al.,2017). Most of these compounds had a furan or cyclopentenone structure. In general, these pyrolysis products were associated with polysaccharides from microbial sources (e.g., algae, bacteria) and vascular plants. In this study, levoglucosan and other anhydrosugars were absent from the pyrolysis products of all the samples, indicating that the carbohydrate pyrolysis products herein originated predominantly from degraded and/or microbial polysaccharides, rather than vascular plant polysaccharides (Kaal et al., 2016). The relative proportions of these compounds were comparable in DOM from the tributaries and lake surface water and were signif icantly higher than in the LGDOM. This suggests that the microbes in groundwater were less active than those in the other types of water because of the high salinity (Curtis and Adams, 1995; Cawley et al., 2016).
The phenol and C1-C3alkylphenols accounted for 12.14%-15.73% of the products, and these proportions were slightly higher than the proportions in most reference samples. The phenolic compounds appeared to have multiple origins (Zhang et al., 2016), and other researchers have frequently described these as the products from the degradation of degraded lignin and proteinaceous biomass (Martin et al., 1979; Page et al., 2003). In our study, however, the lack of N-compounds suggests that these compounds did not derive from proteinaceous biomass and, because the trends were not consistent with methoxyphenols,were not from a lignin source. These compounds therefore probably had microbial or bacterial origins.In addition, the phenol contents were higher in the LGDOM than in the LSDOM, perhaps because there were more labile phenolic components in the LSDOM samples.
Polycyclic aromatic hydrocarbons (PAHs) may be derived from plant resins or coal deposits (González-Pérez et al., 2014; Kaal et al., 2016). The proportions of PAHs (1.89%-7.41%) were generally low in all the samples and were particularly low in the reference samples. Other researchers have reported that MAHs and PAHs were frequently associated with black carbon, which may have originated from wildf ires or the burning of fossil fuels, or perhaps was brought to the study area from other regions by monsoons (Xu et al., 2015; Kaal et al., 2016). It is worth noting that the contents of MAHs and PAHs were both high in RDDOM, and may have been related to coal deposits distributed throughout the drainage basin of the creek.
Aliphatic compounds are based on a polymethylene chain, including homologous series of n-alkanes and n-alkenes (C10-C17), and branched analogues and alicyclic structure compounds. These were much lower in the pyrolysis products from tributary DOM(6.42%-17.29%) than lake DOM (24.24%-25.04%).The results indicated that the chemical composition of the DOM changed obviously as it moved from the river to the terminal saline lake.
Among the derivatized phenolic products, those with a phenol, n-methoxy-structure, mostly originated from methylated guaiacyl lignin (Kaal et al., 2017),which is an important marker of terrigenous sources(Jiang et al., 2017). Buurman et al. (2009) observed that these products were more abundant in soil HAs compared to samples herein. In this paper, these compounds were absent from lake DOM. This result shows that tributary DOM had little eff ect on the chemical structures of DOM in the lake, which largely derived from in situ microbial activity rather than terrigenous plants.
Of the fatty acid compounds, only propanoic acid was observed in the pyrolysis products of RBDOM,which indicates that this group made only a minor contribution, as also found in the reference materials.There were no long chain fatty acids in the samples,suggesting that they were typical of autochthonous(algal or bacterial) sources (Jiang et al., 2017). Of course, it may have been diffi cult to detect fatty acids because of the cyclization products of decarboxylated fatty acids produced in the process of pyrolysis and incompatibilities between the free fatty acids and the apolar columns used in GC (Zhang et al., 2016, 2018;Yang et al., 2017a, b).
Pyrolysis product groups of contaminants (mainly phthalates) may originate from equipment used in sample handling and experimental processes. Finally,compounds with a halogen atom in its structure were also identif ied, and its source remains unknown.
The products from the pyrolysis of DOM from the lake and its tributaries mainly comprised degraded/microbial aromatics (including MAHs, PAHs, and Ph), carbohydrates, aliphatics, lignin derivatives, and N-containing compounds. The relative percentages of these pyrolysis products in reference materials from diff erent DOM sources varied greatly, indicating the complex and heterogeneous nature of DOM in diff erent environment settings (Table 3).
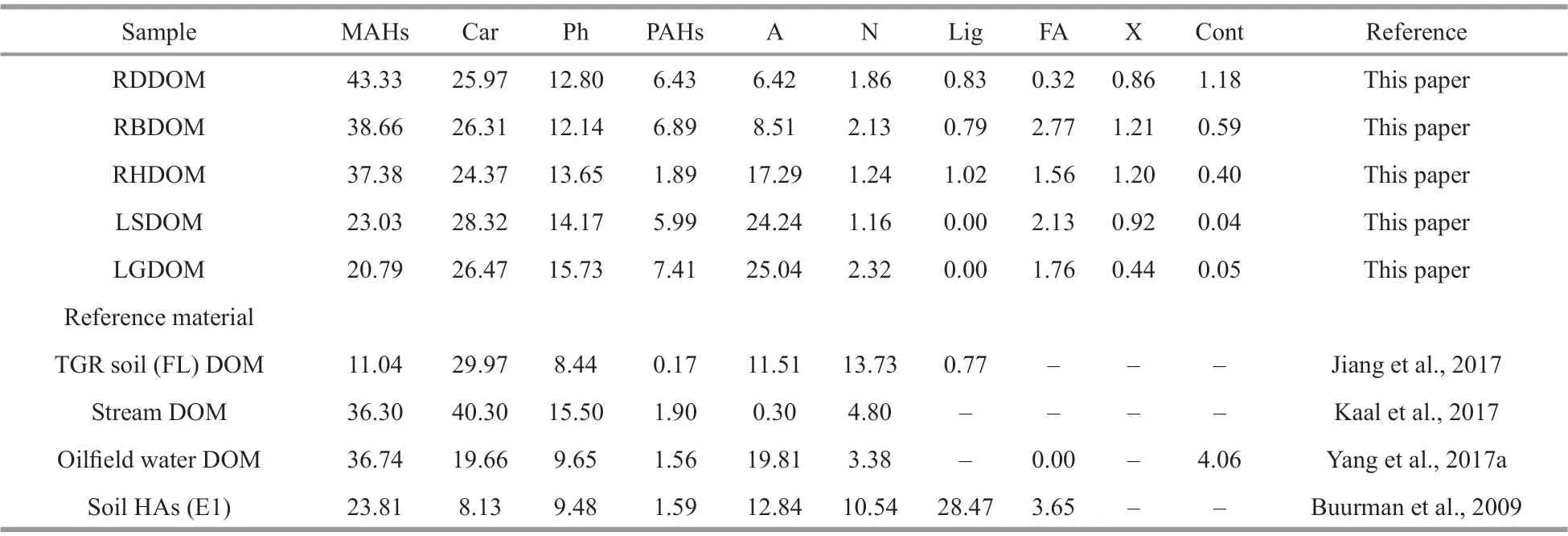
Table 3 Relative percentages (% of total quantif ied peak area, TQPA) of the main classes of compounds identif ied by Py-GC-MS
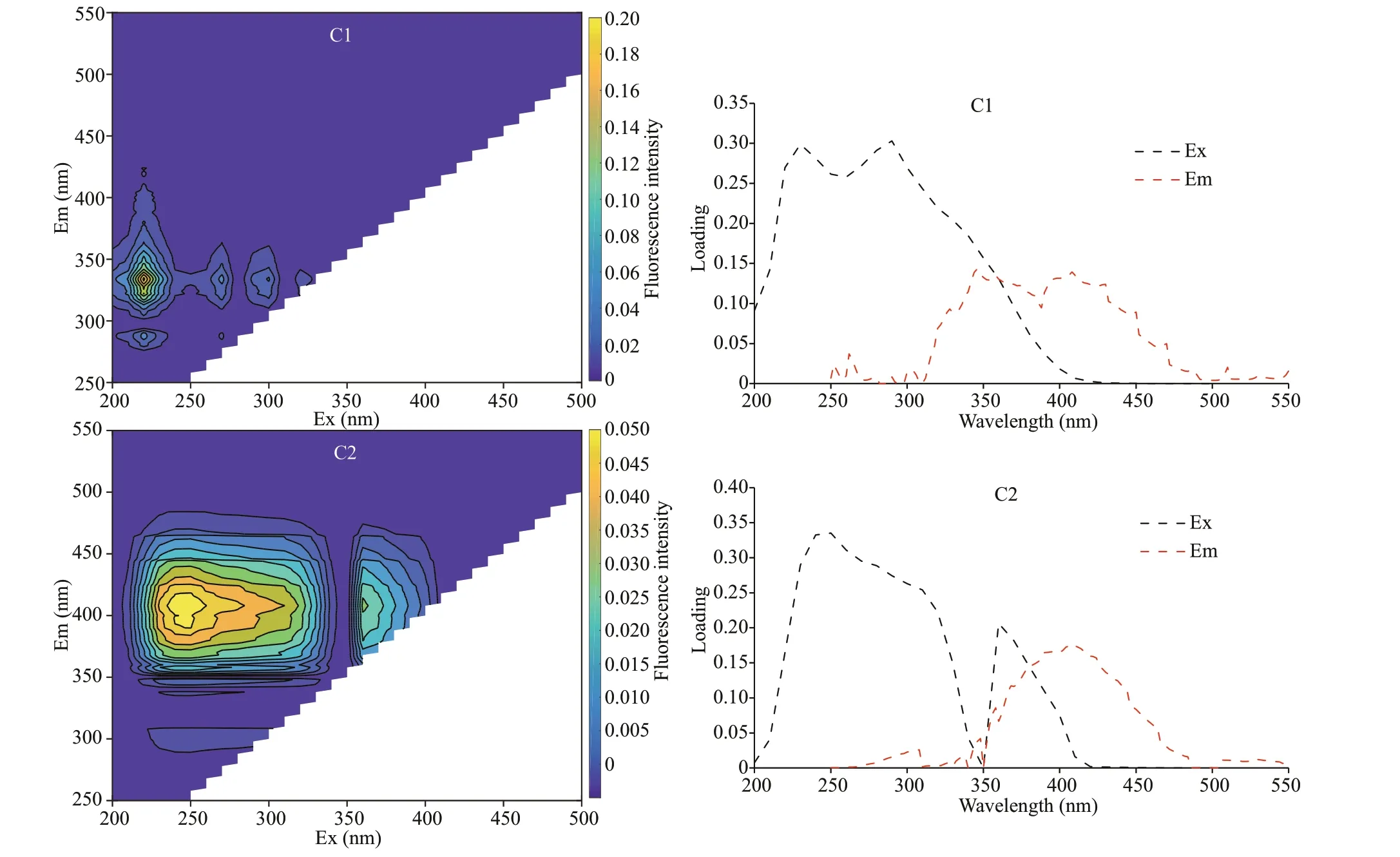
Fig.3 The EEM contours and the Em/Ex loadings of the PARAFAC-derived components C1 and C2
3.5 PARAFAC analysis and binding of PARAFACderived components with heavy metals
PARAFAC analysis was performed on the EEM spectra of the f ive samples titrated with Pb(Ⅱ) at diff erent concentrations to obtain more detailed information about the source-dependent binding behaviors of DOM components with heavy metals. As shown in Fig.3, two PARAFAC-derived components were successfully decomposed by PARAFAC analysis,after split half analysis, residual analysis, and visual inspection. Component 1 (C1) was a protein-like component with an Ex/Em of 230 nm/330 nm(aromatic-like f luorophore) and 300 nm/330 nm(tryptophan-like f luorophore), respectively.Component 2 (C2) may be classif ied as a humic-like component with a main peak (Ex/Em=250 nm/400 nm:humic acid-like f luorophore) and a secondary peak(Ex/Em=350 nm/400 nm: fulvic acid-like f luorophore). These PARAFAC-derived f luorescent components were similar to those in the EEM spectra(Fig.3), and have been frequently reported in other studies of rivers, lakes, and seawater (Coble, 1996;Stedmon et al., 2003; Guéguen et al., 2005; Coble,2007; Xu and Guo, 2017).
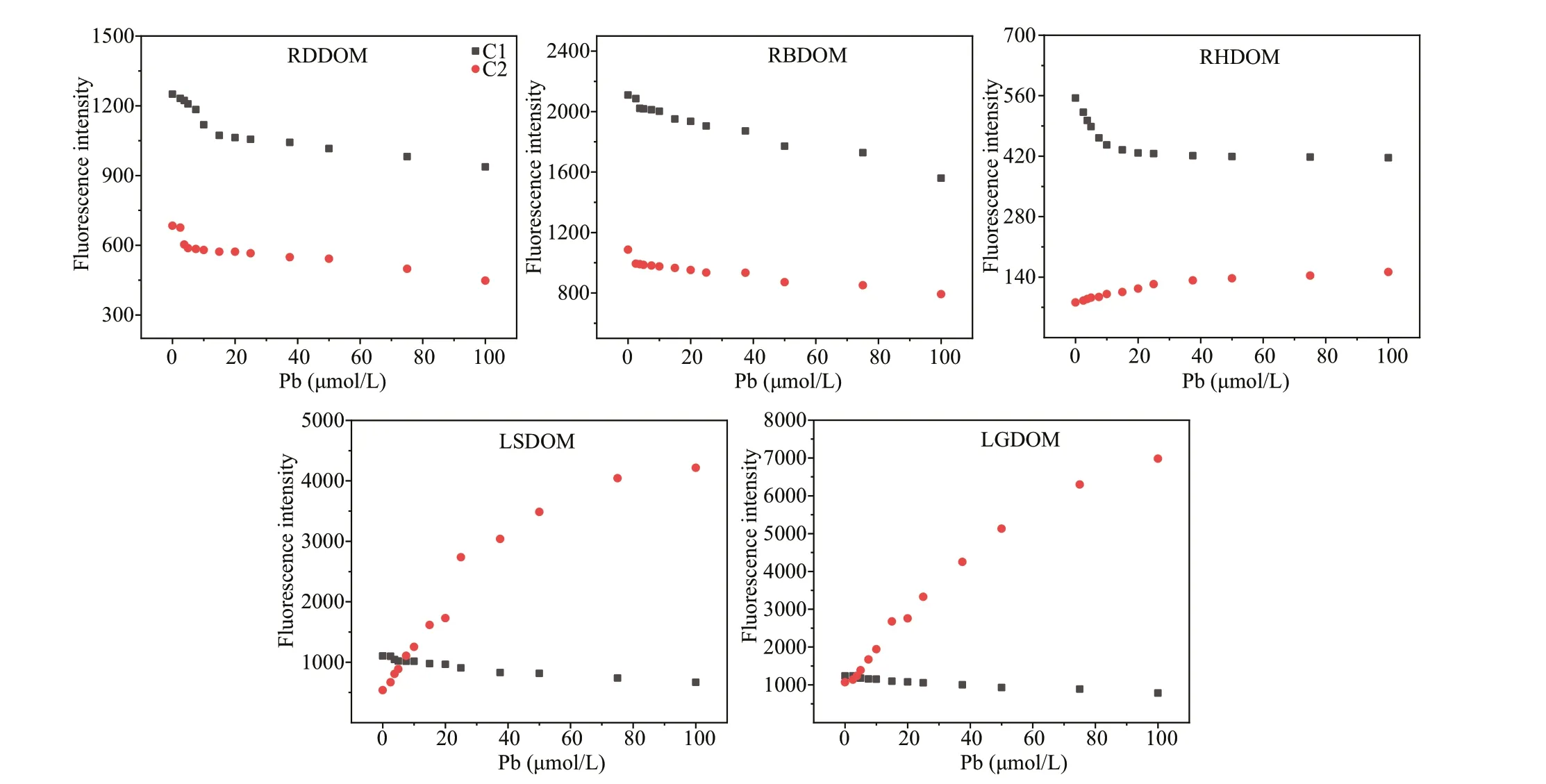
Fig.4 Variations of f luorescent intensities of the components derived from PARAFAC analysis with Pb(II) metal addition
The binding behaviors of each component with Pb(Ⅱ) are shown in Fig.4. As the metal was added, C1 initially decreased and then either declined gradually or stabilized regardless of the type of sample. The f luorescence quenching suggested that the proteinlike components bound eff ectively with the metals.Compared with the f luorescent quenching of C2 in tributary DOM (except the C2 of the RHDOM), the intensities of C2 in the lake DOM (LSDOM and LGDOM) increased as the metal was added, which shows that the DOM f luorophores from the lake and the tributary had diff erent binding mechanisms. Other researchers have observed enhanced f luorescence intensity for protein-like substances (Yamashita and Jaff é, 2008; Xu et al., 2019b), whereas the increases in the f luorescence for the humic substances were observed herein, indicating that the heterogeneities in the heavy metal binding properties depended on the origin.
The stability constants (logKM) were calculatedusing a modif ied version of the Stern-Volmer model to show the binding behaviors of PARAFAC-derived components with metals (Table 4). The logKMvalues ranged from 0.83 to 1.74 and from 0.53 to 1.20 for C1 and C2, respectively. These values were signif icantly lower than those found for Pb binding with DOM from other natural waters (4-6), such as freshwater and wastewater (Wu et al., 2011; Xu et al.,2019a), and were also much lower than those for other heavy metals, which were generally about 5(Yamashita and Jaff é, 2008; Zhang et al., 2010; Yu et al., 2012; Chen et al., 2013). The low binding capacity of DOM in this study may ref lect the fragile environmental system. The logKMvalue of PARAFAC-derived C1 in the LSDOM was lower than that in the LGDOM; this corresponded well with the trend in the aromatics (the sum of MAHs,PAHs, and Ph) in the lake water DOM from the Py-GC-MS analysis, and indicates that aromatics may provide more binding sites for C1. In addition, thefvalues of the f luorescent components ranged from 0.17 to 0.25 for river DOM and from 0.32 to 0.50 for lake DOM herein, respectively. These values are comparable with those found for other natural DOM(Chen et al., 2013; Xu et al., 2018a, 2019b). Thefvalues of PARAFAC-derived C1 and C2 in the DOM from the three tributaries were always lower than those of in the lake, indicating that the DOM binding sites were closely related to its origins. Thefvalue for C1 in the LSDOM was noticeably lower than that in the LGDOM. The binding capacity and stability constants derived from the Stern-Volmer model depend on many factors, such as the pH, temperature,ionic strength, and the metal concentrations (Cao et al., 2004). The results are therefore only valid for the experimental conditions under which they were performed.
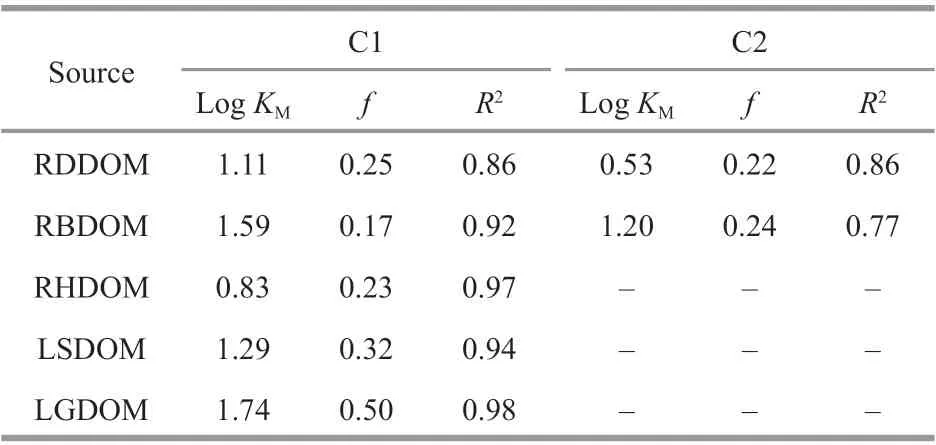
Table 4 Binding properties of Pb(Ⅱ) with DOMs from rivers and lake
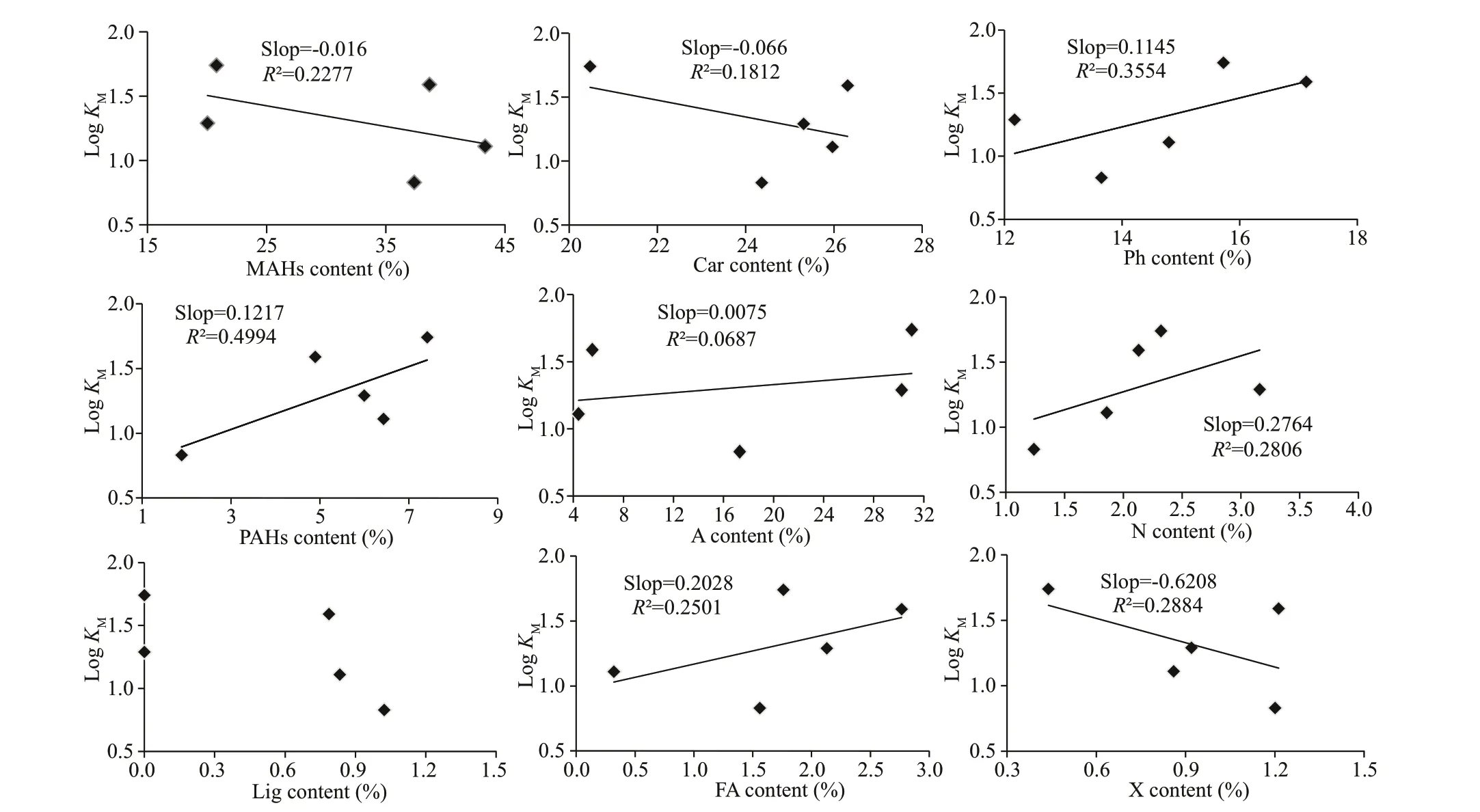
Fig.5 Correlations between the relative abundance of organic components and stability constant log K M of C1 derived from PARAFAC model
3.6 Relationship between pyrolysis groups and the binding behaviors of f luorophores with trace metals
Previously, studies of the binding properties of DOM with metals have often considered the f luorescent component. Here, it was diffi cult to carry out titration experiments and apply metal-DOM binding models of molecular level components or functional groups because of the limited technology.Results fromt-tests (P<0.05) suggest that there were signif icant diff erences in the data for pyrolysis products (except for fatty acids and halogen compounds) and stability constants. We carried out Pearson’s correlations between the diff erent pyrolysis groups and logKMof C1 (the main f luorophore in our samples), as presented in Fig.5. The relative abundances of phenols, PAHs, and N-containing compounds were positively correlated with logKM,which suggests that these groups may enhance the binding behaviors.
Ohno et al. (2014) demonstrated that DOM complexed minerals by ligand exchange reactions with -OH functional groups. We investigated the relationships between diff erent pyrolysis groups and binding parameters, and found that the binding capacity of metal ions was positively correlated with groups of phenols, PAHs, and N-containing compounds. This suggests that the functional groups of -NH or cavity structures of PAHs eff ectively facilitate the binding of DOM with metals.
4 CONCLUSION
The results from various analyses indicate that tributary DOM had a lower DOC content, higher molecular weight, and higher SUVA254than DOM in the lake. The EEM spectra showed that protein-like f luorophores were mainly distributed in the tributary and lake surface DOM, and that lake groundwater DOM was rich in humic-like substances. The FTIR and Py-GC-MS data indicated that DOM from the lake watershed had mainly microbial origins, and was composed primarily of aromatics, carbohydrates, and aliphatics. Further quantitative analysis revealed that the tributary DOM contained more aromatics, lower carbohydrates, and lower aliphatics than that of in the lake. The LSDOM had higher MAHs and carbohydrates than the LGDOM. Using the Pb(II)titration technique coupled with PARAFAC analysis,we found that PARAFAC-derived components with trace metals had relatively low condition stability constants (logKM<2). Of the two types of DOM from the lake, the LGDOM had higher potential to bind Pb(II) than the LSDOM. The organic matter of phenols, PAHs, and N-containing compounds can also contribute to the binding constants of DOM with metals. We investigated the chemical compositions of DOM from a hypersaline lake and inf lowing tributaries and its ability to bind with metals. The results provide insights into the behavior and fate of DOM and metal ions in such watersheds, and also can be compared with the f indings from studies of other natural waters.
5 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
6 ACKNOWLEDGMENT
We would like to sincerely thank the two anonymous reviewers for the valuable comments on an earlier version of the manuscript, and thanks to the editorial offi ce of JOL for the proofreading of references and the f inal version of the manuscript.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Characterization of dissolved organic matter in submarine groundwater from a salt marsh in Chongming Island, China*
