Taxonomy and regeneration of a newly recorded Polychaete Capitella teleta (Annelida, Capitellidae) in the coastal water of Shandong, China*
Qian LI , Yongnan LI , Yue WANG , Xuwen WU ,4,**, Linlin ZHANG ,**
1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology & Center of Deep Sea Research, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Department of Marine Organism Taxonomy & Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
Abstract The polychaete species of Capitella are widely distributed in the China seas, however little is known about Capitella taxonomy, and specimens collected from China have been identif ied as Capitella capitata (Fabricius, 1780) for more than 50 years. C. capitata was considered to be Arctic and subarctic in distribution, therefore the records of C. capitata in the China seas probably represent other species. A taxonomic study based on the samples collected from the northeast coastal water of Shandong Province reveals a diff erent species, Capitella teleta Blake et al., 2009, which is recorded in the China seas for the f irst time. Morphologically, C. teleta can be easily distinguished from C. capitata by the absence of neuropodial capillaries on chaetigers 8 and 9. The identity of C. teleta is further supported by genetic distance and phylogenetic analyses assessed from mitochondrial cytochrome c oxidase subunit I (COI) gene. In addition,the regeneration feature of C. teleta was studied through whole mount immunohistochemistry and chemical staining. After amputation, the wound of C. teleta was healed within 24 h, forming a signif icant regeneration blastema by 3 days post amputation (dpa). By 5 dpa, muscle tissues regenerated, nerve f ibers also extended.By 7 dpa, neurites and muscle tissues are both signif icantly regenerated. Notably, there are more than ten segments regenerated until 16 dpa. As a highly opportunistic species, Capitella teleta is distributed in China,Japan, Korea, North America, and the Mediterranean. It is expected to be an excellent model for studying developmental genetics and evolution of regeneration.
Keyword: Annelid; Capitella teleta; regeneration; Chinese waters
1 INTRODUCTION
Capitellais an elongate, thread-like marine benthic annelid, which is globally known in ecological research as an indicator to marine pollution (Grassle and Grassle, 1976; Dean, 2008).Capitellaspecies are common in the Chinese marine regions, and often dominant in high densities of up to 127 200 inds./m2(Wu, 1964; Yang and Sun, 1988). Wu (1964) reported the f irst species ofCapitellain China,C.capitata(Fabricius, 1780), based on the specimens of the Yellow Sea and the East China Sea. However,Capitellaspecimens collected from diff erent locations of Bohai Sea, Yellow Sea, and the East China Sea exhibit various types of chaetal arrangement,developmental patterns, and ecological characters,which might represent a series of diff erent species(Wu et al., 1988; Lin et al., 2008). Although several sibling species were reported with provisional names in China (Wu et al., 1988), so far, all the specimens ofCapitellahave been identif ied asC.capitata(Fabricius, 1780) without a detailed taxonomic study.

Fig.1 Distribution of the species Capitella teleta based on this study and related literatures
Capitellacapitatawas formerly considered as a cosmopolitan species and had been widely used as an indicator of environmental pollution for a long time.However, various studies over the past 40 years suggested the nominal “C.capitata” include approximately 12-13 sibling species (Grassle and Grassle, 1976; Gamenick et al., 1998; Méndez, 2006).In order to clarify the species, Blake (2009) provided a detailed re-description ofC.capitatabased on a large collection of specimens and indicatedC.capitatais distributed in Arctic and subarctic areas.Therefore, previous records ofC.capitatafrom boreal and temperate localities, including Chinese waters,should be re-evaluated.
Regeneration is a process of re-growing a partial or whole body after being damaged. It is a wide-spread phenomenon in the animal kingdom. However, the regeneration ability is signif icantly diff erent depending on the organism groups. For example,some animals such as planarian and hydra can regenerate their whole body from a fraction (Wagner et al., 2011; Buzgariu et al., 2018), while zebraf ish and salamander are able to regenerate a partial tissue only (Mierzwa et al., 2020; Pronobis and Poss, 2020;Sibai et al., 2020).Capitellahas been recognized as a model in regeneration study for long years as its remarkable regeneration abilities (Seaver, 2016).
Based on the examination of the specimens ofCapitellafrom the coastal water of Shandong, a newly recorded species in China,C.teletaBlake et al., 2009 was verif ied through morphological and molecular analysis. Being one of the original sibling species ofC.capitata,C.teletahas been commonly used as an experimental species under the provisional nameCapitellasp. I for over 40 years. It was described as a new speciesCapitellateletaby Blake et al. (2009). The species is widely distributed along the east and west coasts of North America, Korea, Japan, and the Mediterranean, and considered as a highly opportunistic species. Furthermore, the regeneration process ofC.teletawas studied through whole mount immunohistochemistry and chemical staining (Hill and Boyer, 2001; Meyer et al., 2015). The muscle and nervous tissue ofC.teletaregenerated rapidly, more than 15 segments regenerated at 16 dpa.Capitellateletais expected to be an excellent model in the functional study and regeneration evolution study of marine invertebrate due to its high regenerative ability,easy to culture in lab settings, and wide distribution in coastal waters. This is for the f irst time that the species have been recorded in China seas. We described the morphology ofC.teletain this study, and displayed the phylogenetic analysis ofCapitellaspecies based on the partial sequences of COI gene. We also investigated the rapid regeneration ability of this species.
2 MATERIAL AND METHOD
2.1 Collection, laboratory culture, and morphological examination
Specimens were sampled from the Weifang Port adjacency (119°3′53.54″E, 37°12′20.91″N) and Qingdao Zhanqiao Pier (120°19′15″E, 36°3′40″N),Shandong Province, China on October 30, 2019 and September 9, 2020, respectively (Fig.1). All specimens were taken back to laboratory and cultured in a 16-19 ℃ temperature incubator, where they were kept in fresh sea water and fed with sea bottom mud once a week.
Specimens examined were anesthetized with 7.5%magnesium chloride and then f ixed in 70% ethanol solution (Lewbart and Mosley, 2012). Details of prostomium, nuchal organs, segments, parapodia, and chaetae were observed through a ZEISS DiscoveryV12 stereomicroscope (ZEISS, Germany) and a ZEISS Axio Imager Z2 compound microscope (ZEISS,Germany) according to Blake et al. (2009). Digital images were taken through an AxioCam 512 color camera mounted on the two microscopes. Images of diff erent focal planes were stacked through the extended depth of f ield by the software Helicon Focus 7 (Helicon, Ukraine). The collected specimens were stained with methyl green to increase the contrast and to observe the staining pattern according to Blake et al. (2009). Specimens for SEM (scanning electron microscope) observation were f ixed in 2.5%glutaraldehyde solution at 4 ℃ for 1 h, dehydrated with gradient concentrations of ethanol ending up with 100% ethanol, followed by drying at critical point, coated with gold, and f inally, observed and photographed using the HITACH S-3400N scanning electron microscope (HITACH, Japan).
2.2 DNA extraction and sequencing
Five specimens, consisting of three Weifang worms and two Qingdao worms, were embedded in 0.5%cornmeal agarose plate for 2 h to remove as much gastrointestinal tract matter as possible. Afterwards,the genomic DNA was extracted from the posterior segments using Fastpure®Cell/Tissue DNA Isolation Mini kit (VazymeDC102, Nanjing, China) (Tomioka et al., 2016). The primer pair was jgLCO 1490(5′-TITCIACIAAYCAYAARGAYATTGG-3′) and jgHCO 2198 (5′-TAIACYTCIGGRTGICCRAARAAYCA-3′) (Geller et al., 2013). The 25-μL PCR(Polymerase Chain Reaction) mix contained 8-μL sterile water, 2.5-μL DNA template, 1-μL each primer of 10-μmol/L, 12.5-μL (10 μmol/L) 2X Phanta Master Mix (Vazyme P511, Nanjing, China). PCR was conducted on a T100TMThermal cycler (Bio Rad,USA) with the following thermal conditions: 95 ℃for 3 min (initial denaturation); 35 cycles of 95 ℃ for 15 s (denaturation), 60 ℃ for 15 s (annealing), 72 ℃for 1 min (extension), and a further 5 min elongation at 72 ℃. Subsequently, the PCR products were detected by 1.0% agarose gels (BBI Life Sciences 9012-36-6, Shanghai, China) electrophoresis.Amplif ied DNA was sequenced in Single directions using 3730xl ABI by Sangon Biotech (Shanghai)Corporation.
2.3 Barcoding and phylogenetic analysis
Five sequences ofC.teletain our study were performed for barcoding and phylogenetic analysis.Related sequences ofCapitellafor phylogenetic analysis were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/). The outgroup comprised species ofNereisandSipunculus. The sequences were aligned using MEGA6 with the ClustalW program (Tamura et al., 2013). Genetic distances of mitochondrial cytochrome c oxidase subunit I (COI) within and among the clades ofCapitellawere calculated using MEGA6 with Kimura’s two-parameter model (Tamura et al., 2013).Maximum likelihood (ML) analysis was carried out using MEGA6 with 1 000 bootstrap replicates.
2.4 Antibody staining and phalloidin staining
After anesthesia and amputation, the whole mount immunohistochemistry forC.teletain China was performed according to the method of de Jong and Seaver (2016), followed by f ixation with 4%paraformaldehyde (PFA) (Sigma-Aldrich P6148,Darmstadt, USA) in f iltered sea water (FSW) for at least 40 min at room temperature (RT) for target samples. Samples were subsequently washed with washing solution (PBT: phosphate buff er saline(PBS)+0.1%Triton X-100, Sigma-Aldrich T8787,Darmstadt, USA) for several times. After that, samples were blocked in PBT with 10% normal goat serum for 60 min at room temperature. Then the worms were incubated in primary antibody overnight at 4 ℃ while gently rocking, followed by secondary antibody incubation for 4 h at RT while rocking. After rinsing for several times, samples were labeled by 1꞉400 phalloidin(Thermo Fisher A12379, Massachusetts, USA) in PBT with 10% normal goat serum for 60 min at room temperature while rocking. Finally, all samples were incubated in Hoechst33342 (Thermo Fisher H3570,Massachusetts, USA) overnight at 4 ℃. Then images were taken by confocal microscopy LSM710 (ZEISS).Primary antibody was 1꞉400 mouse anti-acetylated-αtubulin (Sigma-Aldrich 6-11b-1, Darmstadt, Germany USA), and second antibody was 1꞉250 donkey antimouse secondary antibody (Invitrogen A10036,Carlsbad, CA, USA). All f igures were edited in Adobe Photoshop CS6 and Adobe Illustrator CS6.
3 RESULT
3.1 Taxonomy
Family Capitellidae Grube, 1862
GenusCapitellaBlainville, 1828
CapitellateletaBlake et al., 2009
Figs.2a-j, 3a-i; Tables 1-3
Material examined: Samples were collected in the intertidal zone (119°3′53.54″N, 37°12′20.91″E) in Weifang Port adjacency, Weifang, Shandong, China,collected by Qian LI and Yongnan LI on Oct. 30,2019 (WF200726.1-24, 24 specimens); and the intertidal zone (120°19′15″E, 36°3′40″N) in Zhanqiao Pier, Qingdao, Shandong, China, collected by Xiaofei ZHU, Jieyang WENG, and Jie LI, on Sep. 9, 2020(ZQ200909.1-18, 18 specimens). Molecular analysis was conducted for WF200726.22-24 and ZQ200909.14-15; micrographs were taken for WF200726.7-10, 17-21, and ZQ200909.17-18;SEM was done on WF200726.11-16.
Description: Living specimens bright red or light yellow, preserved animals with pale color (Fig.2c-j).5.0-11.0-mm long with 39-60 chaetigers (based on collected material). 0.5-1.1-mm wide. Body slender,slightly wider in anterior segments, gradually narrow posteriorly.
Prostomium f lat, triangular and narrow (Fig.3a).Eyes present in vivo, located in lateral side of prostomium, not observed after preserved.Peristomium and prostomium bounded by nuchal organs. Head composed of peristomium and prostomium, with no appendages, usually longer than the 1stchaetiger (Fig.3a & b). Proboscis not exposed.
Nine thoracic segments with small folds (Figs.2c-e,3b). Chaetal arrangement of thoracic segments on 9 segments varies. Chaetigers 1-7 with capillary chaetae on both notopodia and neuropodia, 3-8 per fascicle (Fig.3b). Capillaries slender, tapering posteriorly (Fig.3d). Chaetigers 8-9 of females with neuropodial hooded hooks, 3-8 per fascicle.Chaetigers 8-9 of males with 2 pairs of genital spines on notopodia (Figs.2a, d, f, j, 3e). Anterior end of hooded hooks curve to hood shape (Fig.3f). Hooded hooks with a robust main fang (Fig.3i) and small teeth around main fang (Fig.3h & i). Genital spines with a sharp and pointy tip. They are diff erent in chaetigers 8 and 9. Chaetiger 8 with six or eight shorter spines,chaetiger 9 with four robust genital spines (Fig.3e).Genital spines on chaeigers 8 and 9 oppositely arranged (Figs.2f & 3e). Thoracic setigers usually wider than abdominal setigers (Fig.3b & c).
Abdominal region with many narrower segments,gradually thinner posteriorly. Abdominal segments with ventral and lateral grooves, more wrinkled than thoracic segments (Fig.3c). Abdominal chaetae all hooded hooks, similar to thoracic hooded hooks;anteriorly 2-4 hooks per fascicle, reduced posteriorly to 1-2 hooks. 1-4 per fascicle (Fig.3g & h). Pygidium with rounded lobes (Fig.3c).
MostC.teletaare gonochoristic. Mature females with pairs of ovaries on some abdominal segments(Fig.2i). Mature males with genital spines on chaetigers 8 and 9 (Fig.2d & j). The average number of embryos produced per female less than one hundred within a brood tube. Embryos f irstly develop in a brood tube and then release until the late stage of larvae. It will take 7-9 days to f inish a transition from single-cell embryo to late trochophore. In addition, no food is required during this period, and then followed by living benthic life, become reproductive approximately 8 weeks after fertilization.
Methyl green staining: Males were dyed blue except for the chaetiger 10 (Fig.2a). The thoracic staining of the female was not obvious, only light staining was observed on chaetigers 5-8. Abdominal part from chaetiger 15 to few chaetigers before pygidium were stained blue (Fig.2b). Four worms were treated with methyl green dye, including 3 females and 1 male. In 3 females, methyl green staining pattern (MGSP) was consistent.
Remark: Although some Capitellidae species have been found (Lin et al., 2019a, b),C.capitatawas the only recorded species ofCapitellain the whole China Seas according to the description given by Yang and Sun (1988), “C.capitata” is characterized by the following chaetal arrangement: chaetigers 1-7 of both females and males with capillaries; chaetigers 8-9 of females with hooded hooks on notopodia and neuropodia, and notopodial hooded hooks replaced by genital spines in males. However, these characters are consistent withC.teletain this study. It indicates that the specimens ofC.teletawere formerly misidentif ied asC.capitataby Chinese authors.According to Blake (2009),C.capitatas. str. is considered Arctic and subarctic in distribution,therefore early records ofC.capitatafrom the China should be re-examined.
Morphologically,C.teletacollected from Shandong exhibited some diff erences from American, Korean, and Japanese populations in chaetal number per fascicle, methyl green staining pattern, and number of genital spines of males (Table 1), which represents intraspecif ic variations among diff erent populations ofC.teleta.
3.2 Barcoding and phylogenetic analyses
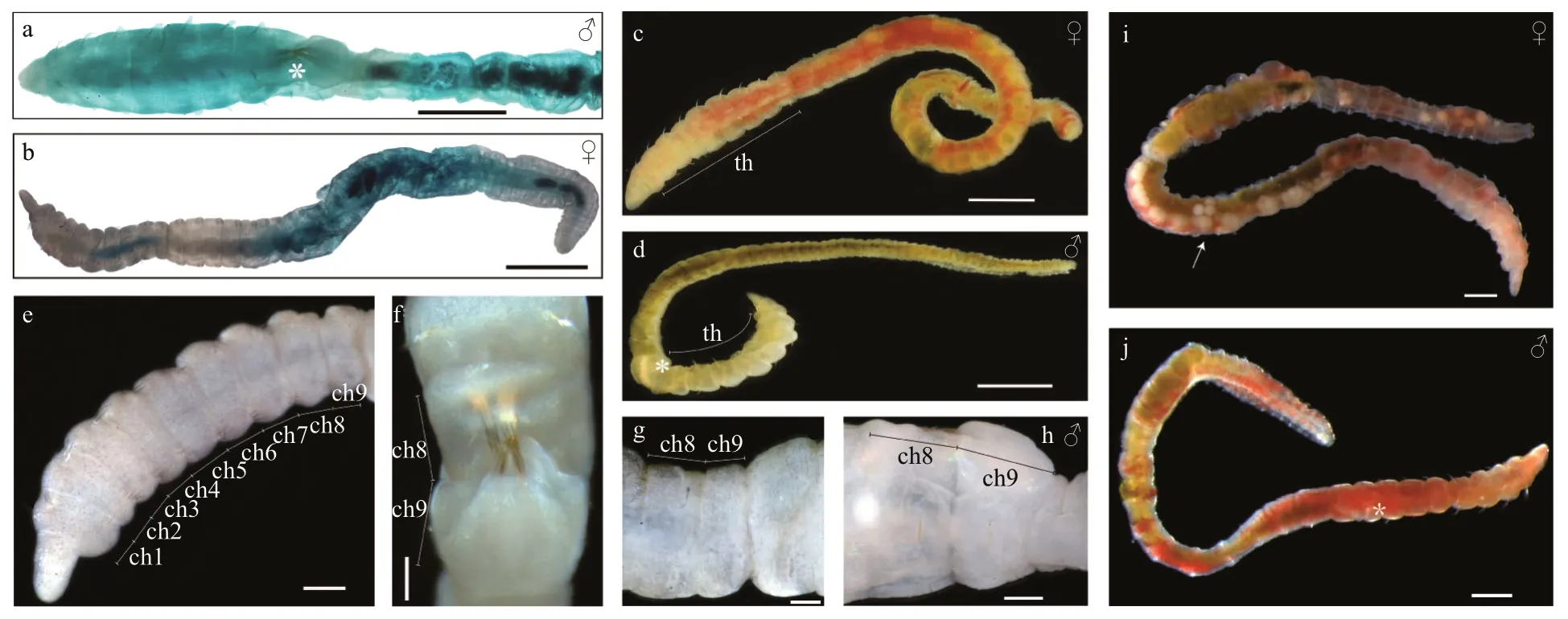
Fig.2 Micrographs of Capitella teleta
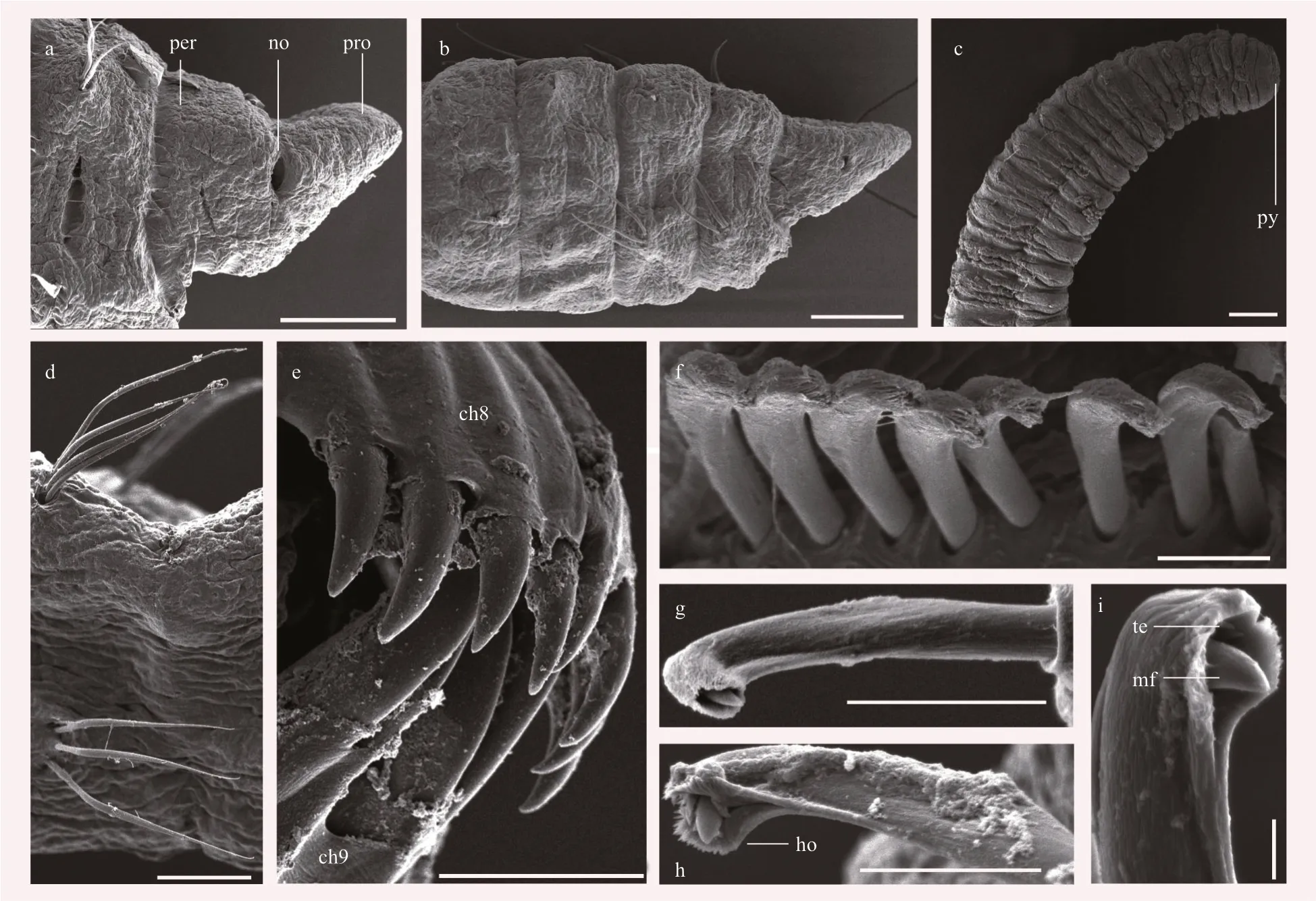
Fig.3 Scanning electron micrographs of Capitella teleta
The genetic distances and phylogenetic analyses assessed from COI (Fig.4; Table 2) were performed to clarify the status ofC.teletain this study.Capitellaspecies were grouped into 6 clades based on the ML phylogenetic tree (Fig.4). Populations ofC.teletafrom China, Japan, Korea, America, and Italy were clustered together in Clade 1 with high node support(Tomioka et al., 2016; Jeong et al., 2018); Clade 2 consisted of a species closely related toC.teletafrom Gamo of Japan with an inconclusive taxonomic identity; Clade 3 included an unknown species ofCapitellafrom Japan; members of both Clade 4 and Clade 5 were identif ied asC.capitata, collected from Canada and India, respectively. Strangely, the worms,both from Canada, were grouped into 2 clades, this suggested one of populations ofC.capitatamight represent another species and warrant reinvestigation.Clade 6 contained twoCapitellaspecies from the United States.
K2P distances within and among the 6 clades were calculated (Table 3). The COI genetic distances among the species ofCapitellaranged from 3.7% to 23.7% (12.4%-23.7%, excluding Clade 2), while the intraspecif ic distances within 6 clades were very low(0.2%-1.9%). Excluding Clade 2, there was a clear barcoding gap (1.9%-12.4%) for the species ofCapitella. The K2P distances among the Clade 1 ranged from 0.000 to 0.017 (mean 0.003), indicatingthat our specimens from Shandong represent the same species as in Japan, Korea, America, and Mediterranean, and should be identif ied asC.teletawith no doubt.
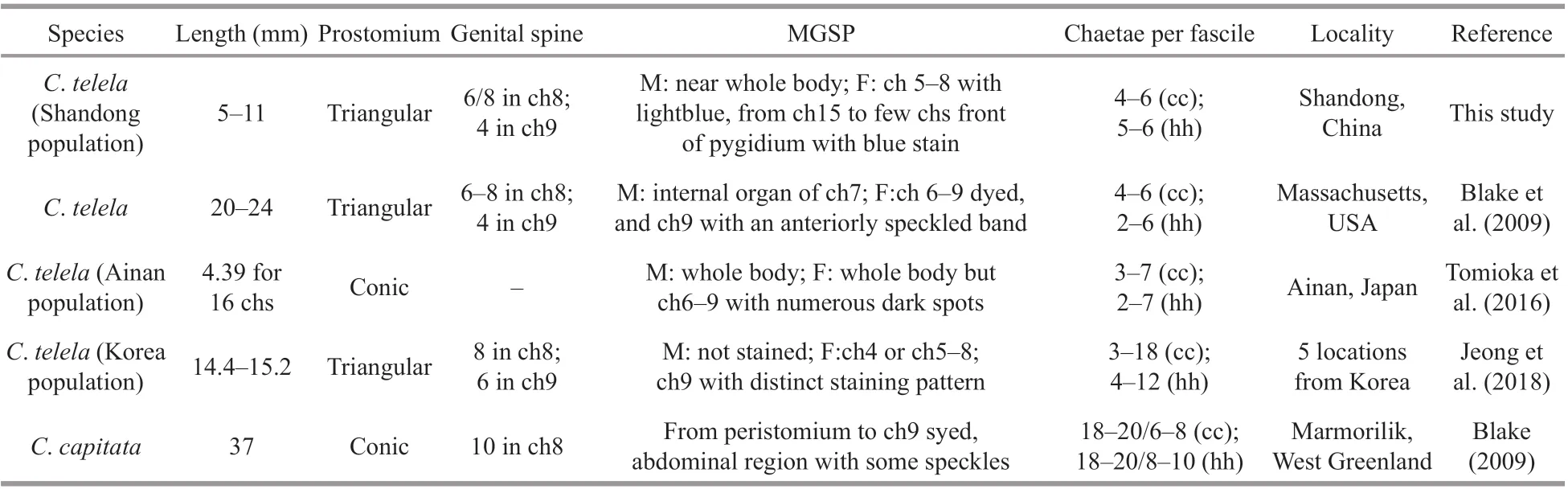
Table 1 Morphological diff erences between C. capitata and C. teleta species from diff erent regions
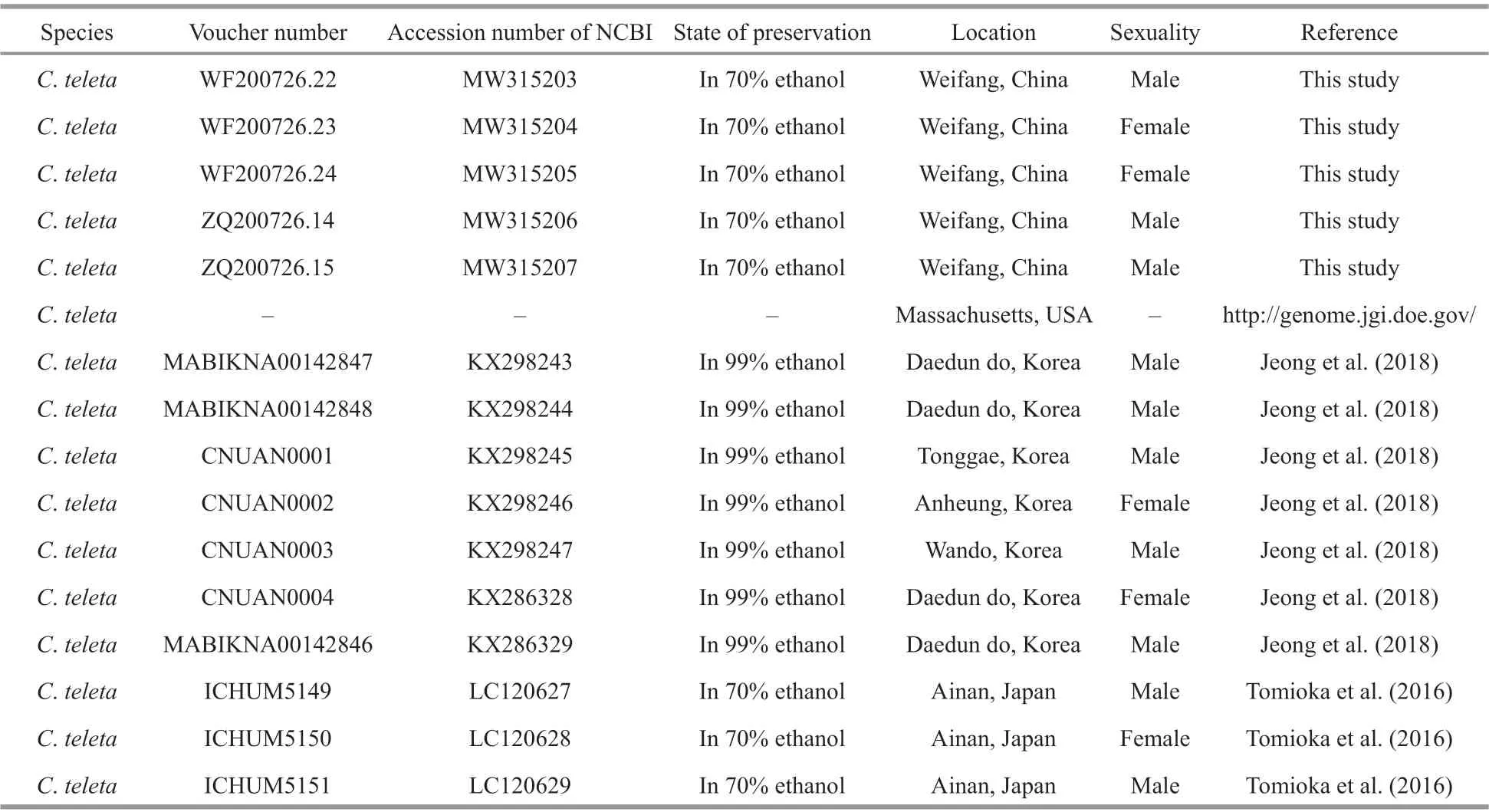
Table 2 Sampling information of Capitella species used for barcoding and phylogenetic analyses
To be continued
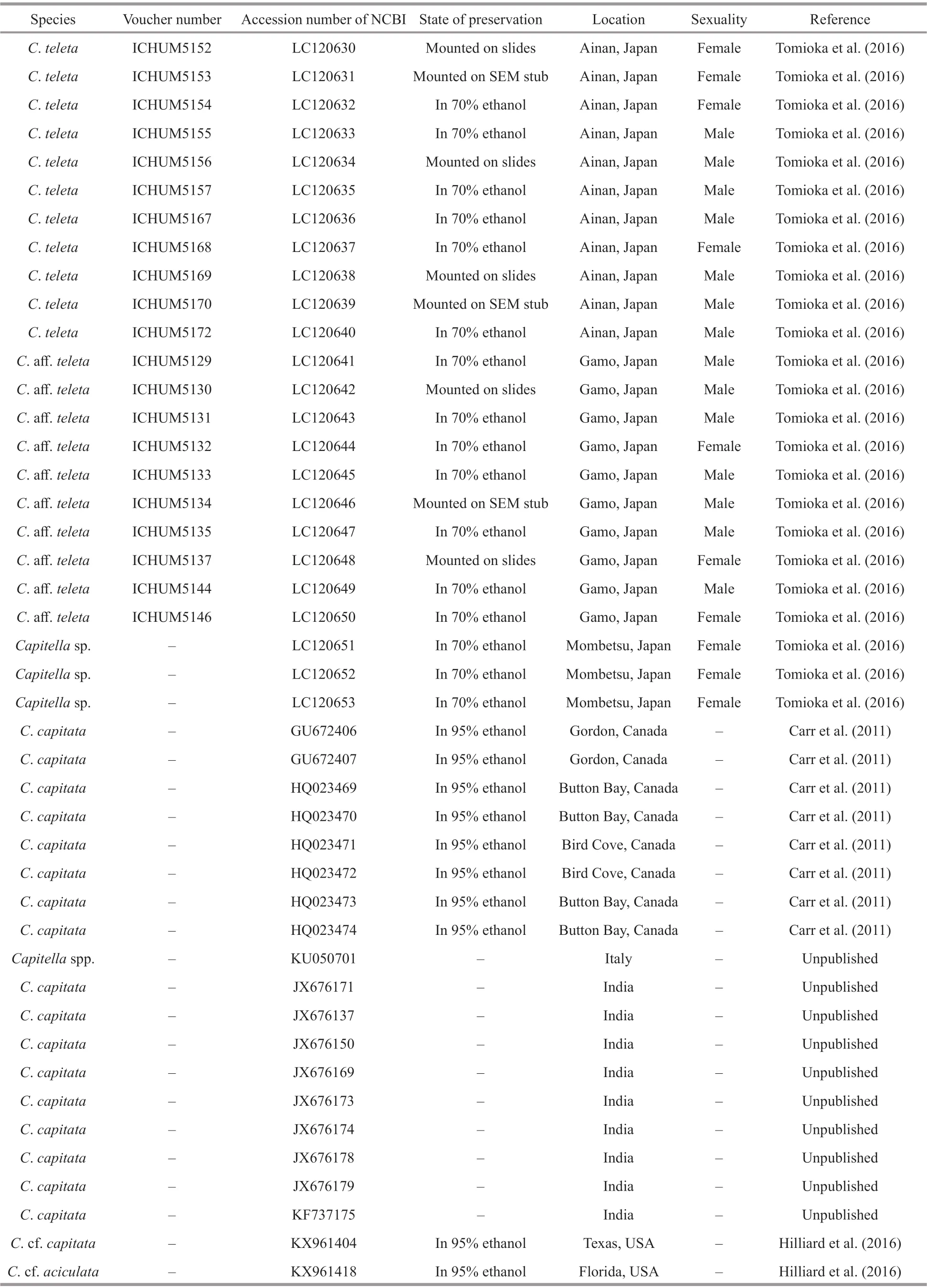
Table 2 Continued
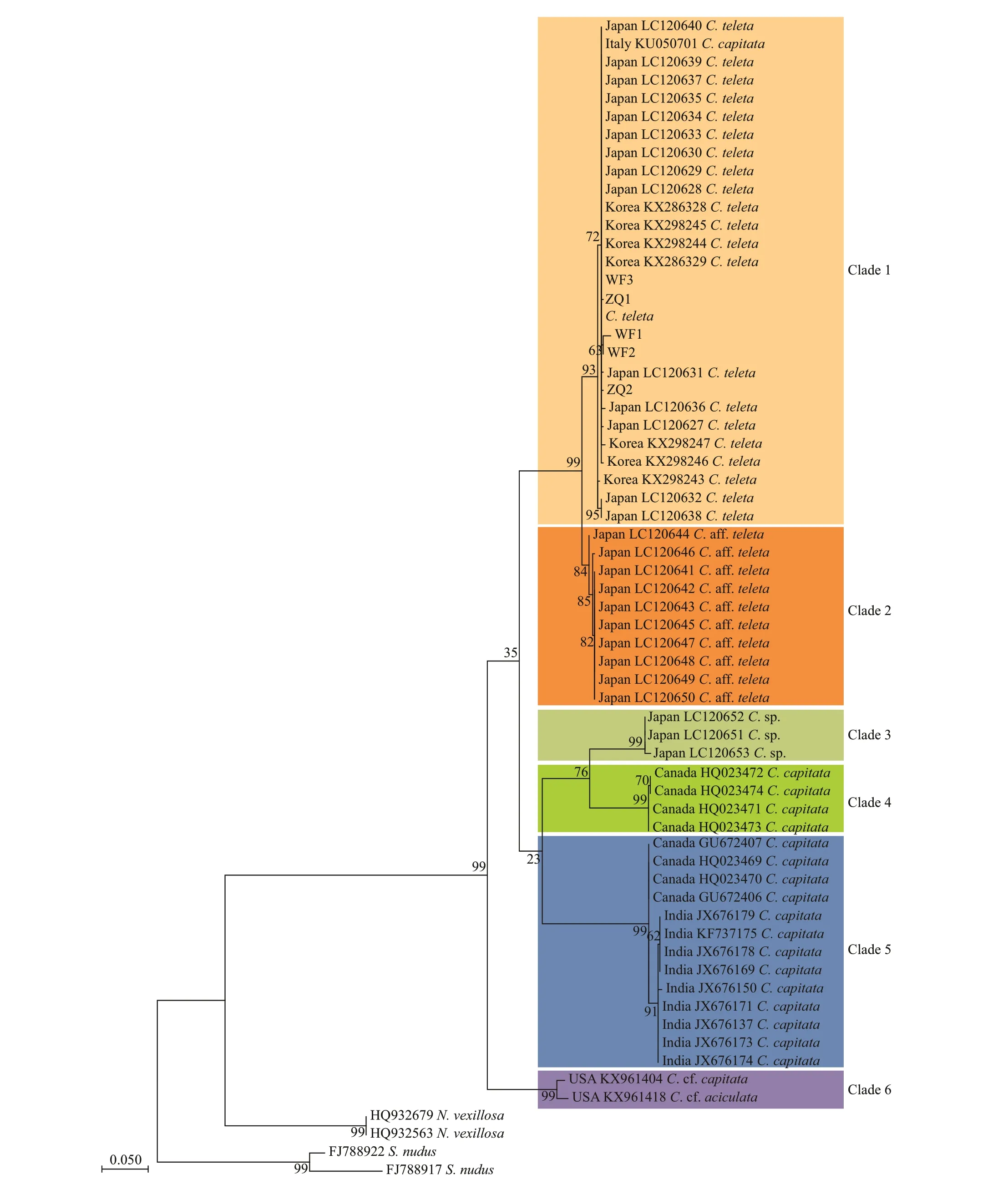
Fig.4 Maximum-likelihood (ML) tree based on COI gene sequences of Capitella teleta and the relatives
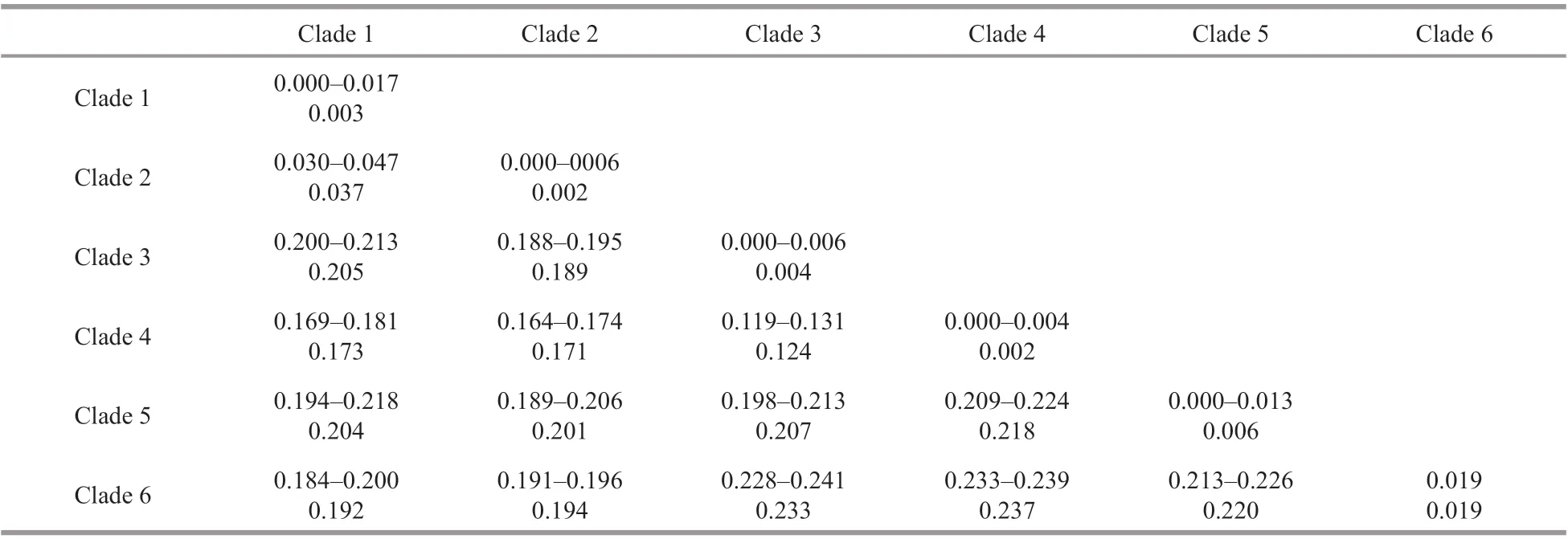
Table 3 Genetic distances of COI between diff erent clades of Capitella based on K2P distance
3.3 Regeneration feature of C. teleta in China
To investigate the regeneration ability ofC.teletain China, the whole mount immunohistochemistry and phalloidin staining were performed. At 0-h post amputation (hpa), cells labelled evenly distributed through the whole body, and sizes were uniform and round (Fig.5a). The longitudinal muscles seemed to disappear at the cut site, while only circular muscles existed (Fig.5a′). The ventral nerve cord (VNC) was arranged in the trunk part, attaching to the anterior brain (Fig.5a″). VNC likely to have an obvious termination at the cut site, which was viewed as a symbol of amputation location (de Jong and Seaver,2018). At 3 hpa and 12 hpa, no epithelium to seal the wound was observed at the surgery site, which meant that the wound healing process might not have been completed (Fig.5b & c). Similarly, muscles and neurites seemed to stop at the cut site (Fig.5b′, b″, c′,c″). From 12 to 24 hpa, the wound completely healed.At 24 hpa, a group of labeled cells gathered around the wound, which meant the regeneration of a small new blastema (Fig.5d). However, no extension of muscles or nerves were found (Fig.5d′, d″). At 3 dpa,a larger regenerated blastema was observed, which consisted of a group of cells (Fig.5e). The muscles had some messy extension growth (Fig.5e′). VNC displayed slight increases well (Fig.5e″). At 5 dpa,muscles obviously regenerated (Fig.5f′), but only a few nerve f ibers regenerated (Fig.5f″). At 7 dpa, a mass of cells gathered at the wound site (Fig.5g), and both longitudinal muscles and multiple rings of circular muscles near the pygidium were observed(Fig.5g′). Although the extension and growth of VNC was remarkable, the mature ganglia had not yet formed (Fig.5g″), which indicated that it failed to regenerate a complete chaetiger at present. Re-grew segment numbers were counted with mature ganglion labeled by anti-acetylated α-tubulin. Up to 16 dpa,approximately 17 segments regenerated (Fig.5h′) and the muscle tissue completely re-grew (Fig.5h). The muscle architecture ofC.teletaconsists of circular,longitudinal, and helical muscles (Fig.5h).
4 DISCUSSION
In this study, we verif ied the taxonomic status ofC.teletafrom Shandong through morphological examination and phylogenetic analysis.Capitellateletafrom Shandong is the second reported species ofCapitellain Chinese waters. It is consistent withC.teletafrom Korea, Japan, and the United States in most of the characters. However, there are some morphological diff erences among these populations.For example, there are 6(8) and 4 genital spines on chaetigers 8 and 9 in Shandong population,respectively, while 8 and 6 genital spines are found in American, Japanese, and Korean populations.Besides, the methyl green staining patterns vary among diff erent populations. In the American population, the internal organ in chaetiger 7 of males was stained, chaetigers 6-9 of females remain stained.In the Japanese population, both females and males were dyed all over the body. In the Korean population,chaetigers 5-9 were stained in females but not in males. Almost the whole body of the males was stained except for the chaetiger 10, while abdominal segments of the female was signif icantly stained in the Chinese population. We assumed that the morphological diff erences amongC.teletafrom diff erent areas probably resulted from intraspecif ic variations.Capitellateletais considered as a cosmopolitan species that is known to be distributed in the Mediterranean, the United States, Japan, Korea,and China (Blake et al., 2009; Tomioka et al., 2016;Jeong et al., 2018; this study). The worldwide distribution might be related to anthropogenic dispersal, as proposed by Tomioka et al. (2016).C.teletais gonochoristic in a normal situation, embryos would f irst develop in a brood tube that is around the pregnant female, and would release from the tube until they develop into the late stage of larvae.C.teletain Shandong has a relative short reproductive cycle of about 2 months, with relatively fewer embryos produced each time compared toC.teletain USA, which can produce 100-250 embryo once.
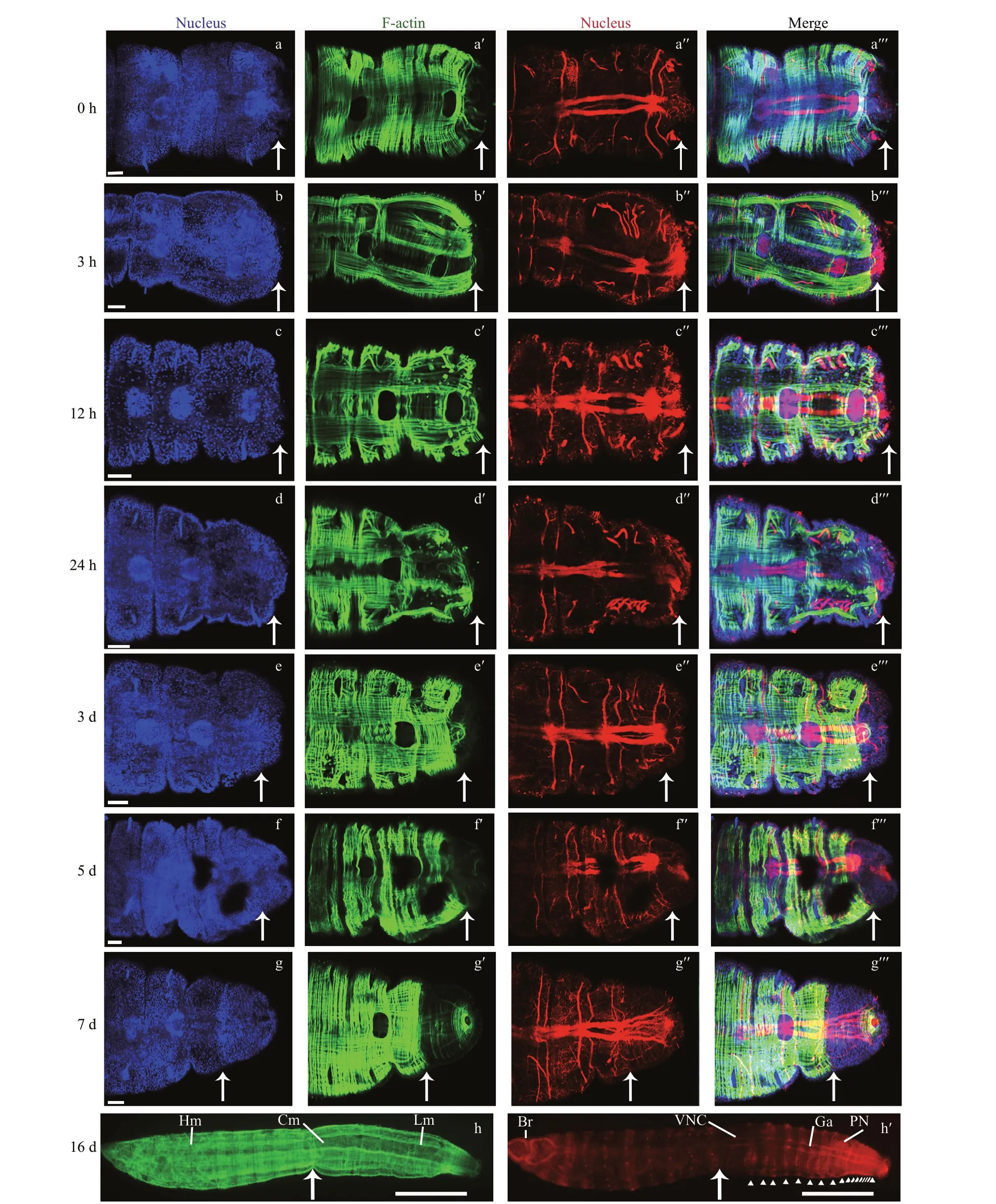
Fig.5 Muscle and nervous regeneration during posterior regeneration process of Capitella teleta
Lin et al. (2008) collected numerousCapitellaspecimens with various kinds of thoracic chaetal arrangements along the Chinese coastal waters. It indicates that there might be many unknown species ofCapitellain the China seas. However, all the specimens ofCapitellahave been identif ied asC.capitata(Fabricius, 1780) since Wu (1964) f irstly reported the species in China. The chaetal arrangement of “C.capitata” reported in China is the same asC.teleta(chaetigers 1-7 with capillaries,chaetigers 8-9 with hooded hooks and genital spines). However,C.teletacan be easily distinguished via the absence of neuropodial capillaries on chaetigers 8 and 9 (vs. present inC.capitata). Besides,C.capitatas. str. is considered to be Arctic and subarctic in distribution according to Blake (2009), while most specimens ofCapitellain China are collected in lower latitude regions from 20°N to 40°N. Therefore, it seems that records ofC.capitatain China might represent other species and need to be re-investigated.
The regeneration ofC.teletain China conforms to a strict timeline and process, and posterior part can be regenerated. In a few days, the diff erent tissues could regrow. Besides, Giani et al. (2011) described the regeneration process ofC.teleta, at 1 dpa, the wound was completely close, and with a small blastema.Within 3 dpa, the blastema became bigger, and at 5 dpa, axons extended into the blastema, likely entering into the regeneration tissue. About 20 segments regenerated at 18 dpa. The regeneration process is similar to our worms, with no signif icant diff erences and some subtle diff erences that may be related to the environment. Many members of polychaeta are able to regenerate, some species can regenerate both head and tail, or only tail. For example, the f irewormEurythoe(Annelida) can complete the anterior and posterior regeneration within 50 days (Yáñez-Rivera and Méndez, 2014).Regeneration ability is benef ic to the benthic worms and can improve the survival rate of its population. It is reported that some asexually reproductive worms can autotomize to regenerate (Ribeiro et al., 2018);however, the relative between asexual reproduce and post-injury regeneration is controversial. Although autotomy seems similar to regeneration, as far as we concern, regeneration should be initiated by injury,which is a kind of passive behavior rather than active.
The process in polychaeta regeneration usually include wound healing, formation of a regeneration blastema, cell diff erentiation, and segments addition.However, some worms regenerate without a blastema,such asSpirobranchuslamarcki, the opercular regenerate is based on non-blastema (Bubel et al.,1985). The blastema is a regeneration specif ic structure fulling with the mass of cells. In many cases,regeneration generally involves a regeneration blastema, such as planarian (Tanaka, 2016; Planques et al., 2019). Exceptionally, the regeneration of ctenophore is based on non-blastema (Ramon-Mateu et al., 2019). However, the cellular sources and potential fates of source-cells of blastema need to further address.
5 CONCLUSION
In conclusion, the worms found in Qingdao and Weifang areCapitella teleta, which was identif ied by morphological descriptions and genetic evidence.Surprisingly enough, this worm is likely to be a model for function study and regeneration evolution study due to its rapid regeneration ability.
6 DATA AVAILABILITY STATEMENT
The data generated and/or analyzed in this study are available from the authors on reasonable request.
7 ACKNOWLEDGMENT
We thank Jieyang WENG, Jie LI, Xuegang WANG,and Xiaofei ZHU for their assistance in sampling specimens, and are very grateful to the staff of the SEM room for their help. We also express our gratitude for the support by Oceanographic Data Center, IOCAS.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- Spatial diff erence in net growth rate of Yesso scallop Patinopecten yessoensis revealed by an aquaculture ecosystem model*
- Bacterial community composition in gut content and ambient sediment of two tropical wild sea cucumbers ( Holothuria atra and H. leucospilota)*
- Comparative transcriptomic analysis of Macrobrachium nippon ense in response to Aerom onas veronii or Staphylococcus au reus infection*
- Dietary intake of bamboo vinegar and charcoal powder (BVC)enhances resistance of African catf ish Clarias gariepinus to bacterial pathogen*
- Cloning of catalase gene and antioxidant genes in Scophthalmus maximus response to metalloprotease of Vibrio anguillarum stress*
- Five new records of Xanthidae (Crustacea: Brachyura) from Hainan Island, China*
