Comparison of the photo-acclimation potential of f loating and benthic thalli of Sargassum horneri (Phaeophyta) during autumn and winter*
Jingjing LI , Yunlong PANG , Song QIN , Zhengyi LIU ,**, Zhihai ZHONG ,Wanlin SONG , Longchuan ZHUANG
1 Key Laboratory of Marine Hazards Forecasting, Ministry of Natural Resources, Hohai University, Nanjing 210098, China
2 Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China
3 Weihai Vocational College, Weihai 264200, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Sargassum horneri is a foundational species and an important contributor to the f loating seaweed stock along the northeastern coast of Asia. In this study, benthic and f loating thalli of S. horneri were collected from Changdao Island (37°54′N, 120°43′E), Bohai Bay, China. We conducted an in-situ and an indoor experiment to study the acclimation potential in S. horneri to abiotic conditions at sea surface in autumn and winter. Both benthic and f loating thalli were cultured in situ for two months (from October to December) at diff erent depths: 0 m above sea level (masl) and 3 m below sea level (mbsl), and their growth rate, biochemical content, and photosynthetic performance were compared. During the f irst month of culture, the relative growth rate of f loating thalli was 2-fold greater than that of benthic thalli at 0 masl. The photosynthetic rate of most thalli was signif icantly higher at 0 masl than at 3 mbsl. In the indoor experiments,f loating and benthic thalli were exposed to high light intensity (400 μmol photons/(m 2·s) photosynthetically active radiation (PAR)) for 21 d, and their photo-acclimation capacities were compared. Under high light intensity, the two types of thalli showed low maximum quantum yield ( F v/ F m) and light utilisation effi ciency( α) but high light saturation point ( E k). Floating thalli showed higher photosynthetic rate and photoprotective ability than benthic thalli at high light intensity. The eff ective quantum yield of photosystem II [Y(II)] of both types of thalli recovered after a 6-day treatment with low light intensity (40 μmol photons/(m 2·s)).These f indings suggest that S. horneri is highly acclimated to the sea surface environment, which possibly contributes to its rapid accumulation and long free-f loating periods at the sea surface.
Keyword: chlorophyll- a f luorescence; growth; photosynthesis; photosynthetic pigments; Sargassum horneri
1 INTRODUCTION
The frequency of green and golden seaweed tides,mainly caused by the seaweed taxaUlvaandSargassum, has increased in the past decades because of climate change and human activities (Smetacek and Zingone, 2013). Unlike most seaweeds, which rely on hard substrata, f loating species increase their biomass by making new thalli and/or off spring during the f loating period (Komatsu et al., 2008; Wang et al.,2015). Positive buoyant seaweeds and hitchhiking species extend their distribution range by long-distance rafting with the currents (Macreadie et al., 2011;Bertola et al., 2020). Thus, the movement and longevity of f loating seaweed rafts may aff ect the connectivity of coastal ecology (Rothäusler et al., 2015).
The longevity of f loating seaweeds is determined by the balance between their acclimation potential and the biotic/abiotic stress at the sea surface (Talaet al.,2017). The survival of f loating seaweeds depends on several biotic and abiotic factors, such as temperature, photosynthetically active radiation(PAR), ultraviolet radiation (UVR), and grazing animals (Rothäusler et al., 2011a). High photoacclimation potential ref lects the ability to minimise the damage caused by ambient light conditions and to retain photosynthetic responses to the changing environment (Wu et al., 2015). This property is important for f loating seaweeds, as at the sea surface,seaweeds are exposed to a combination of high light intensity stress, UVR, and changing sea surface temperature, which are distinct from those in benthic habitats (Zhao et al., 2019). UVR is another important factor that aff ects the photosynthetic performance and survival of intertidal and f loating seaweeds (Koch et al., 2016; van Hees et al., 2019). Some seaweed produce UV-absorbing compounds, such as mycosporine-like amino acids (red and green seaweeds) and phlorotannins (brown seaweeds), to avoid damage caused by UVR (Quintano et al., 2019).To cope with high levels of PAR and UVR, f loating seaweeds employ some photoprotective strategies;for example, accumulation of UV-absorbing compounds (van Hee et al., 2019), dissipation of excess light energy as heat (Rothäusler et al., 2018),and reducing the content of accumulated pigments(Zhao et al., 2016).
Sargassum horneri(Turner) C. Agardh is a common macroalgal species found along the coast of China that forms extensive subtidal forests, especially in off shore islands in the Northwest Pacif ic (Komatsu et al., 2008; Li et al., 2020). Large f loating patches ofS.horneriare often found on the continental shelf west of the Kuroshio Current. Previously, the bloom ofS.horneriin 2017 in the Yellow Sea of China was attributed to high water temperature and increased light availability (Qi et al., 2017). In Bohai Bay,vesicles ofS.horneriare often found in September(autumn boreal, f ield observation), and patches of f loating thalli appear from September to May the following year. After the reproductive stage, the majority of plants begin to age and lose biomass during the time from May to August. Remote sensing data indicate that f loatingS.horneripatches in Bohai Bay drift southwards with the currents in winter (Xing et al., 2017). Yatsuya (2008) reported thatS.horneri,which detached off the central part of Honshu Island facing the Sea of Japan in January, stayed f loating for more than three months. Based on this study, the f loating period ofS.horneriis much longer than that of the other three Sargassacean species, includingMyagropsis myagroides(Mertens ex Turner) Fensholt,Sargassum patensC. Agardh andSargassum siliquastrum(Turner) C. Agardh(Yatsuya, 2008). The longevity of f loating thalli of these species is also greatly aff ected by the reproductive stage, as thalli begin shedding and withering after sexual reproduction. Thalli ofS.horneridetached before maturation had a f loating period of 8-14 weeks, while those detached during and after maturation had a f loating period of 3-8 weeks and 2 weeks or less,respectively (Yatsuya, 2008).
Our experiments onS.horneriwere conducted from October to December, which is the rapid growing stage of the species to avoid the eff ects of reproductive development (Yu et al., 2019). Because the f loating and benthic thalli ofS.hornerioccupy diff erent ecological niches characterised by contrasting environmental factors, particularly light intensity, we investigated whether these two thalli types exhibit diff erent photo-acclimation potentials to diff erent light intensities. Both thalli types were cultivated at diff erent depths in situ and under diff erent light intensities in the laboratory to elucidate the photo-acclimation mechanisms of this golden tide species.
2 MATERIAL AND METHOD
2.1 Sample collection
AllS. hornerisamples were collected from Changdao Island, China (37°54′N, 120°43′E) on October 20, 2018. Approximately 20S.horneriadults, ranging in size from 0.6-1 m, were detached from a nearbySargassumforest by scuba diving to a depth of 2-3 m. All benthic thalli were cut from the bottom (without holdfast) with knife. Approximately 20 free-f loating thalli were collected from the f loating mat near the shore (ca. 17 km from the coast).
2.2 Experimental design
2.2.1 In-situ experimental design
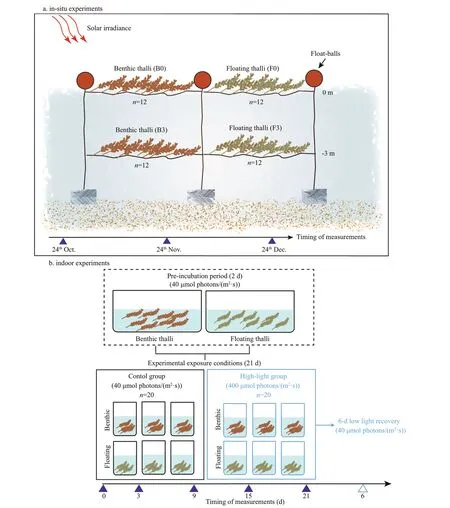
Fig.1 Experimental design for in-situ and indoor experiments
The in-situ experiment was conducted in a kelp aquaculture farm in a protected bay. Each thallus was rinsed in seawater to remove epiphytes and sediments.The rinsed thalli were twisted into nylon ropes and suspended in the sea horizontally at 0 m above sea level (masl) or 3 m below sea level (mbsl) using rope and f loat-balls. Four treatments were established(n=12 for each treatment; Fig.1a): f loating thalli cultured at 0 masl (F0); benthic thalli cultured at 0 masl (B0); f loating thalli cultured at 3 mbsl (F3);and benthic thalli cultured at 3 mbsl (B3). The suspended thalli were cultured in the sea from 24 October 2018 to 24 December 2018.
Hydrological parameters, including temperature,salinity, dissolved oxygen, and pH, at each depth were measured every month using an YSI probe(Professional Plus; YSI Inc., USA) (Supplementary Table S1). Light intensity was measured daily (early morning) throughout the culture period at a location near the site of the in-situ experiments using a microquantum sensor associated with a diving-pulse amplitude modulated (PAM) f luorometer (WALZ Germany) (Supplementary Fig.S1). The light intensity at 3 mbsl was calculated using the following equation:
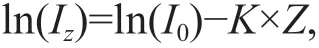
whereIzis the light intensity at depthZ;I0is the surface light intensity; andKis the light extinction coeffi cient (K=1.83, calculated using the Secchi disk depth) (Supplementary Fig.S1).
2.2.2 Indoor experimental design
Benthic and f loating thalli were rinsed in seawater to remove epiphytes and sediments. The rinsed thalli were stored in a foam plastic box containing crushed ice to control the temperature, and then transferred to the laboratory. Indoor experiments were conducted to compare the responses of benthic and f loating thalli to high light intensity. Based on the measurement of light intensity in situ (ranging from 300 to 500 μmol photons/(m2·s) (Supplementary Fig.S1), high light intensity was set at 400 μmol photons/(m2·s). Both types of thalli were cultured in 100-L plastic tanks containing f iltered seawater for 2 d (pre-incubation).The seawater was continuously aerated with an air pump, and thalli were cultured in a growth chamber at 15 °C under 10-h light/14-h dark photoperiod and 40 μmol photons/(m2·s) light intensity (PAR). Then,fragments (ca. 8 cm each) were obtained from the upper portion of each thallus and cultured in sterile f iltered seawater in two growth chambers (GXZ-280C; Ningbo Jiangnan Instrument, China)maintained at 15 °C, 10-h light/14-h dark photoperiod and either 400 μmol photons/(m2·s) (high-light group;n=20) or 40 μmol photons/(m2·s) (control group;n=20) (Fig.1b). The f iltered seawater was replaced every 2 d. After 21 d, thalli from the high-light group were allowed to recover by placing under 40 μmol photons/(m2·s) light intensity for 6 d.
2.3 Relative growth rates (RGRs)
The length of each thallus (from the base to the top) was measured on 24 October, 24 November, and 24 December duringin-situ experiments. The RGR(%/d) in each month was calculated using the following equation (Rueness and Tananger, 1984):
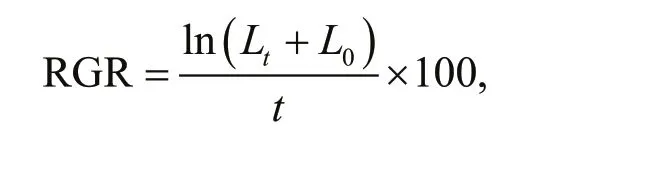
whereL0is the initial thallus length;Ltis the f inal length aftertdays; andtis the number of days.
2.4 Biochemical analyses
Before and after the in-situ experiments, thalli fragments (measuring ~5 cm) were cut from the top of each individual thallus, stored in a box containing crushed ice and then transferred to the laboratory for biochemical analyses. Chlorophylla(Chla) and carotenoid (Car) contents were estimated as described previously (Miki et al., 2016). Approximately 500 mg of thallus (wet weight, ww) was ground thoroughly in 5 mL of 90% acetone and then centrifuged. After storage in the dark at 4 °C for 24 h, the absorbance of the supernatant was measured at 470, 663, and 646 nm(A470,A663,A646). Pigment concentrations (mg/g ww)were determined according to the following equations(Wellburn, 1994):

The soluble phlorotannin contents of freeze-dried seaweed were extracted and measured using the Folin-Ciocalteu method (van Alstyne, 1995). Tissue(10 mg, dry weight, dw) was ground to f ine powder in 3 mL of 80% acetone and extracted overnight.Approximately 300 μL of extract (diluted 1꞉5 with reagent-grade water) was added to 100 μL of the Folin-Ciocalteu reagent (Sigma-Aldrich, Seelze,Germany), and the sample was mixed by shaking for 5 min. The solution was made alkaline by adding 200 μL of 20% sodium carbonate (NaCO3), and shaken then for an additional 30 s. Subsequently, the sample was incubated in the dark at room temperature(RT) for 45 min and then centrifuged at 2 000×gfor 3 min at RT. The absorbance of the supernatant was measured at 730 nm using a microplate spectrophotometer (Eon, BioTek, USA). Purified phloroglucinol (Sigma-Aldrich, Seelze, Germany)was used as a standard. Values were expressed as mg/g dw.
2.5 Chl- a f luorescence measurements
The Chl-af luorescence of samples cultured in situ and in indoor experiments was measured early in the morning during the f irst 2 h of the light period. Prior to measuring Chl-af luorescence, samples were adapted to the dark for 20 min. Chl-af luorescence(Schreiber et al., 1995) was measured using a Diving-PAM f luorometer (WALZ, Germany). The device employs a red light emitting diode (LED) with emission maximum at 650 nm to excite f luorescence.
The maximum quantum yield (Fv/Fm) of PSII was calculated using the following equation:
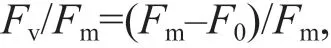
whereF0is the initial f luorescence determined under 3-7 μmol photons/(m2·s) light intensity;Fmis the maximum f luorescence induced after a saturating pulse (5 640 μmol photons/(m2·s)).
The coeffi cient of photochemical quenching (qP)(Schreiber et al., 1986; Kramer et al., 2004) was measured and calculated as follows:
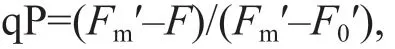
whereFis the steady-state f luorescence at a given actinic light (104 μmol photons/(m2·s));Fm′ is the maximal f luorescence induced after a saturating pulse(5 640 μmol photons/(m2·s));F0′ is the minimal f luorescence induced after a 3-s pulse of far-red light(735 nm; ~1 μmol photons/(m2·s)).
The eff ective PSII quantum yield [Y(II)] (Schreiber et al., 1995) was calculated as follows:
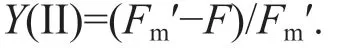
Once in-situ measurements for each treatment were completed, the samples were immediately returned to the seawater.
After 20-min dark adaptation, rapid light curves(RLCs) were measured with a light intensity gradient,comprising nine diff erent light intensities (0, 104,186, 322, 463, 621, 893, 1 189, and 1 754 μmol photons/(m2·s)), using a f luorometer (Diving-PAM;Walz, Germany); each light intensity was maintained for 10 s. The relative electron transport rate (rETR;μmol·electrons/(m2·s)) was calculated according to the following equation (Genty et al., 1989):

where PAR is the photosynthetically active radiation(μmol photons/(m2·s)), andFIIis the fraction of chlorophyll associated with PSII being 0.8 in brown seaweeds (Orzymski et al., 1997).
Photosynthetic parameters, namely, maximum relative electron transport rate (rETRm) and light utilisation effi ciency (α) were derived from RLCs using the following equation (Platt and Gallegos,1980):
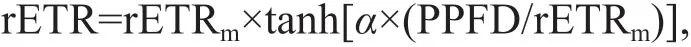
where PPFD represents the photosynthetic photon f lux density of RLCs.
The light saturation point (Ek) was calculated as follows:
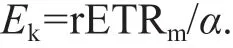
During the measurement of RLC, nonphotochemical quenching (NPQ) (Bilger and Björkman, 1990) was calculated as follows:
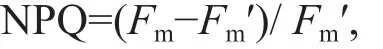
whereFmandFm′ values were obtained from the RLCs.
2.6 Statistical analysis
Prior to all statistical analyses, the homogeneity of variances was verif ied with Levene’s test. Statistical signif icance of the in-situdata (RGRs, photosynthetic parameters and Chla, carotenoid, and phlorotannin contents) was analysed by two-way analysis of variance (ANOVA), withS.horneriincluding thallus type (benthic and f loating) and sampling depth as f ixed factors. For signif icant diff erences, a post-hoc Tukey’s honestly signif icant diff erence (HSD) test was applied. Statistical signif icance of the variation in photosynthetic parameters of indoor experiments was analysed with repeated measures ANOVA (RMANOVA), followed by Tukey’s HSD post-hoc test.Statistical signif icance was considered atP<0.05. All statistical analyses were conducted using the statistical software package SPSS 24.0
3 RESULT
3.1 In-situ experiment and environmental conditions
Light intensity declined dramatically with an increase in depth (Supplementary Fig.S1). No signif icant diff erences were observed in sea temperature, pH and salinity at diff erent depths(Supplementary Table S1). The sea temperature decreased from October (ca. 17.6±1.4 °C) to December (ca. 4.9±1.3 °C) (Supplementary Table S1). The dissolved oxygen level increased from October to December at 0 masl (from 6.9 to 12.0 mg/L)and 3 mbsl (from 7.0 to 9.1 mg/L).
In most treatments, the RGR was lower in December than in November (Fig.2). The results of ANOVA revealed a signif icant interaction between sampling depth and thallus type in each month(November:F=936.658,P<0.001; December:F=91.954,P<0.001) (Supplementary Table S2). Both types ofS.hornerithalli at each depth showed positive RGRs, except the f loating thalli at 3 mbsl (F3) (Fig.2).In November, the RGRs of f loating thalli cultured at 0 masl (F0) were approximately 2-fold higher than those of benthic thalli cultured at the same depth (B0)(F=9.579,P<0.001), whereas in December, the RGRs of F0 were lower than those of B0 (F=371.720,P<0.001) (Fig.2, Supplementary Table S2). Benthic samples cultured at diff erent depths showed similar RGRs in November; however, B0 samples grew faster than B3 samples in December (Fig.2).
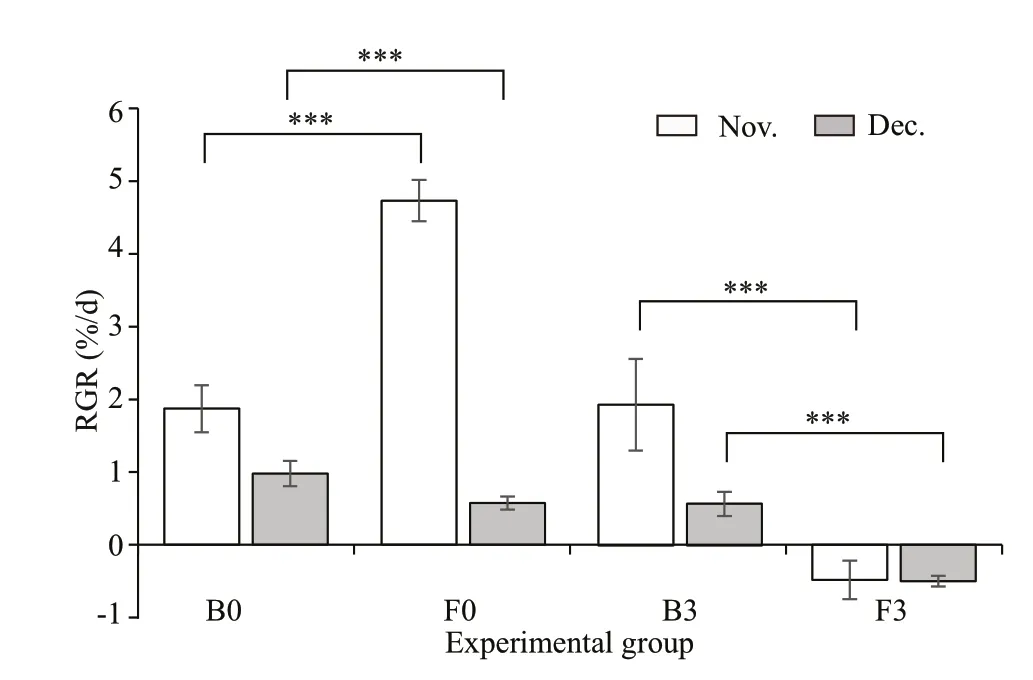
Fig.2 Relative growth rates (RGRs) of benthic and f loating S. horneri thalli cultured at 0 masl and 3 mbsl during in-situ experiments
Before the in-situ experiments, benthic thalli showed signif icantly higher Chlaand carotenoid contents than f loating thalli (Table 1). After a twomonth culture period, the Chlaand carotenoid contents of all samples (except F3) decreased (Table 1). The Chlacontent of B3 and F3 samples was approximately 5-fold and 6-fold higher than that of B0 and F0 samples, respectively (Table 1). The carotenoid contents of B3 and F3 samples were approximately 2-fold higher than those of B0 and F0 samples (Table 1). The initial phlorotannin concentrations in the benthic and f loating thalli were 7.613±0.006 and 8.877±0.012 mg/g dw, respectively(Table 1). Higher phlorotannin concentrations were also detected in the thalli cultured at 0 masl in December (P<0.05; Supplementary Table S3).
At each depth, benthic thalli displayed signif icantly higherFv/Fmvalues than f loating thalli during in-situ experiments, except for B3 vs. F3 in November(P<0.05; Fig.3, Supplementary Table S4).Y(II)values in F0 were signif icantly higher than those in B0 (P<0.05; Fig.3). Additionally,Y(II) values of F0 were declined from October to December. During the two-month culture period, both thalli showed a depth adjustment in the qP values (P<0.05; Supplementary Table S4), with higher values in thalli cultured at 0 masl that those cultured at 3 mbsl (Fig.3). In addition, qP values of B3 samples were higher thanthose of F3 samples from November to December(P<0.05; Fig.3).
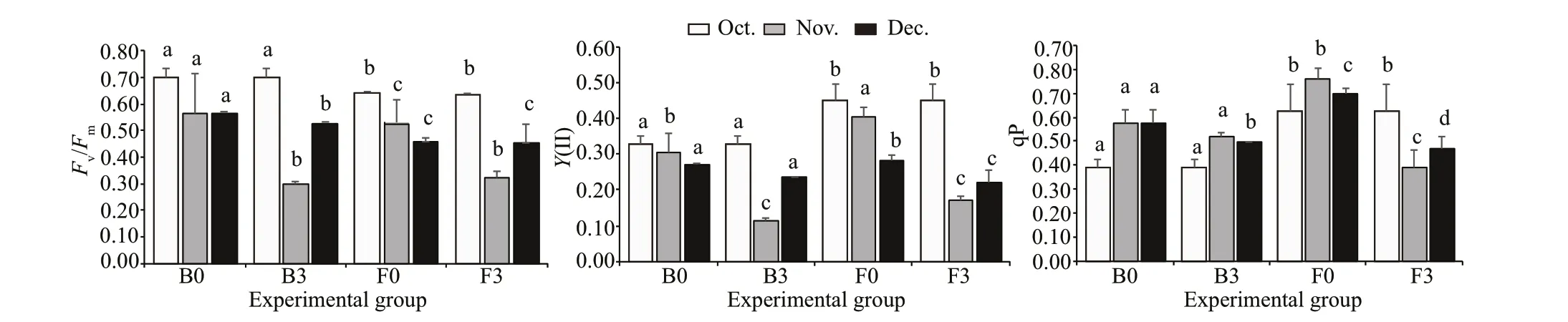
Fig.3 Photosynthetic parameters of in benthic and f loating S. horneri thalli cultured in situ at 0 masl and 3 mbsl from October to December in 2018
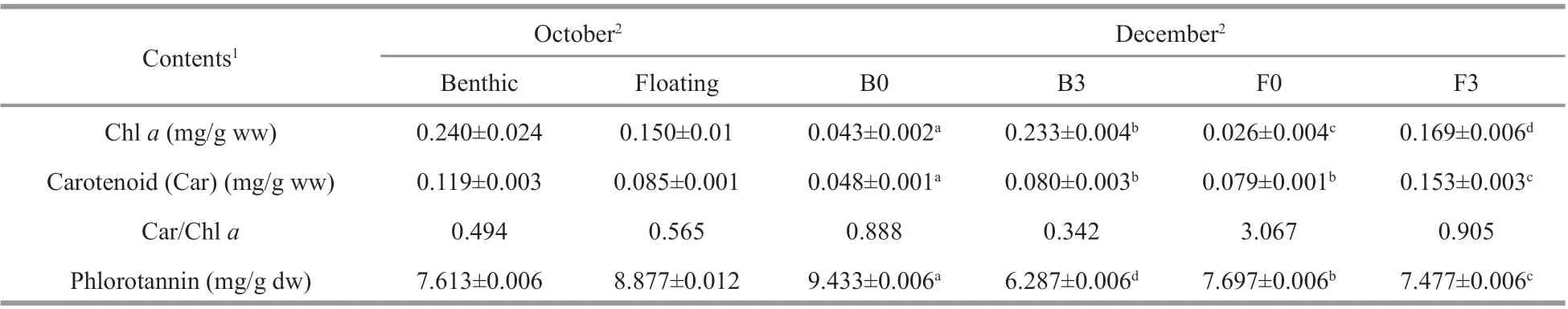
Table 1 Pigment (Chl a and carotenoid) and phlorotannin contents of benthic and f loating Sargassum horneri thalli at diff erent water depths measured during in-situ experiments in October and December 2018

Fig.4 Variation in F v/ F m, Y(II), rETRm, α, E k, and NPQ of benthic and f loating S. horneri thalli in the high-light (400 μmol photons/(m 2·s)) and control (40 μmol photons/(m 2·s)) groups during the indoor experiment
3.2 Indoor experiment
After 21 d of high-light treatment, the photosynthetic performance of both types of thalli decreased, as shown by the decreasingFv/FmandY(II) values(Fig.4). By contrast, only slight changes inFv/Fmwere found in the control group during the incubation time. TheY(II) values of f loating thalli were higher than those of benthic thalli during the f irst 9 d;however, both thalli types showed similar photosynthetic performance in subsequent days(Fig.4). In the control group, theY(II) values of f loating thalli were higher than those of benthic thalli(Fig.4). TheY(II) values of f loating and benthic thalli at each treatment are signif icantly diff erent(F=437.788;P<0.05; Supplementary Table S5).
The rETRmof f loating thalli was higher than that of benthic thalli in both high-light and control groups(Fig.4). However, no signif icant diff erences were detected between diff erent thalli at each treatment(F=0.971,P>0.05; Supplementary Table S5). The light utilisation effi ciency (α) of f loating thalli was higher than that of benthic thalli in both high-light and control groups (Fig.4). Within the high-light group, theEkvalue of f loating thalli was higher than that of benthic thalli, except on the day 3 (Fig.4).After 21 d of indoor culture, theEkvalues of both thalli were higher in the high-light group than in the control group (Fig.4).
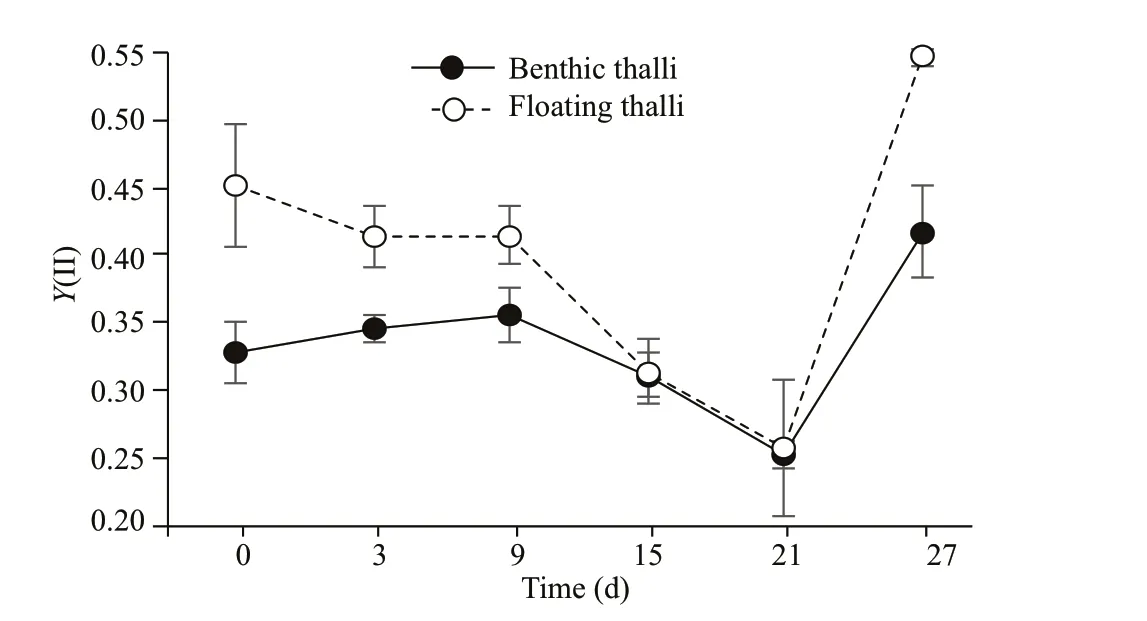
Fig.5 Values of Y(II) in benthic and f loating S. horneri thalli of after high-light intensity treatment and 6-d recovery
The NPQ, which represents heat dissipation ability,of both types of thalli decreased with time in the highlight group (Fig.4). During the f irst 9 d, the NPQ of f loating thalli was higher in the high-light group than in the control group. By contrast, the NPQ values of benthic thalli in the high-light group were lower than in the control group (Fig.4).
After 6 d of exposure to low light intensity(40 μmol photons/(m2·s)), both types of high-lighttreated thalli recovered gradually, and the eff ective quantum yieldY(II) exceeded the initial level (Fig.5).
4 DISCUSSION
New environmental conditions related to the ongoing rapid global climate change are expected to cause shifts of species range and abundance (Duarte et al., 2018; Martínez-Minay et al., 2019), which may benef it seaweeds with high environmental acclimation potential and dispersal ability.Sargassum hornerithalli were assumed to employ effi cient mechanisms to respond to changes in solar irradiance at diff erent water depths. Our data suggest thatS.horneripossess high photo-acclimation potential that enables adjustments to changes in air-sea interface conditions.The increased photosynthetic rates and various photoprotective strategies may contribute to the longterm persistence and rapid growth of f loatingS.horneriat the sea surface.
Temperature and solar irradiation are the main factors aff ecting the reproduction and growth of f loating seaweeds at the sea surface (Tala et al., 2016).In this study, high solar irradiance increased the growth rates ofS.horneriat the sea surface from October to November when the temperature was approximately 11-18 °C (Supplementary Table S1), which is within the optimal growth temperature (Topt) range (13-18 °C)ofS.horneri(Sun et al., 2008). Lower temperatures may buff er the negative eff ects of high light intensity(Graiff et al., 2013; Tala et al., 2019) because respiration rates seem to be sensitive to increasing temperature, especially when the temperature exceeds theToptfor net photosynthesis (Staehr and Wernberg,2009). By contrast, the reduction in RGRs ofS.horneriin December may be attributed to the low ambient temperature (4.94±1.3 °C), which is much lower than theToptofS.horneri. Choiet al. (2008) showed that the RGRs ofS.hornerigermlings were greater at higher temperatures (15-25 °C) than at 10 °C.
Sargassumhornerithalli cultured at diff erent water depths for two months showed diff erent acclimation responses, probably because of changes in variable Chl-af luorescence of PSII, photosynthetic pigment contents and phlorotannin content. The highest RGR was detected in F0 in November; however, the RGR decreased in December. Accordingly, a reduction in photosynthetic parameters was observed in F0 from November to December. Photo-acclimation potential is usually linked to photosynthate production and/or maximal electron transport (Quintano et al., 2019).Among all treatments, F0 showed the highestY(II)and qP values, suggesting that high light intensity enhances the plastoquinone acceptor (QA) reoxidation capacity and CO2assimilation rates (Hollis and Hüner, 2017). Besides, during indoor experiments,higher rETRmvalues were detected in the f loating thalli than the benthic thalli, an outcome which could be related to an effi cient use of energy (Koch et al.,2016). Similarly, rETRmis high in apical fronds than in middle and basal fronds of brown seaweedMacrocystis pyriferaat diff erent water depths,ref lecting apical fronds are better acclimated to high light (Marambio et al., 2017).
The ability to adjust pigment concentrations ref lects the ability to balance the harvesting of light energy with the dissipation of excess excitation energy (Koch et al., 2016), and this ability contributes to the successful persistence ofS.horneriat the sea surface.Sargassunhornericultured at 3 mbsl showed higher Chlaand carotenoid contents than those cultured at the sea surface, implying that thalli at 3 mbsl can make more effi cient use of low irradiance than those at 0 masl (Xu and Gao, 2008). Severe high levels of solar irradiance may destroy photosynthetic pigments of thalli at the sea surface. A previous study showed that the contents of Chla, Chlc, and carotenoids declined inM.pyriferaaf loat on the sea surface for 15 d (Rothäusler et al., 2011b).Phlorotannins (polyphenol), the UV-absorbing compounds in brown seaweeds, exhibit high antioxidant and photoprotective capacities.Phlorotannins show dynamic changes with depth,suggesting that the variation is induced by the dose of radiation and UVR, as revealed in other brown seaweeds (Figueroa et al., 2014; Tala et al., 2017).Thus, high photosynthetic rate, increased UVabsorbing compounds and eff ective energy dissipation contribute to the long-term persistence and accumulation ofS.horneriat the sea surface.
To further understand the acclimation strategies ofS.horneri, we tested the photoprotective and recovery abilities ofS.horneriby placing both types of thalli under high light intensities in the laboratory. After 21 d of indoor culture, both types of thalli showed an increase inEkand decrease inαunder high light intensity, which are the general characteristics of sunadapted alga (Necchi, 2004). Once excess light has been absorbed, it can be dissipated via several routes,e.g. through the thermal dissipation (represented by NPQ) of excess excitation energy via the xanthophyll cycle (Jahns and Holzwarth, 2012). In the xanthophyll cycle, de-epoxidation of violaxanthin to zeaxanthin effi ciently promotes the thermal dissipation of excess excitation energy (Demmig-Adams and Adams III,1996). Dissipating energy as heat could be an effi cient way to prevent the generation of reactive oxygen species (ROS), which increase the extent of photoinhibition by inhibiting the repair of PSII(Takahashi et al., 2009). In the indoor experiments,f loating thalli showed stronger thermal dissipation capacity under high light intensity than benthic thalli,indicating that most of the absorbed energy may be passively dissipated in the form of heat and f luorescence (Figueroa et al., 2019). Furthermore, in the present study, rapid recovery rates ofY(II) in benthic and f loating thalli indicate thatS.hornerithalli adjust their physiological states rapidly to the changing environment.
The values ofY(II), rETRm, andαwere higher in f loating thalli than in benthic thalli in indoor experiments, indicating f loating thalli utilise the absorbed light through the photochemical reaction more effi ciently than benthic thalli (Rothäusler et al.,2011b). In addition, during indoor experiments, the rETRmof f loating thalli was signif icantly higher than that of benthic thalli under both high and low light intensities, an outcome that could be related to an effi cient use of energy (Li et al., 2014). Floating thalli decayed at lower water depth with low light irradiance during in-situ experiments; therefore, we inferred that high light irradiance is needed for the f loating thalli to maintain balance between light energy absorbed versus energy utilised. In the f ield bottom trawl survey, Liu et al. (2018) observed that thousands of tons ofS.horneribiomass sank to the bottom of the Yellow Sea of China. Apart from the reproductive stage, low light may also inhibit these thalli to grow and f loat to the surface again.
5 CONCLUSION
In this study,S.horneridemonstrated a high photoacclimation potential, which is essential for biomass accumulation and long-term persistence at the sea surface. When af loat,S.hornerithalli were characterised by increased photosynthetic capacity,photoprotective pigments, and UV-absorbing compounds, which prevent the damaging eff ects of high solar irradiance. Future studies should explore the eff ects of multiple factors on positive buoyant seaweeds to understand their f loating and adaptive strategies to new environmental conditions as well as evaluate and forecast their accumulation time and f loating longevity in the near future.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- Spatial diff erence in net growth rate of Yesso scallop Patinopecten yessoensis revealed by an aquaculture ecosystem model*
- Bacterial community composition in gut content and ambient sediment of two tropical wild sea cucumbers ( Holothuria atra and H. leucospilota)*
- Comparative transcriptomic analysis of Macrobrachium nippon ense in response to Aerom onas veronii or Staphylococcus au reus infection*
- Dietary intake of bamboo vinegar and charcoal powder (BVC)enhances resistance of African catf ish Clarias gariepinus to bacterial pathogen*
- Cloning of catalase gene and antioxidant genes in Scophthalmus maximus response to metalloprotease of Vibrio anguillarum stress*
- Taxonomy and regeneration of a newly recorded Polychaete Capitella teleta (Annelida, Capitellidae) in the coastal water of Shandong, China*
