Animal exercise studies in cardiovascular research:Current knowledge and optimal design—A position paper of the Committee on Cardiac Rehabilitation,Chinese Medical Doctors’Association
Yua B,L Wan,Rnn Dn,Ln C,Zqn Fan,W Ga,Q Lan,Snu Ln,Suxn Lu,Xa Lu,Yuqn Sn,Guu Wu,Jan Yan,Guln Zan,W Za,Lan Gu,*,Jun Xa*
a Institute of Geriatrics,Affiliated Nantong Hospital of Shanghai University,Sixth People’s Hospital of Nantong,School of Medicine,Shanghai University,Nantong 226011,China
b Cardiac Regeneration and Ageing Lab,Institute of Cardiovascular Sciences,Shanghai Engineering Research Center of Organ Repair,School of Life Science,Shanghai University,Shanghai 200444,China
c Department of Rehabilitation Medicine,Nanjing University of Chinese Medicine,Nanjing 210023,China
d Department of Cardiology,Peking University People’s Hospital,Beijing 100044,China
e Department of Cardiology,Tongji Hospital Affiliated to Tongji University,Tongji University School of Medicine,Shanghai 200065,China
f Department of Cardiology,Daqing Oilfield General Hospital,Daqing 163000,China
g Department of Cardiology and Institute of Vascular Medicine,Peking University Third Hospital,NHC Key Laboratory of Cardiovascular Molecular Biology and Regulatory Peptides,Key Laboratory of Molecular Cardiovascular Science,Ministry of Education,Beijing Key Laboratory of Cardiovascular Receptors Research,Beijing 100191,China
h Department of Rehabilitation Medicine,First Affiliated Hospital of Sun Yat-Sen University,Guangzhou 510080,China
i School of Medicine,Huaqiao University,Quanzhou 362021,China
j Division of Cardiac Rehabilitation,Department of Physical Medicine and Rehabilitation,Xiangya Hospital of Central South University,Changsha 410008,China
k Department of Rehabilitation Medicine,First Affiliated Hospital of Nanjing Medical University,Nanjing 210029,China
l Department of Cardiology,Eighth Affiliated Hospital of Sun Yat-Sen University,Shenzhen 518033,China
m Guangdong Innovative Engineering and Technology Research Center for Assisted Circulation,Sun Yat-Sen University,Shenzhen 518033,China
n NHC Key Laboratory of Assisted Circulation,Sun Yat-Sen University,Guangzhou 510080,China
o Department of Rehabilitation Medicine,Shanghai Xuhui Central Hospital,Shanghai 200031,China
p Cardiac Rehabilitation Department,Guangdong Cardiovascular Institute,Guangdong Provincial People’s Hospital,Guangdong Academy of Medical Sciences,Guangzhou 510080,China
Abstract Growing evidence has demonstrated exercise as an effective way to promote cardiovascular health and protect against cardiovascular diseases However,the underlying mechanisms of the beneficial effects of exercise have yet to be elucidated.Animal exercise studies are widely used to investigate the key mechanisms of exercise-induced cardiovascular protection. However, standardized procedures and well-established evaluation indicators for animal exercise models are needed to guide researchers in carrying out effective,high-quality animal studies using exercise to prevent and treat cardiovascular diseases.In our review,we present the commonly used animal exercise models in cardiovascular research and propose a set of standard procedures for exercise training, emphasizing the appropriate measurements and analysis in these chronic exercise models. We also provide recommendations for optimal design of animal exercise studies in cardiovascular research, including the choice of exercise models, control of exercise protocols, exercise at different stages of disease, and other considerations, such as age, sex, and genetic background. We hope that this position paper will promote basic research on exercise-induced cardiovascular protection and pave the way for successful translation of exercise studies from bench to bedside in the prevention and treatment of cardiovascular diseases.
Keywords: Animal studies;Cardiovascular disease;Cardiovascular research;Exercise;Exercise models
1. Introduction
Exercise has long been proved to be an effective way to promote cardiovascular health and protect against cardiovascular diseases (CVDs). Regular exercise reduces the risk of CVDs and improves cardiac function in patients.1Exercise capacity and cardiorespiratory fitness are also powerful predictors of CVD events and all-cause mortality in patients and in healthy populations.2There is solid evidence demonstrating the beneficial effects of exercise on hypertension, coronary heart diseases,and heart failure.3-5Properly designed exercise training has become a well-established intervention for patients with heart failure.6,7Indeed,there has been increasing interest in and investigations focusing on the vital mechanisms of exercise in protecting the cardiovascular system.
The benefits of exercise are multifaceted.8Exercise have direct effects on the cardiovascular system, leading to physiological cardiac growth(cardiomyocyte hypertrophy and proliferation) and adaptive angiogenesis.9-11Exercise can also regulate cardiac excitation/contraction coupling and mitochondrial energy metabolism and can attenuate cardiac myocyte death and fibrotic process in response to pathological stimulus.12,13Besides the cardiovascular system, the benefits of exercise are also associated with neurohumoral regulation and systemic organ communications.14-16These investigations not only improve our understanding of the mechanisms of exercise but also help to identify novel therapies that can mimic the benefits of exercise for patients with CVDs.17
2. Purpose and current status of animal exercise studies
2.1. Purpose of animal exercise studies
Clinical exercise prescription aims to realize an objective scientific exercise-training program for patients.18These programs may vary in exercise modality,intensity,frequency,and duration according to the differing physical conditions and disease states of patients. Despite increasing evidence demonstrating the benefits of regular and chronic exercise in the prevention and treatment of CVDs, the underlying mechanisms have yet to be elucidated.8
Preclinical animal studies are useful and practical for establishing programmed exercise training and for examining the effects of exercise interventions in various experimental cardiac-injury models. Aerobic exercise training (e.g., treadmill running, voluntary wheel running, and swimming) and resistance training (e.g., squat-training, ladder climbing) have most frequently been adopted in rodent studies19and have demonstrated that exercise training is capable of protecting the heart from a variety of cardiac injuries, such as myocardial infarction, cardiac ischemia-reperfusion injury, diabetic cardiomyopathy, atherosclerosis, cardiac fibrosis, and heart failure.20-25These positive findings have encouraged more and more scientists to focus their research on this field.
2.2. Current status of animal exercise studies
Exercise can be generally categorized as dynamic exercise or static exercise. Dynamic exercise (e.g., treadmill running,voluntary wheel running, and swimming), commonly applied in experimental animal studies, has multiple advantages, such as enhancing the capacity of oxygen transport in the cardiovascular system and muscles,improving hemodynamics and exercise capacity, and regulating autonomic functions. Indeed,dynamic exercise is a useful strategy for reducing cardiovascular risk factors and adverse events.26Static exercise(e.g.,resistance training)helps to increase muscle mass,enhance muscle strength and contractility, and improve basal metabolic rate.25Thus, different animal exercise models lead to different systemic and cardiac adaptations and should be carefully chosen according to the targeted organs and functions studied in CVDs.
Different exercise intensity, frequency, and duration can substantially influence the systemic effects of exercise as well as its benefits to the heart.19,27Long-term moderate-intensity aerobic exercise or interval high-intensity aerobic exercise are the most commonly used exercise modes for studying the cellular and molecular mechanisms of exercise-induced cardiac protection.28,29Short-term high-intensity or strenuous exercise is a good way to assess cardiopulmonary fitness, exercise capacity, and rapid metabolic changes in cardiac and skeletal muscles derived from exercise.30However, to achieve an effective exercise protocol,exercise validity and efficacy need to be proved. One example of exercise validity would be assessing physiological cardiac growth when studying exercise-induced cardiac hypertrophy.21For exercise efficacy,it is important to both unveil its functional protection and to identify its underlying cellular and molecular mechanisms in CVDs.8Comparisons of different modes of exercise in preventing and treating CVDs also help translate results from basic research to clinical exercise prescriptions.31,32
Because researchers from different groups may use their own conventional exercise protocols and evaluation standards in their animal exercise studies,it is sometimes difficult to reproduce and compare the results from different groups. For example, in addition to running speed, running time,running frequency,and running duration, the inclination of treadmill lanes is also a critical parameter that influences animal-exercise performance and muscle strength after treadmill running.33However,measurements of maximal oxygen uptake(VO2max),time to exhaustion from exercise(endurance capacity),and grip strength help to compare equitably the results obtained from various laboratories.34,35
Under these circumstances, it is necessary to obtain expert opinions and make scientific statements about the standardization and optimization of animal exercise studies in cardiovascular research. Guidelines for animal exercise and training protocols for cardiovascular studies,published in the American Journal of Physiology—Heart and Circulatory Physiology,
presents the most commonly used animal exercise models,ranging from small animals (rats and mice) to large animals(horses, pigs, dogs, and rabbits).36The guidelines provide excellent and very useful steps that researchers can take to perform and control the quality of their animal exercise studies,especially when standardizing procedures and applying rigorous quality controls to animal-exercise models with the intention of analyzing kinetic parameters and reproducing tests of physiological functions affected by exercise.36In our statement,we provide rules for best practices and for optimal selection,design,and control of animal-exercise studies,especially insofar as these studies relate to chronic exercise used for exercise-induced cardioprotection. Our statement also summarizes and identifies useful evaluation indicators for cardiovascular responses to different types of exercise. Standardized procedures and well-established evaluation indicators for animal exercise models are greatly needed to help scientific researchers to carry out effective and efficient animal exercise studies that can be used for developing prevention and treatment techniques for CVDs.
3. Animal-exercise models used in cardiovascular research
Rodent exercise training models are the most commonly used animal models in studying the effects of exercise on CVDs.However,large-animal exercise models are more practical for providing translational research of exercise interventions because the cardiovascular systems of large animals have more similarities to the human cardiovascular system. Below, we propose standard procedures for exercise training and identify appropriate evaluation measurements and methods of analysis in commonly used animal models employing chronic exercise, including treadmill running, voluntary wheel running,swimming exercise,and resistance exercise,in cardiovascular research(Fig.1).
3.1. Treadmill running
3.1.1. Model establishment
Treadmill running is among the most commonly used exercise modalities used in rodents for studying the effects of endurance running on skeletal muscles and the cardiovascular system.37,38Although treadmill-running protocols vary among investigation groups, they can be categorized basically as either moderate-intensity continuous running or interval highintensity running.39,40The categorization of exercise intensity is generally dependent on the VO2max, which represents the greatest possible amount of oxygen supply during exercise.35Generally,exercise in which 50%-70%of VO2maxis reached is defined as moderate-intensity exercise, and exercise in which 85%-90% of VO2maxis reached is defined as highintensity exercise.40-42However,for those individuals or animals who do not attain a VO2plateau during exercise, the highest VO2attained is termed peak oxygen uptake(VO2peak).Increasing evidence has demonstrated that critical power(CP)and critical speed (CS) are more sensitive functional parameters of exercise performance than VO2max.43The hyperbolic power/speed-duration curve is constructed by the subject performing exercise at a constant power or speed to the point of exhaustion.44,45The power asymptote is known as CP (or CS when exercise intensity is measured by speed), which is a“fatigue threshold” that distinguishes exercise performance with a sustainable physiological response to exercise (<CP)from the development of fatigue during exercise(>CP).45For example, rats that run below CS have sustained exercise performance, while those that run above CS have significantly disproportionate blood flow, especially in highly glycolytic muscle fibers.46CS can also be determined in mouse treadmill running,which is estimated to be ~75%of VO2max.47
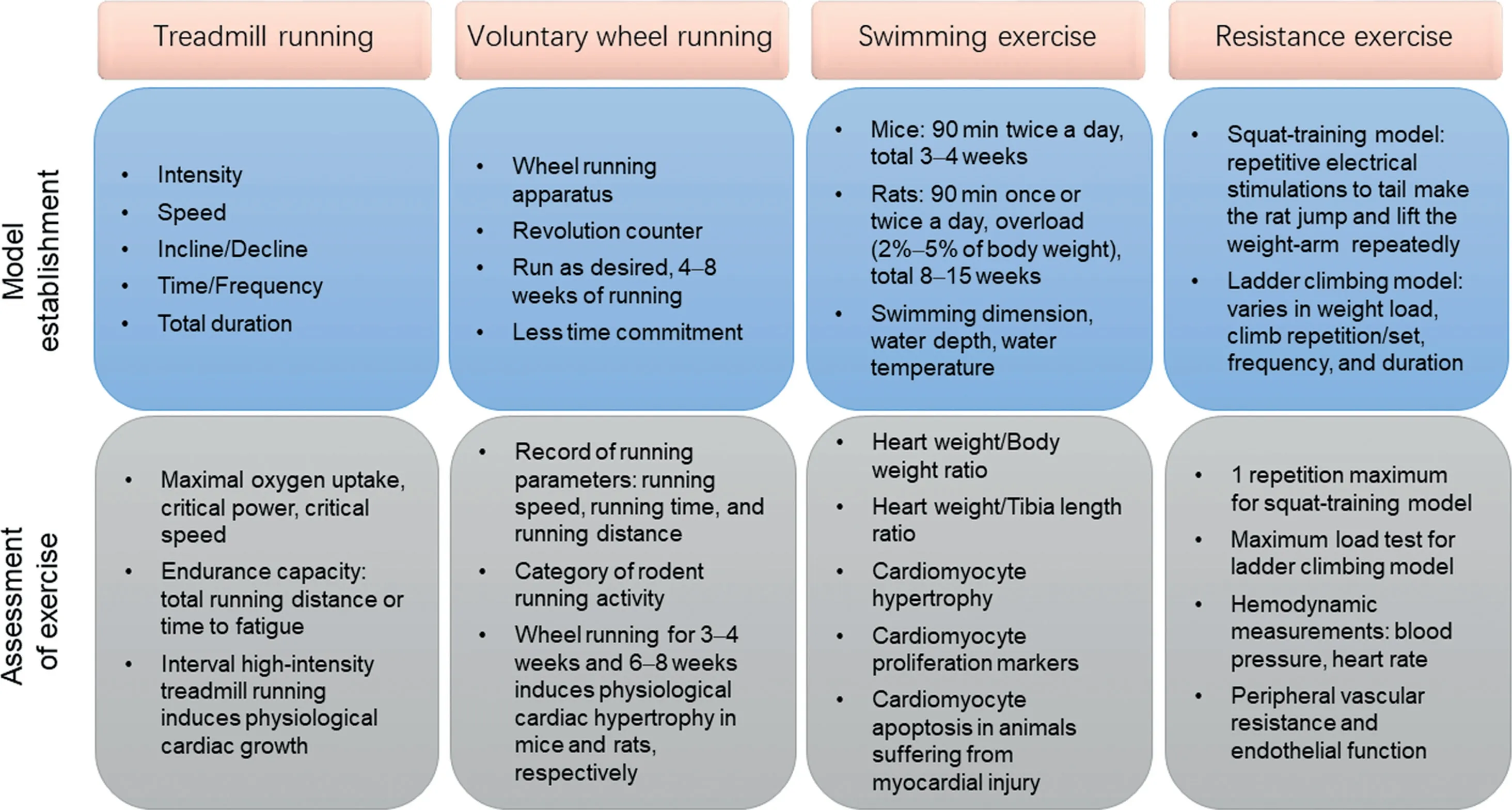
Fig.1. Animal exercise models commonly used in cardiovascular research.Model establishment and assessment of exercise for treadmill running,voluntary wheel running,swimming exercise,and resistance exercise.
The optimal protocols for measuring VO2maxof rats and mice during treadmill running in a metabolic chamber can be applied by warm-up running for 15 min at 40%-50%of VO2maxbefore the VO2maxprotocol, which is then increased by 0.03 m/s every 2 min in settled slopes.41,42Intensity-controlled treadmill running in rats and mice indicates that the inclination of the treadmill significantly affects VO2max. For example, an incline of 25° yields the highest VO2maxin rats and the best cardiovascular load in mice.41,42Researchers should determine the inclination of treadmill in association with running speed. For moderate-intensity continuous treadmill running,rodents should run at an incline of 0°-30°.38,48-52For interval high-intensity treadmill running, an incline of 25°has proved to be efficient for inducing cardiovascular responses.41,42
Moderate-intensity continuous treadmill running (e.g., for adaptive training of mice) should begin with 5 m/min and 10 min of running on the first day and increase progressively by 2 m/min and 10 min/day until the running speed reaches 10-20 m/min with a duration of 30-120 min/day.38,52,53The daily running duration (30-120 min), running frequency(once or twice per day), and total running duration (weeks to months) vary depending on disease models, functions investigated, and scientific questions posed. Along with an increase in animal exercise capacity, running speed should be progressively elevated weekly.54,55Although various treadmill-running protocols have been reported in the literature,investigators should always make the running behaviors of mice or rats consistent by using bottle brushes,puffs of air,or electrical shocks.Animals that fail to learn proper running protocols on the treadmill should be excluded from the study.
Compared to moderate-intensity continuous treadmill running,interval high-intensity treadmill running requires the rodents to have adaptive training to ensure that they can run at 50%-60%of VO2maxfor at least 30 min.After their adaptation to this running period, mice or rats should have a daily warm-up training session for 10-20 min at 50%-60% of VO2max, followed by 5-8 intervals alternating between 4-8 min of high-intensity running(at 85%-90%of VO2max)and 2-3 min of recovery running(at 50%-60% of VO2max).40-42Functionally, aerobic interval high-intensity treadmill running has been shown to increase cardiomyocyte contractility and calcium sensitivity.40,56Interval high-intensity treadmill running post myocardial infarction is protective for attenuating cardiac remodeling and improving cardiac function.57
3.1.2. Assessment of exercise
Intensity-controlled treadmill running provides reliable and well-controlled endurance training when a VO2maxmeasurement is used.41,42With treadmill running, VO2maxprogressively increases when measured at the start of every week. Indeed, the workload(usually adjusted by running speed)is increased accordingly throughout the running period. It has been reported that at low to moderate exercise intensity, there is a close correlation between running speed and VO2max.58However, this correlation is not accurate enough to control the exercise intensity strictly because the correlation is not present within a range of 85%-90%of VO2max.58Thus,accurate control of exercise intensity should be ensured by adjusting the running speed on the basis of serial measurements of VO2max.41,42
Endurance exercise can significantly increase VO2maxas well as endurance capacity.59Endurance capacity in treadmill training is determined by the total running distance or the time to fatigue when mice or rats are exhausted and cannot run any farther or fail to keep pace with the treadmill.60For mice or rats finishing a total duration of moderate-intensity continuous running training and after adapting to the running environment,they should begin running at the same speed as usual (e.g.,15 m/min), which should be increased progressively (e.g.,by 1 m/min every 4 min). After the mice or rats are exhausted and cannot keep running despite being pushed,the total running distance or exercise time is determined in order to evaluate endurance capacity.61The exhaustion of rats can be confirmed by placing the rats on their backs and determining that the rats cannot turn over immediately.60The exhaustion of mice can be determined when a mouse fails to get back on the treadmill after 3 consecutive attempts to continue running.62
Because mice and rats are nocturnal animals,running training during their dark cycle is more effective.Measurements of VO2maxand endurance capacity should also be taken at a fixed time to ensure the reproducibility and reliability of data. For example,rat endurance capacity can be influenced by liver and skeletal muscle glycogen concentrations that may fluctuate during a day.63,64Moreover, the running environment should not be noisy,and the room temperature should be kept between 20°C and 22°C.
The reported effects of continuous treadmill running on physiological cardiac hypertrophy have been controversial,39,51,65,66and they may be related to different strains of rodents used as well as the exercise intensity employed in exercise protocols.48,67,68For example, C57BL/6J mice showed no significant increase in exercise capacity or cardiac growth after moderate-intensity treadmill running,whereas FVB/NJ mice developed obvious cardiac growth using this protocol.49However, interval high-intensity treadmill running in rats and mice controlled by VO2maxmeasurements has been shown to be effective in increasing ventricular weight.41,42In addition to promoting peripheral vascular effects and systemic metabolic regulation, treadmill running also brings about improvements in cardiac contractility and myofilament calcium sensitivity,69,70reduces mitochondrial reactive oxygen species production and mitochondrial permeability transition pore opening,71-73attenuates endoplasmic reticulum stress,74,75and inhibits cellular apoptosis and aging signaling in the heart.76
3.2. Voluntary wheel running
3.2.1. Model establishment
The wheel-running model is a voluntary running exercise applicable to both rats and mice.77-79The wheel-running apparatus for each animal is set in a single cage that is connected to a transducer and revolution counter for calculation and record of running parameters. Because animals are allowed to run at the speed, duration, and frequency they desire,voluntary wheel running requires less investigator time commitment, which is particularly applicable for long-term studies, including those on aging.80As nocturnal animals,rodents usually run during their dark cycle.Indeed,wheel running is good for rodents in that they run voluntarily during their active times each day,and it is important for investigators to facilitate the light-dark cycle of rodents.An unexpected light exposure will alter their voluntary running performance. The investigator should also check the record of running activity and ensure that all running wheels and revolution counters work well. The hygiene of animals using running wheels and the health status of these animals,including paw or toenail injuries,should be carefully checked.
3.2.2. Assessment of exercise
It is essential to record the running parameters of animals every day,including running speed,running time,and running distance.81Rodents should be adapted to the running wheel for 1-2 days before initiating a record of running parameters.Increasing evidence has demonstrated that the running distance usually peaks at ~4 weeks of voluntary wheel running and will remain stable or slightly decline thereafter.81The record of running parameters also helps to categorize rodents’running activity. Variations have been found in the voluntary wheel running of adult rats;these variations have been categorized as low activity (2-5 km/day), medium activity(>5-≤11 km/day), and high activity (>11 km/day).82It has been reported that adult male C57/BL6 mice run an average distance of 6.8 km/day(3.1-16.2 km/day).83Animals that are not willing to run on a voluntary wheel (e.g., C57/BL6 mice that run <3.1 km/day) could be excluded from the study.Although the frequency of exclusions of rodents from studies has not yet been fully determined, increasing the number of animals used for voluntary wheel running in a study helps to reduce the impact of exclusions.84
Although it is not practical to measure exercise capacity in voluntary wheel-running rodents,growing evidence has shown that voluntary wheel running can significantly increase VO2maxin these rodents,although to a much less extent than treadmill running.81Unlike the intensity-dependent effects of treadmill running on physiological cardiac hypertrophy,voluntary wheel running for 3-4 weeks in mice83,85,86and 6-8 weeks in rats87-90can induce physiological cardiac hypertrophy. Voluntary wheel running-induced physiological cardiac hypertrophy shows different transcriptomic and metabolomic profiles compared to pathological hypertrophy.91Voluntary wheel running for 4-8 weeks can also reduce myocardial infarction and cardiac ischemia/reperfusion injury,which have been found to be related to endothelial nitric oxide synthase expression and β3-adrenergic receptor activation.78,79
3.3. Swimming exercise
3.3.1. Model establishment
Swimming training of rodents is a commonly used exercise model for studying the physiological adaptations of skeletal muscles and the cardiovascular system to exercise, including physiological cardiac growth.92,93Mice that received a programmed swimming-exercise protocol starting with 10-min sessions twice a day and increasing to 90-min sessions twice a day, and then maintaining 90-min sessions twice a day,5-7 days/week for 3-4 weeks, had increased heart weight and developed physiological cardiac hypertrophy.94,95It is important that investigators respect a standard protocol in order to observe significant physiological cardiac growth after swimming exercise.
Although the swimming apparatus is inexpensive, it is important to ensure that the swimming dimensions, water depth, and water temperature remain constant.96For a swimming tank or bucket with a diameter of 65-80 cm, a total of 8-10 mice should swim together because mice tend to swim more vigorously when grouped together.However,it is crucial to avoid“gang swimming”,which causes animals to push one another under the water. The water depth should be set to>10-12 cm, and the water temperature should be set to 30°C-32°C.This helps to ensure an efficient swimming exercise and prevents mice from diving to the bottom or changing swimming speed because of lower temperatures.97A thermometer should be kept in the water during the whole course of swimming, and warm water should be added carefully into the bucket to maintain the water temperature at 30°C-32°C.Additionally, the swim buckets can be placed on large heat pads to help maintain a constant temperature. Mice should have a 5-min swimming adaptation session on the day before the formal exercise program begins. A protocol that begins with 10 min of swimming twice a day and increases by 10 min each day until two 90-min sessions each day are reached is important for training mice to be proficient swimmers.Although various mouse strains have been used in swimmingexercise models,98-100the ages and the initial body weights of mice should be randomized so that they are identical in the sedentary and swimming groups.C57BL/6 mice that are 8-10 weeks old and weigh 24-25 g are suitable for beginning a swimming-exercise program.95When the mice are swimming,investigators should be vigilant regarding their swimming behavior and use a bottle brush when it is necessary to keep them swimming. Some mice may also need a little rest to avoid any adverse animal events. They can be picked up for 30 s or so and rest on the palm of the researcher’s hand before being placed back in the water. Keeping notes on mice that continually float or need rest is also important because cardiac hypertrophy at the endpoint may not be expected for those mice. After their swimming exercise, mice need to be dried with a towel and ventilated in a warm place.Any environment that is either too hot or too cold will cause unexpected illness in the animals.Mice should rest at least 4 h between the 2 daily swimming sessions. Mice that are subjected to an exhaustive swimming exercise program should swim with an overload of 5% of body weight. Exhaustion can be confirmed when a mouse fails to swim to the surface of the water within 7 s.101
Compared to mice, rats used in swimming exercises produce and accumulate more air bubbles in their fur, which results in more floating or bobbing behaviors instead of actual swimming. Thus, rats usually need an overload (2%-5% of body weight) that is effected by attaching a balance weight to the chest or about 5 cm from the end of the tail during swimming.102Exhaustion can be defined as rats’ being unable to swim to the surface for breathing within 10 s.102-104The swimming dimension should be 1000-1500 cm2per rat, and the water depth should be greater than the length of the rat from nose to tail.105,106A swimming tank with a diameter of 65-80 cm is suitable only for 2 rats swimming at the same time. It is also important that rats have a 5-min swimming adaptation session on the day before the formal exercise program begins. Then rats begin with 10-min swimming on the first day of the formal exercise program, and an overload will be added on the third day of swimming. Swimming duration should increase by 10 min/day until a total of 60-90 min is attained (once or twice a day). The total swimming duration can vary from 8 to 15 weeks, which is much longer than the mouse swimming protocol.107-110
3.3.2. Assessment of exercise
After the swimming exercise,the heart weight,heart weight body weight ratio (HW/BW), and heart weight to tibia length ratio (HW/TL) should be determined.111Both the HW/BW ratio and HW/TL ratio are commonly used to determine exercise-induced cardiac growth,although the increase in the HW/BW ratio may be overestimated due to the change in body weight after exercise.For swimming-trained mice and rats,an increase of about 12%-15% or more in HW/BW ratio (or HW/TL ratio) can be considered to be effective physiological cardiac growth due to exercise.21,98,112,113According to the literature, there is no significant difference in the the degree of cardiac hypertrophy after swimming exercise in rats and mice.92,98,108-110,113-117Heart rate can be reduced or remain unchanged, but blood pressure is not altered in swimmingexercised rodents.98,114-117It is also recommended that citrate synthase activity in mixed skeletal muscles be measured,including the tibialis anterior and gastrocnemius.98,112A similar increase in citrate synthase activity indicates that there is no difference in exercise capacity between differently exercised groups.98,112,118
Multiple factors, such as genetic modification of animals,disease conditions,and exercise at differing stages of disease,can influence the effects of exercise, so it is not practical to set uniform, predetermined exclusion criteria for exercise studies. However, animals that exhibit certain exercise behaviors indicating that they cannot adapt to the swimming environment, including drowning, continually floating, or losing more than 15% of their body weight, can be excluded from the analysis.
Histological analysis of the cross-sectional area of the cardiomyocytes should be conducted to confirm the hypertrophic growth of the heart.95The pathological hypertrophy-marker genes,atrial natriuretic peptide and brain natriuretic peptide,do not increase with exercise training.21In addition to physiological hypertrophy,swimming training can promote cardiomyocyte proliferation and renewal.119Cardiac cell proliferation and renewal have been reported to be necessary to mediate the protective effect of swimming training in the murine model of cardiac ischemia-reperfusion injury.120Interestingly, some of the best-characterized pathways in physiological cardiac growth (e.g., insulin-like growth factor 1/phosphoinositide 3-kinase/protein kinase B (IGF1/PI3K/AKT), downregulated CCAAT/enhancer binding protein β(C/EBPβ)and upregulated Cbp/p300-interacting transactivator with ED-rich carboxy-terminal domain-4 (CITED4), and non-coding RNAs, such as microRNA-222 (miR-222) and miR-17-3p), also show beneficial effects against pathological cardiac remodeling.21,92,95,119The mechanisms underlying the cardioprotective effects of swimming exercise could be related to the improved survival(pro-proliferative and anti-apoptotic effects)of cardiomyocytes,cardiac electrical and contraction functions, myocardial glycolipid metabolism,and mitochondrial adaptations.8
3.4. Resistance exercise
3.4.1. Model establishment
Compared with aerobic exercise,resistanc e exercise has been much less studied in cardiovascular adaptations and diseases.The most commonly used methods of resistance training in animal models include squat-training and ladder climbing.Squat-training requires a specially designed apparatus first described by Tamaki et al.121Rats wear a canvas jacket that is connected to a wooden bar with a loading weight on it.When the rat tail is stressed by an electrical stimulation (20 V, 0.3-s duration, at 3-s intervals), the rat jumps and lifts the weight-arm of the apparatus repeatedly.122Rats should be preconditioned to this apparatus for about 2 weeks. The squat-training requires 12 repetitive stimulations per set, with 90 s of rest after each set. The sets should be administered 4 times/day and last for 4 weeks (5 days/week).122The weight loads should be regulated to 65%-75% of 1 repetition maximum (1 RM; for details, see “3.4.2 Assessment of exercise”).122This model was first used to analyze skeletal muscle adaptations and metabolic changes in response to resistance training123,124and was later used to analyze physiological cardiac hypertrophy and cardiac diseases.25,55,125
Compared with the special apparatus required for squat-training,the equipment used for ladder climbing or grille climbing is less complicated. For ladder-climbing exercise, the ladder is about 1 m in length with an 80°-85° incline, and the interval between steps is generally 0.5-1.0 cm. The animals need to be adapted to the climb for 1 week,with gradually increasing loads(usually beginning with 10%-20% of body weight). However,climb repetitions (8-15 repetitions per set), number of climb sets (e.g., 4 sets/day), frequency of climbs (3-5 days/week),and duration of climbs (from weeks to months) can vary depending on the type of research being conducted. Likewise,the weight load attached to the tail can be maintained at 40%-60% of the maximum load55or can be gradually increased to 90%-100% of the maximum load after each climb set.126Because of the differing parameters used in ladder climbing, the data reported by different groups are difficult to compare.
3.4.2. Assessment of exercise
The weight loads added to the animals should be regularly controlled and adjusted for both squat-training and ladder climbing.For squat-training,1 RM is defined as the maximum weight lifted when the animal is able to jump in response to an electronic stimulation.121The 1 RM should be measured every 2 weeks;this serves as a basis for controlling the weight loads(usually set at 65%-75% of 1 RM) and represents an important index for characterizing training load.122For ladder climbing,the maximum load test needs to be conducted at the start,during (every 45 days), and after the completion of resistance training.127After adapting to the climbing activity for 5 consecutive days,rats or mice should climb the ladder with an initial load of 75% of body weight, which is increased by 15% of body weight after finishing each climb.128The weight load when the animal cannot climb the entire ladder after 3 successive stimulations to the tail is defined as the maximum load.55
Hemodynamic measurements after resistance exercise training should include the arterial systolic and diastolic blood pressure and heart rate.129Resistance training can reduce blood pressure (diastolic pressure and mean arterial pressure)and peripheral vascular resistance and improve endothelial function in hypertensive and diabetic rats.129-131The squattraining(load of 65%-75%of 1 RM,for 4 weeks)can induce a slight increase in the left ventricle weight.122However, the effect of climbing-exercise training on cardiac hypertrophy has been much less studied.132Nevertheless, resistance exercise has been found to be beneficial in preventing and treating hypertension, myocardial infarction, ischemia reperfusion injury,heart failure,and diabetic cardiomyopathy.72,133,134
4. Optimal design of animal-exercise studies in cardiovascular research
Various exercise models have been used in studies of cardiovascular physiology and CVDs. For optimal design of animal-exercise studies in cardiovascular research, we provide consensus recommendations concerning the choice of exercise models, the control of exercise protocols, exercise at different stages of disease, and other considerations that should be marked. An instructive outline of the optimal design of animal-exercise studies in cardiovascular research, with an example of studying exercise-induced physiological cardiac growth and its related beneficial effects against CVDs, is presented in Fig. 2.
4.1. Choice of exercise models
Dynamic exercise,especially treadmill running,is the most commonly used exercise model in the study of CVDs.Treadmill running, conducted either before or after the onset of CVDs,has been shown to have protective effects in various CVDs, such as myocardial infarction, ischemia-reperfusion injury,atherosclerosis,hypertension,diabetic cardiomyopathy,and heart failure.19In addition, swimming training protects mice from pathological remodeling and signs of heart failure in response to aortic-banding, myocardial infarction, and ischemia-reperfusion injury.21,112,120Numerous studies have also reported the beneficial effects of exercise in CVDs using other exercise models, such as voluntary wheel running and resistance training.122,135
A primary issue in current exercise studies in cardiovascular research involves choosing an exercise model. The choice of exercise models can be associated with multiple important factors, including the scientific question posed and the functions investigated. One representative example of this is exercise-induced physiological cardiac growth, which is characterized by a physiological process without cardiac fibrosis and cardiac dysfunction.98,136Understanding whether and how exercise-induced physiological cardiac growth can protect the heart may provide new opportunities for identifying novel therapeutic targets for CVDs.137Using swimming training and voluntary wheel-running exercise models, scientists have proved that such aerobic dynamic training can induce both cardiomyocyte hypertrophy and proliferation.21,119Although interval high-intensity treadmill running has been shown to induce physiological cardiac growth,41,42moderate-intensity continuous treadmill running or resistance training are not very efficient exercise models for inducing cardiac growth and cardiomyocyte proliferation.39,65,66Protocols and effects of different types of exercise on cardiac physiological hypertrophy and other cardiovascular responses are listed in Supplementary Tables 1 and 2.
4.2. Exercise intensity,frequency,and duration
Exercise intensity, frequency, and duration are essential parameters that should be seriously considered and decided upon before animal studies start. Some exercise models require strict control of exercise parameters.For example,mice that swim for 90 min twice a day,5-7 days/week,for 3-4 weeks can develop obvious physiological cardiac growth.21,92,95Because evidence has shown that the voluntary running distance usually peaks at~4 weeks, the commonly used running duration is 4-8 weeks for the voluntary wheel-running model.81It is quite important to adhere to a standardized exercise protocol in order to achieve the obvious exercise-associated phenotypes.
Exercise parameters can vary among research groups.In all research reports,it is quite important that the exercise parameters be described in detail in Methods section. For example, a large number of studies using treadmill running do not or cannot measure VO2maxbut describe only the running speed instead. This absence of parameters, especially for interval high-intensity treadmill running (which requires regular measurements of VO2max), is insufficient for controlling exercise intensity during the study.58As for voluntary wheel running, the running distance can vary substantially, even in a single group of animals.82However, experimental data (e.g.,running speed,running time,and running distance) about voluntary wheel running are not always provided by the investigators. A clear presentation of these exercise parameters is necessary for promoting the standardization of exercise models and facilitating data comparisons among the various research groups.

Fig.2. Optimal design of animal studies in cardiovascular research.CVD=cardiovascular disease;I/R=ischemia-reperfusion;VO2max=maximal oxygen uptake.TagedEnd
Spontaneous or modified animal models of CVDs are widely used in cardiovascular research.Low-to moderate-intensity aerobic exercise attenuates cardiac remodeling in spontaneously hypertensive rats,and the settings of running speed,duration,and frequency are not significantly different from those of healthy wild-type animals.138-140The beneficial effects of aerobic exercise have been shown to exist in other spontaneous disease models,such as reduced obesity in fatty Zucker rats(by improving insulin resistance), increased functional vasodilation, and reduced heart glycogen content and mitochondrial apoptotic signaling.141-143However, compared to lean Zucker rats, obese Zucker rats have lower VO2max.141Although VO2maxcan be increased by treadmill running in both lean and obese Zucker rats,VO2maxstill remains lower in trained obese Zucker rats than in trained lean ones.141In addition to VO2max,determinations of the maximal lactate steady state and the speed at maximum exercise test help to prescribe and evaluate exercise in obese rat models.144-147Indeed, some disease models may influence animal exercise capacity.In these circumstances,measurements of exercise capacity are necessary to ensure accurate control of exercise intensity and proper presentation of results and conclusions.148
4.3. Exercise at various stages of disease
Although a growing number of CVD studies have shown the benefits of exercise in animal models, the clinical translational potential must be more carefully examined. For example, exercise carried out at differing stages of a disease is an important issue that should be considered in designing the study. Exercise before and after the onset of heart failure is categorized as primary and secondary prevention of heart failure, respectively.18Despite the fact that preventive exercise interventions have been widely proved to attenuate cardiovascular injury and preserve cardiac function, exercise interventions occurring after the onset of CVDs may have differing effects.55Aerobic and resistance exercise conducted in rats 3 months after myocardial infarction improved exercise capacity and maximum load carrying,respectively.55However, the onset of exercise 3 months after myocardial infarction failed to reduce infarct size or attenuate cardiac dysfunction in rats.55Indeed, exercise training as a therapy carried out after cardiac injury deserves further investigation. The mechanisms related to the therapeutic effects of exercise post injury need to be explained and differentiated from the effects of preventive exercise interventions.9,57,126
4.4. Other considerations
4.4.1. Age
Most exercise studies use young or adult animals in cardiovascular research.Because aging is an independent risk factor for CVDs,it is important to use aging animals in studying the effects of exercise.149Young and middle-aged mice (up to 20 months) may perform treadmill running at the same running speed. However, older mice (24 months of age) have reduced exercise capacity,and their running speed should be regulated according to VO2maxmeasurements.150Old mice (24-30 months of age)that run 45 min at 10 m/min at a 10°incline,5 days/week for 8 weeks,have improved exercise capacity,diastolic function, and contractile reserves as well as increased capillary density and reduced pulmonary congestion.76Old animals can also be used in studies involving voluntary wheel running.76Thus, the effects of exercise need to be explored through the use of aging animals in CVD studies.
4.4.2. Sex
Sex-related differences in cardiac structures and functions,as well as in cardiovascular pathophysiology, have been studied increasingly.151-153Males and females can have different exercise capacities as well as systemic and cardiac responses when involved in exercise training.134Although some early studies showed that running exercise induced similar skeletal muscle adaptations in male and female rats, only the isolated hearts from male rats showed higher stroke work,stroke volume,coronary flow,and myocardial oxygen consumption.154In exercise models, female mice showed better exercise performance than males after treadmill running and voluntary wheel running,and female mice had greater physiological cardiac hypertrophy than male mice after voluntary wheel running.155Studies of sex differences in response to exercise,as well as the separate underlying mechanisms,are strongly warranted.
4.4.3. Genetic background or pharmacological treatment influence
Genetic manipulations in rodents and even in large animals can be used to identify the the mechanisms related to exerciseassociated effects in CVD studies.However,genetic backgrounds as well as pharmacological interventions in animals may influence their exercise capacities. One study found that endothelial nitric oxide synthase or β3-adrenergic receptor-deficient mice exercised to a lesser degree than wild mice during a 4-week voluntary wheel-running exercise.78Measurements of running parameters (e.g., running distance) and VO2maxare necessary to assess the impact of genetic background on exercise capacity in animal-exercise models. Indeed, myocardial-specific genetic manipulations are a better strategy for avoiding unexpected influences on other cell types, organs, or systems, which may also have less impact on exercise capacity.98,111
4.4.4. Other animal models
Although rodents are the most commonly used species in animal-exercise studies, other species, such as zebrafish, rabbits,dogs, swine, and horses, can also be used for exercise training.156-159More details about large-animal exercise models can be found in the Guidelines for animal exercise and training protocols for cardiovascular studies,published in the American Journal of Physiology—Heart and Circulatory Physiology.36Using largeanimal exercise models has obvious advantages over small-animal models because large animals have cardiovascular structures and functional responses to exercise that are more similar to those of humans. However, the laboratory facilities, breeding, and maintenance of large animals, the exercise protocols used, and the measurements to be taken are all factors that influence researchers’ abilities to carry out large-animal studies.Nevertheless,large-animal exercise models are valuable in promoting both an understanding of the mechanisms at work and the translational value of CVD studies related to exercise.
The zebrafish genome has a close relationship to the human genome.160Zebrafish exercise models have been increasingly used in studies of exercise physiology, CVDs, and toxicology.161-165A swimming protocol for zebrafish larvae,in which they swam at 5 body lengths/s (BL/s), 18 h/day, for 7 days for 9-day-old larvae and 12 days for 21-day-old larvae,did not influence cardiac activity such as heart rate, systolic and diastolic ventricular volume, and cardiac output.166Instead, this swimming protocol improved the oxygen-carrying capacity of blood and increased the capillarization and mitochondrial density in muscle tissues, as measured by red blood cell count,cast of the vascular bed,and electron microscopy.166In another study, zebrafish that were 14 days old began with 3 BL/s swimming for the first week and then maintained swimming at 5 BL/s after the second week,for 6 h/day for 10 weeks.167The result was that they had upregulation of the muscle growth factor myogenin and proliferating cell nuclear antigen in both the heart and axial muscle.167This swimming protocol enhanced slow aerobic-muscle development in the axial musculature,while the heart muscle showed a shift toward a faster phenotype but did not become more aerobic.167In yet another study, adult zebrafish swimming at 13 BL/s,6 h/day,5 days/week for 4 weeks,had increased proliferation of cardiomyocytes under normal condition.163Zebrafish swimming using this protocol for 13 experimental days also displayed increased cardiomyocyte proliferation and improved cardiac recovery and function after cryoinjury.163Indeed, zebrafish swimming is emerging as an interesting model for studying the effects and mechanisms of physical exercise on the heart and CVDs.
4.4.5. Adverse effects of excessive exercise
Although most studies are designed to study the beneficial effects of exercise, it is also important to highlight the adverse effects caused by excessive exercise. In 1 study, high-intensity running exercise(e.g.,28 m/min for 60 min,5 days/week for 16 weeks)increased atrial fibrillation susceptibility in rats,which has been associated with autonomic changes,atrial dilation,and atrial fibrosis.168Likewise, mice swimming in a steady water current(90 min per session,daily for 6 weeks)or running on a treadmill with a 30° incline (at 21 m/min for 120 min, daily for 6 weeks)increased atrial arrhythmia susceptibility.169
In models relying on forced exercise (e.g., the exhaustive exercise test), experienced laboratory personnel should observe the animals’ exercise behaviors closely. The animals should not be forced to engage in exercise when exhaustion occurs.In addition,responses to improper exercise such as cessation of swimming, constant floating, increased defecation,hyperalgesia, or biomarkers of peripheral muscle fatigue,should be assessed because improper exercise may cause psychological distress in animals.170-172Although the exact impact of psychological distress on experimental results has not yet been fully determined,it has been reported that forced treadmill running,but not voluntary wheel running,effectively induces neuroprotection in stroke.173Certainly,it is important to choose proper exercise protocols in order to reduce pain and distress in animal-exercise experiments.
5. Functional experiments to determine targets in response to exercise
Because of the rapid development of omics technology,such as genomics, proteomics, and metabonomics, as well as microarray profiling and in silico analysis, more and more potential targets in response to exercise are able to be revealed.21,95,119First, it is critical to confirm the expressions of predicted targets in the hearts of exercised animals using real-time quantitative polymerase chain reactions and Western blot analysis. Specific biochemical validation experiments are needed for noncoding RNAs, such as circular RNAs and long non-coding RNAs,that are identified in the hearts of exercised animals.174,175Next, it is essential to conduct functional experiments in order to determine the roles of these targets in the hearts of exercised animals. Increasing evidence indicates that targets identified in exercised animal hearts have the potential to mitigate cardiac injury in multiple ways.176Molecules that are regulated in exercised animal hearts may promote physiological hypertrophy of cardiomyocytes and may have the potential to enhance the expression of proliferation markers of cardiomyocytes and prevent cardiomyocyte apoptosis as well.9
For studies of exercise-induced physiological cardiac hypertrophy, the molecules that were screened out need to be further investigated based on functional experiments. In in vitro experiments,primary cultured neonatal rat or mouse cardiomyocytes are essential for determining the functional roles of exercise-responsive molecules in regulating the physiological hypertrophy and the proliferation markers in cardiomyocytes.119,175The oxygen glucose deprivation/reperfusioninduced apoptosis model can be used to examine whether these molecules have antiapoptosis effects in cultured neonatal cardiomyocytes.21,95It is noteworthy that cardiomyocytes derived from human embryonic stem cells or human-induced pluripotent stem cells are valuable in confirming these functions in human cardiomyocytes.177,178In in vivo experiments, exercise-associated cardiac phenotypes, such as physiological cardiac hypertrophy (without dysregulation of pathological hypertrophy markers, e.g., atrial natriuretic peptide and brain natriuretic peptide) and increased expression of proliferation markers (e.g., 5-ethynyl-2’-deoxyuridine, Ki-67, phosphohistone H3,and Aurora B)in the myocardium deserve to be validated in exercised animal models such as murine models of swimming exercise by using genetically engineered or modified animals.92,98,175,179A deep understanding of the functions and mechanisms of exercise-responsive molecules paves the way for identifying novel myocardial protective or therapeutic targets for CVDs.8,137,176
6. Translational values and conclusions
Animal studies using exercise interventions have greatly enlarged our understanding of the beneficial effects of exercise in preventing and treating CVDs. It is noteworthy that the involved cellular and molecular mechanisms contributing to exercise-induced cardiovascular adaptations are potential interventional targets that can be used to provide cardiovascular protective effects.17,180The molecule-based therapies regulating the PI3K/Akt signaling pathway or calcium handling and the gene therapies or RNA-based therapies targeting the key molecules identified in animal exercise models, such as miR-222,miR-17-3p,and sarco(endo)plasmic reticulum Ca(2+)-ATPase 2a, may mimic, to at least some degree, the effects of exercise.21,95,112The molecule mechanisms identified in the control of physiological cardiac growth have the potential to mitigate detrimental hypertrophy and heart failure.137Additionally, exercise-induced regulation of circulating components such as cytokines, myokines, microRNAs, and other non-coding RNAs,may serve as biomarkers for risk stratification, treatment,and prognosis of CVDs.181-183Indeed, animal studies using exercise interventions have great translational value for identifying novel targets and developing promising clinical approaches to mitigate CVDs.
Despite the intensive interest in studying the beneficial effects and mechanisms of exercise on CVDs, the successful translation of basic research into clinical applications is still limited.Multiple factors contribute to this,including the inappropriate use of animal exercise models,which may influence the evaluation of exercise-induced effects on CVDs and limit the comparison of results from different studies.We hope that this scientific statement will help scientists to design and perform high-quality animal studies better by using exercise interventions that will help in the treatment of CVDs. Rigorous respect for the use of standardized procedures in exercise training and good adherence to exercise protocols during the study are warranted. Collectively, a well-designed, well-performed,and quality-controlled animal exercise study and a deep understanding of the mechanisms of exercise-associated cardioprotection will pave the way for successful translation of exercise studies from bench to bedside in the prevention and treatment of CVDs.
Acknowledgments
This work was supported by grants from the National Key Research and Development Project (2020YFA0803800 to YB), National Natural Science Foundation of China(82020108002 and 81911540486 to JX, 81772444 to LW,81772466 to RD),Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-09-E00042 to JX),Science and Technology Commission of Shanghai Municipality (18410722200 and 17010500100 to JX), and “Dawn” Program of the Shanghai Education Commission(19SG34 to JX).We thank all members of the Committee on Cardiac Rehabilitation,Chinese Medical Doctors’Association.
Authors’contributions
JX and LG designed the concept and structure of the position paper and provided substantial revisions to the draft manuscript;YB and LW designed the concept and structure of the position paper. All authors were actively involved in writing and composing subsection of the position paper. All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.08.002.
 Journal of Sport and Health Science2021年6期
Journal of Sport and Health Science2021年6期
- Journal of Sport and Health Science的其它文章
- The association of grip strength with cardiovascular diseases and all-cause mortality in people with hypertension:Findings from the Prospective Urban Rural Epidemiology China Study
- Cardiorespiratory fitness measured with cardiopulmonary exercise testing and mortality in patients with cardiovascular disease:A systematic review and meta-analysis
- The relationships between step count and all-cause mortality and cardiovascular events:A dose-response meta-analysis
- IGF1-PI3K-induced physiological cardiac hypertrophy:Implications for new heart failure therapies,biomarkers,and predicting cardiotoxicity
- The epigenetic landscape of exercise in cardiac health and disease
- Exploring the impact of COVID-19 on the movement behaviors of children and youth:A scoping review of evidence after the first year
