Cardiorespiratory fitness measured with cardiopulmonary exercise testing and mortality in patients with cardiovascular disease:A systematic review and meta-analysis
Ysmin Ezztvr,Mikel Izquierdo,Julio N´u~nez,Joqu´ın Cltyud,Robinson Rm´ırez-V´elez,Antonio Grc´ı-Hermoso,f,*
a Exercise Intervention for Health Research Group(EXINH-RG),Department of Physiotherapy,Universitat de Val`encia,Valencia 46010,Spain
b Navarrabiomed,Navarra Hospital Complex(CHN),Public University of Navarra(UPNA),Navarra Medical Research Institute(IdiSNA),Pamplona 31008,Spain
c CIBER of Frailty and Healthy Aging(CIBERFES),Instituto de Salud Carlos III,Madrid 28029,Spain
d Department of Cardiology,Valencia University Hospital,Biomedical Research Institute(INCLIVA),Valencia 46010,Spain
e CIBER in Cardiovascular Diseases(CIBERCV),Madrid 28029,Spain
f Sciences of Physical Activity,Sports and Health School,University of Santiago of Chile(USACH),Santiago 71783-5,Chile
Abstract
Keywords: Cardiopulmonary fitness;Coronary artery disease;Exercise capacity;Heart failure;Survival
1. Introduction
Despite noteworthy advances in management and treatment over the last decades, cardiovascular disease (CVD) remains the leading cause of mortality worldwide.1The early identification of modifiable risk factors for CVD can improve longterm survival,2which places emphasis on the importance of developing biomarkers that can predict lifetime CVD risk.Regarding risk prediction, a recent scientific update from the American Heart Association has stated that cardiorespiratory fitness (CRF) may provide additional prognostic value for CVD and associated mortality risk beyond traditional cardiovascular risk factors, such as hypertension, smoking, obesity,hyperlipidemia,and type 2 diabetes mellitus.3
A wealth of evidence from many large (retrospective and prospective)epidemiological cohort studies underpins the link between CRF and health outcomes, including the risk of allcause, CVD, and cancer mortality in apparently healthy and clinical populations,which includes patients with diabetes,4those who are overweight,5or who have hypertension.6Nes et al.7found that in healthy men (n=18,348) and women (n=18,764)<60 years of age, each 1 metabolic equivalent (1-MET)increase in exercise capacity from baseline reduced the risk of CVD mortality by 21%. Additionally, higher levels of CRF in midlife have been associated with lower risk of heart failure,myocardial infarction, and stroke.8To date, however, little is known about the predictive value of CRF levels in adults with CVD.
It is well-established that exercise—through increases in CRF—has quantifiable biological effects on the cardiovascular system in terms of structure and function, and it is considered a cornerstone of cardiac rehabilitation programs.9Increases in CRF have been shown to provide substantial health benefits in patients with CVD, including reduced risk of heart failurerelated hospitalization in later life,10improved short-term mortality after coronary artery bypass grafting,11lower rates of recurrent myocardial infarction, and decreases in both CVD and all-cause mortality.12,13
Despite the recognition of the utility of CRF measurements in many CVD management guidelines,3,14these assessments are not frequently included in routine clinical health screenings and,when performed,are rarely used for risk stratification.15,16This lack of use might be explained by the paucity of information endorsing the role of CRF in predicting long-term mortality in adults with CVD. It has been proposed that the use of CRF as a biomarker should be included as a standard part of clinical encounters (e.g., an accepted “vital sign”).3,14Understanding the associations between CRF and cardiovascular health could help identify individuals at high risk, improve care delivery,and promote policy recommendations for physical fitness and exercise.Thus,the present study aimed to quantify the association between CRF in adults with established CVD and the risk of all-cause and CVD mortality.
2. Methods
The present systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses(PRISMA)guidelines and recommendations.17Patient consent and ethical committee approval were not required to conduct the present study. Neither patients nor the public were involved in the design, conduct,reporting, or dissemination plans of our research. The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number:CRD42020203619).
2.1. Search strategy
The search was conducted independently by 2 investigators(YE and AGH)using MEDLINE,Embase,and SPORTDiscus electronic databases, from inception to May 2021. Additionally, a gray literature search of abstracts and conference proceedings from the European Society of Cardiology, American Heart Association,American College of Sports Medicine,and the American College of Cardiology conventions from the last 5 years was undertaken. The following string of medical subject headings terms was used to identify all possible studies investigating the predictive value of CRF on mortality in patients with CVD: peak oxygen, oxygen consumption, aerobic capacity, exercise capacity, aerobic fitness, cardiopulmonary fitness, cardiovascular fitness, cardiac rehabilitation,major adverse cardiovascular events,mortality,death rate,prospective, longitudinal, follow-up, heart failure, CVD, peripheral artery disease, coronary heart disease, myocardial infarction,coronary artery disease,cardiomyopathy,and acute coronary syndrome (Supplementary File 1). Searching was restricted to published articles in the English and Spanish languages. A medical librarian was consulted to audit the quality of the search strategy. Reference lists of eligible articles were manually scrutinized for further identification of relevant articles. Any disagreement was resolved by consensus with a third author(RRV).
2.2. Selection criteria
After reviewing the title and abstract, 2 investigators (YE and AGH) systematically assessed the full text of identified articles for eligibility.To be eligible for inclusion in the metaanalysis, studies needed to meet the following criteria: (a)exposure: CRF assessed directly from expired gas analysis or estimated through various cardiopulmonary maximal or submaximal exercise tests using a treadmill or cycle ergometer;(b)participants:patients with any type of CVD(i.e.,heart failure,coronary artery disease,peripheral arterial disease,or cardiomyopathy); (c) outcomes analyzed: all-cause mortality,and/or overall survival, and/or CVD mortality; and (d) study design:prospective cohort studies with at least 6 months of follow-up. Studies were excluded if they: (a) did not report data regarding the variables of interest;(b)reported estimated CRF through non-exercise prediction algorithms; (c) did not assess CRF directly from a cardiopulmonary exercise test using a treadmill or cycle ergometer;or(d)reported insufficient information for calculating hazard ratios(HRs)and 95%confidence intervals (95%CIs). In the case of duplicate studies, the most recent version was included. Any disagreement was resolved by consensus with a third author(RRV).
2.3. Data collection process and data items
Data collection was conducted independently by 2 investigators (YE and AGH), using a Microsoft Excel spreadsheet(Microsoft Corp., Redmond, WA, USA) specifically designed for the present study.The following information was extracted from each study that met the selection criteria: (a) study characteristics (first author’s name, publication year, study location, sample size, number of females, study design, follow-up duration, and cut-offs defining CRF categories); (b) participants’ information (sex, age, and number of death events);(c) assessment details (measurement of peak oxygen uptake,maximal oxygen consumption (VO2max), and estimation of METs); and (d) statistical analysis and study results (confounding factors, outcome of interest, and main results).Missing data from the studies were requested from the corresponding authors of the original published papers.
2.4. Risk of bias in individual studies
The Quality Assessment Tool for Observational Cohort and Cross-sectional Studies was used to determine the risk of bias of each study.The checklist comprised 14 items for longitudinal studies.Each item of methodological quality was classified as“yes”,“no”,or“not reported”.
2.5. Summary measures
The a priori plan was to conduct a one-step individual participant data meta-analysis.All analyses were carried out using STATA (Version 16.1; STATA Corp., College Station, TX,USA). Meta-analysis was performed when at least 3 studies provided data. HRs with associated 95%CIs were extracted from studies for mortality (all-cause and CVD), and pooled HRs were then calculated using the random-effects inversevariance model with the Hartung-Knapp-Sidik-Jonkman adjustment.18HRs were pooled,comparing the highest vs.the lowest CRF category in relation to all-cause and CVD mortality. Hazard estimates that were provided in units other than per 1-MET increments(e.g.,per 1-mL/kg/min)were converted into 1-MET increments using exponential functions. When studies presented several statistical risk-adjustment models,only HRs associated with the statistical models that contained the highest number of additional covariates were considered.
2.6. Synthesis of results
The percentage of variation across studies was estimated using the inconsistency index(I2)derived from the Cochran Q statistic,19considering I2values of 25%, 50%, and 75% as low,moderate,and high inconsistency,respectively.19
2.7. Risk of bias across studies
Small-study effects and publication bias were examined using the Doi plot and Luis Furuya-Kanamori (LFK) index,both of which have been shown to be superior to the traditional funnel plot and Egger’s regression intercept test.19Values of-1,between-1 and-2,and >-2,are considered to represent no,minor,and major asymmetry,respectively.20
2.8. Additional analysis
A sensitivity analysis was conducted to assess the robustness of the summary estimates, i.e., to determine whether or not a particular study accounted for the heterogeneity.Thus,to examine the effects of every individual result from every individual study on the overall findings, results were analyzed with each study deleted from the model once.
A subgroup analysis according to CVD type(i.e.,coronary artery disease and heart failure)was conducted.
Finally, random-effects meta-regression analyses using the Hartung-Knapp-Sidik-Jonkman adjustment18were used to independently evaluate whether or not results differed according to the length of follow-up(in months).
3. Results
3.1. Study selection
The electronic search strategy retrieved 3675 studies.After removing duplicates and screening titles and abstracts, 348 studies were assessed for eligibility based on their full text. A reference list of excluded articles and the reasons for their exclusion based on the full text is reported in Supplementary File 2. The PRISMA flow diagram illustrating the number of studies excluded at each stage of the systematic review and meta-analysis is shown in Fig.1.
3.2. Study characteristics
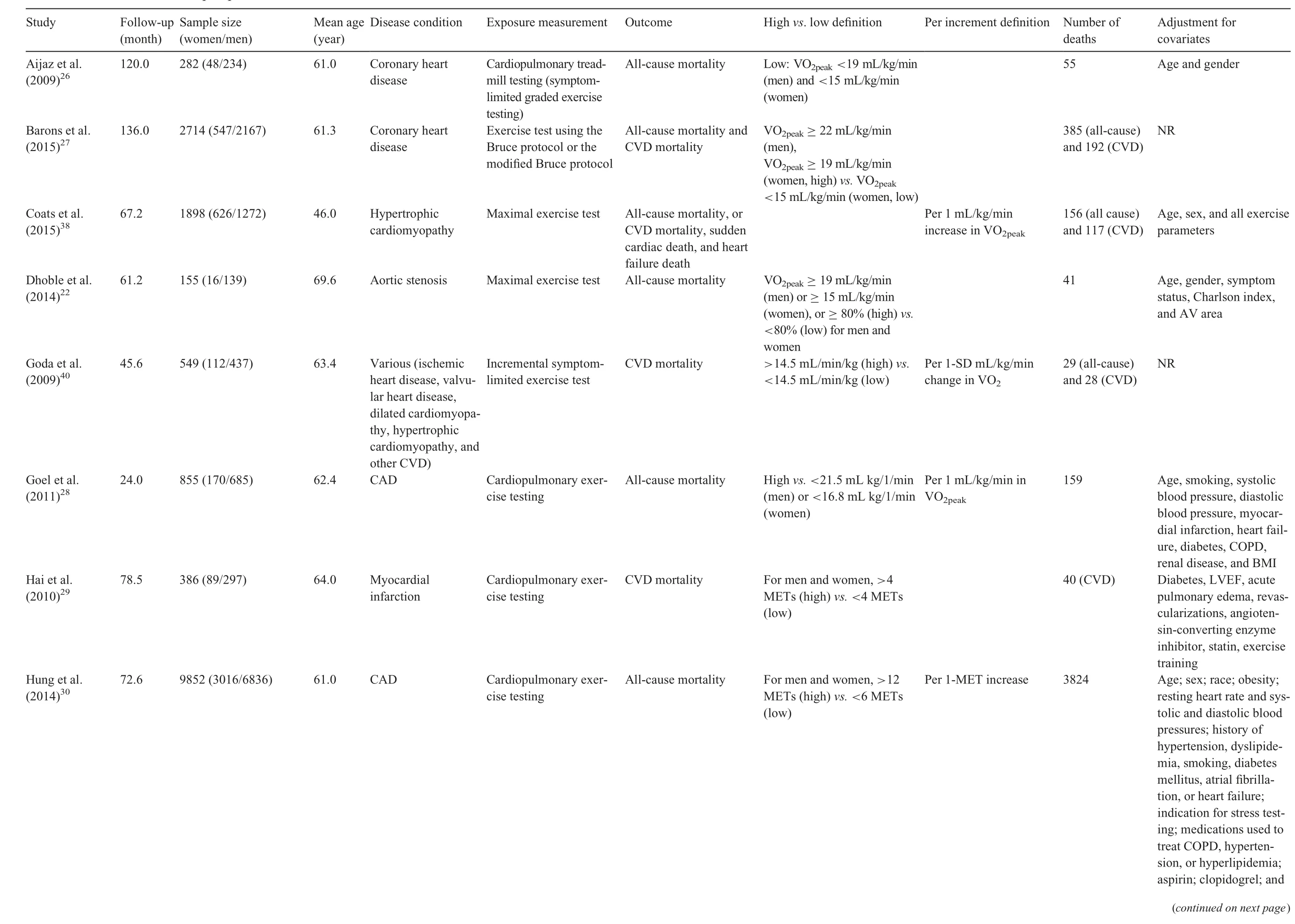
Twenty-one prospective cohort studies were included in the qualitative synthesis, although only 20 were analyzed in the quantitative synthesis(1 study21was excluded because participants were not exclusively patients with established CVD).Our study comprised a total of 156,371 patients diagnosed with CVD (38.1% female), with a mean age of 61.4 years.Sample sizes across studies ranged from 15522to 122,00721patients. The follow-up length ranged from 1223to 16824months. The most common CVDs were coronary artery disease23-32and heart failure,33-37and other studies included patients diagnosed with aortic stenosis,22hypertrophic cardiomyopathy,38peripheral artery disease,39and a combination of various pathologies.21,40-42General characteristics of the 21 included studies are summarized in Table 1.
3.3. Summary measures(exposure)
CRF was measured using cardiopulmonary exercise testing and analyzed through the measurement of peak oxygen uptake(the value representing the highest volume of oxygen uptake attained during the test),VO2max(the value representing the point that oxygen uptake reaches a maximum beyond which no increase in effort can augment it), or estimation of METs. Cut-off points for determining CRF categories varied across studies(Table 1).
3.4. Risk of bias within studies
All studies met at least 10 out of the 14 ite ms included in the Quality Assessment Tool for Observational Cohort and Crosssectional Studies and were considered to have fair-to-good methodological quality. The average score was 10.61/14.00(Supplementary Table 1).
3.5. Synthesis of results
All-cause (HR=0.42; 95%CI: 0.28-0.61, p < 0.001,I2=94.6%) and CVD (HR=0.27; 95%CI: 0.16-0.48,p=0.005, I2=0.0%) mortality was lower in individuals with high CRF than in individuals with low CRF.Cochran’s Q statistic for statistical heterogeneity was 185.81 (p <0.001) and 1.08(p=0.782)for all-cause and CVD mortality,respectively(Fig.2).(Supplementary Fig.2).In the same manner,for every 1-MET increase of CRF, the length of follow-up was not associated with all-cause mortality(β=0.002;p=0.053)or CVD mortality(β=0.001,p=0.808)(Supplementary Fig.3).
With each study deleted from each model once, non-overlapping 95%CIs were observed across all deletions.
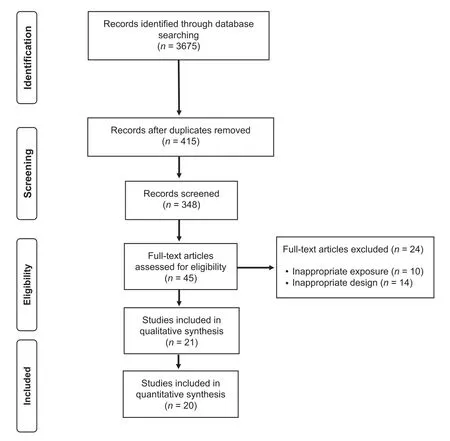
Fig 1. PRISMA flow diagram of literature search and study selection.PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Pooled HRs for the reduction in all-cause mortality risk per 1-MET increase were also statistically significant (HR=0.81;95%CI: 0.74-0.88,p <0.001, I2=80.8%),whereas for CVD mortality they were not (HR=0.75; 95%CI: 0.48-1.18,p=0.138,I2=89.8%)(Fig.3).Cochran’s Q statistic for statistical heterogeneity was 57.34 (p <0.001) and 29.32 (p <0.001)for all-cause and CVD mortality,respectively.
According to pathology, patients with coronary artery disease with high CRF had a lower risk of all-cause mortality(HR=0.32; 95%CI: 0.26-0.41, p < 0.001, Q=7.23,I2=30.9%)than did patients with low CRF.Also,higher CRF(per 1-MET increase) was associated with lower all-cause mortality risk among patients with coronary artery disease(HR=0.83; 95%CI: 0.76-0.91, p=0.003, Q=21.86,I2=77.1%) but not among patients with heart failure(HR=0.69; 95%CI: 0.36-1.32, p=0.134, Q=11.94,I2=83.3%).
3.6. Risk of bias across studies
Asymmetry suggestive of small-study effects was observed for all-cause mortality(LFK index=-5.07)and CVD mortality (LFK index=3.32), taking into account high vs. low CRF categories. These results were similar for every 1-MET increase in relation to mortality (all-cause, LFK index=1.50;CVD mortality,LFK index=-2.18)(Supplementary Fig.1).
3.7. Additional analysis
For high vs. low CRF, there were no significant effects on mortality according to length of follow-up(all-cause mortality,β=-0.001, p=0.665; CVD mortality, β=-0.001, p=0.729)
4. Discussion
The main finding of the present study is that adults diagnosed with CVD with high CRF have substantially reduced all-cause mortality (58%) and CVD mortality risk (73%) than do their least fit counterparts. According to the dose-response analyses, for each 1-MET increase in CRF there was a 19%reduction in risk for all-cause mortality but not CVD mortality.These results support the use of CRF measurements as a prognostic health indicator,which may help to identify individuals with CVD who are at high risk and then guide clinical decision-making to improve their CRF.
Cross-sectional population studies have suggested that higher CRF is associated with more favorable coronary or cardiovascular risk factor profiles.43,44This accords with a previous observation showing stronger associations between CRF and all-cause mortality than between CRF and self-reported physical activity in 42,373 men and women.45Diverse physiological mechanisms have been proposed to explain these associations. For instance, insulin sensitivity and high blood pressure are major determinants of CVD.It is well-established that CRF is associated with insulin sensitivity,46and a significant inverse association between CRF and incident hypertension in both men and women has also been recognized.47,48Likewise, CRF is central to the management of different cardiometabolic risk factors (i.e., glycemic control, lipid profiles, blood pressure).Improving these clustered cardiometabolic risk variables associated with increased risk of diabetes,CVD,and all-cause mortality49,50may partly explain the survival benefits of CRF in this population.
There is increasing evidence of an inverse relationship between CRF and all-cause and cardiovascular mortality in healthy people.51In our analysis,the survival benefit of a better CRF in patients with coronary artery disease equated to a 68% reduction in all-cause mortality risk when compared to their unfit counterparts and a 17% lower all-cause mortality risk for each 1-MET increase in CRF. Similar observations were noted in a longitudinal study of men with a median follow-up of 20 years,52which found that for each 1-MET increase in CRF level there was a 12% reduction in all-cause mortality. This was reaffirmed in a different retrospective longitudinal study with a follow-up of 9 years,53which found that a change in CRF from low to intermediate/high resulted in a significant reduction of death risk for black(45%) and white (59%) patients. A meta-analysis of observational studies by Kodama and colleagues51showed that for every 1-MET increase in CRF in healthy people there was 13% reduction in all-cause mortality and a 15% reduction in CVD mortality. This finding has important clinical implications, as patients with established coronary arterydisease have an increased risk of new cardiovascular events.54It reinforces the need for risk stratification in this population and confirms the great potential for patient health gain through the promotion of CRF.
TTagedEndable 1 General characteristics of the prospective cohort studies included.
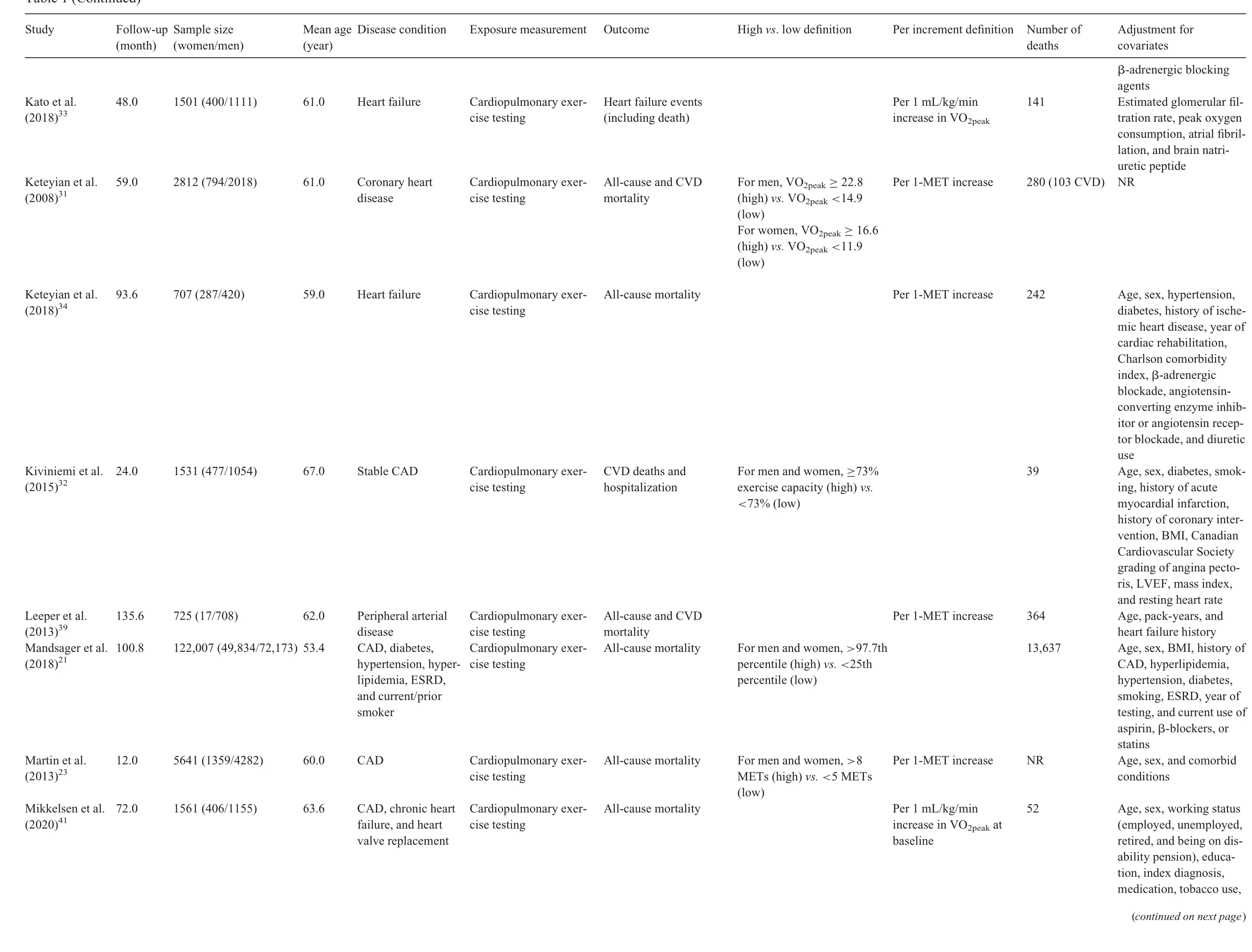
Table 1(Continued)
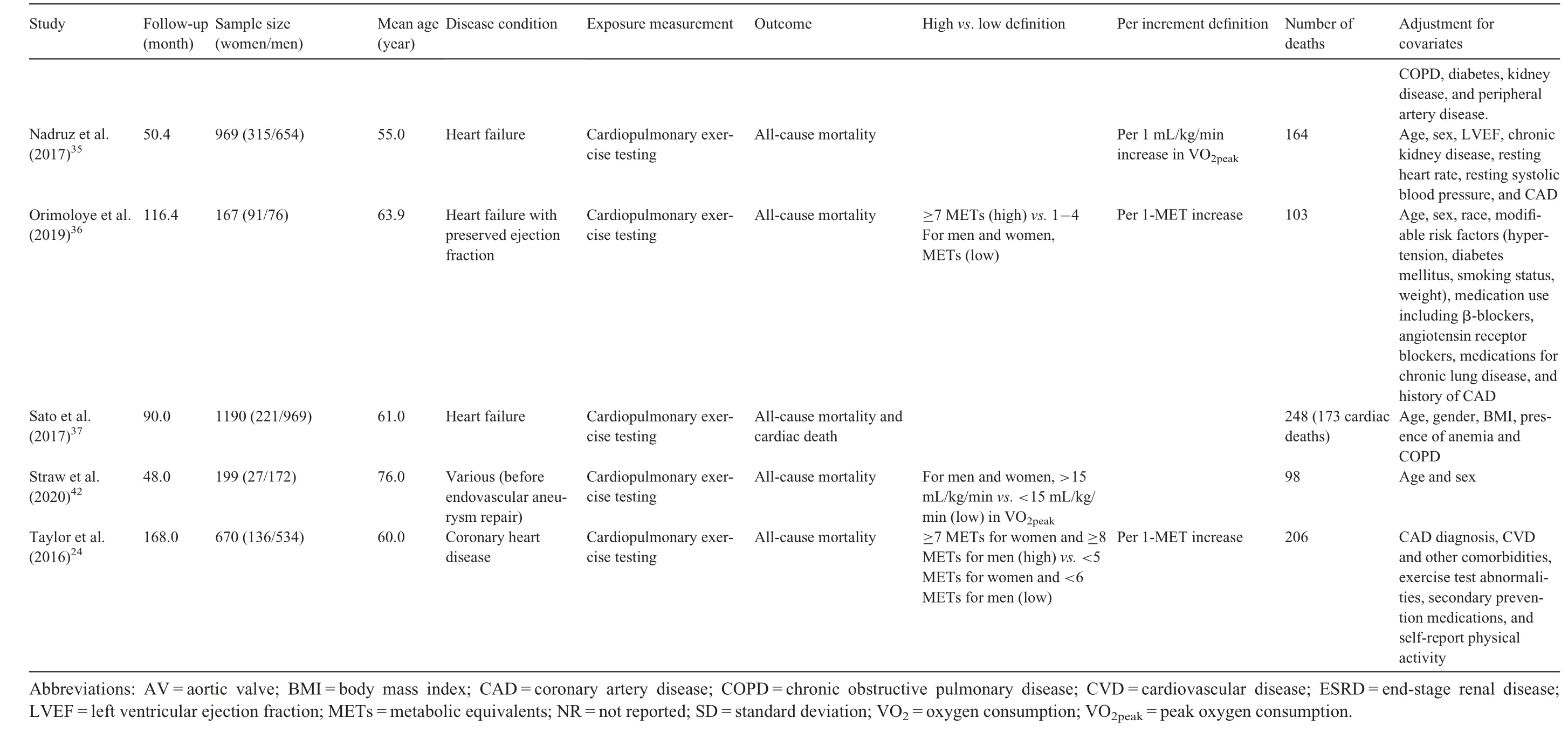
Table 1(Continued)
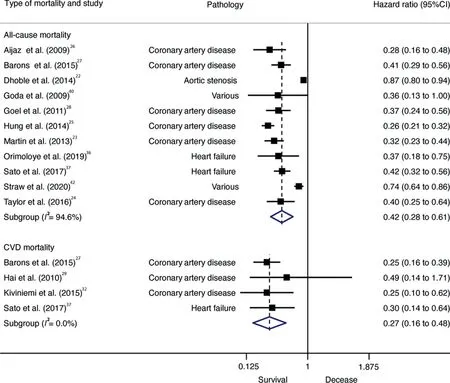
Fig 2. Forest plot showing the hazard ratios of all-cause mortality in patients with CVD,comparing high vs.low CRF.Weights are from random-effects model.95%CI=95%confidence interval;CRF=High cardiorespiratory fitness;CVD=cardiovascular disease.TagedEnd
Our meta-analysis revealed a positive association between higher CRF levels in patients with heart failure and a reduction in their mortality risk.This result could be explained in part by the small number of analyzed studies (n=3). Additionally,because heart failure is a common endpoint for various CVDs and typically appears in aged individuals, it could be argued that changes in CRF by themselves could confer the same survival benefit as they do in other forms of CVD.Similarly,other studies have reported positive findings. In a population-based follow-up study of 1873 men aged 42-61 years without heart failure or chronic respiratory disease,152 incident heart failure events were recorded after a mean follow-up of 20.4 years.The age-adjusted heart rate per unit increase (mL/kg/min) in CRF was 21%,which was minimally attenuated to 6%after further adjustment for established heart failure risk factors(body mass index,systolic blood pressure,history of CVD,diabetes,heart rate, and left ventricular hypertrophy).55Similarly, associations between low CRF and a substantially higher risk for heart failure hospitalization later in life have been found in healthy,middle-aged adults.8Other cohort studies have reported similar associations between physical activity, CRF, and incident heart failure.56,57
Interestingly, patients living with CVD experience marked reductions in their CRF. Abnormal endothelial function,impaired stroke volume response, ventilatory dysfunction,chronotropic incompetence, and abnormal peripheral oxygen utilization have been highlighted as potential contributors to exercise limitation.58,59Among the different strategies aimed to manage the deleterious effects of these disease-related changes (frequently in combination with age-related changes), increasing physical activity has shown to be one of the most effective approaches by far. This is mainly because of its ability to elicit protective benefits in the development of subclinical changes in heart structure and function in patients with CVD, including improvements in endothelial function,60,61left ventricular distensibility,62and diastolic function.63Regular physical activity also promotes biological adaptations in skeletal muscle through increased size and number of skeletal muscle mitochondria64and increased muscle capillary density,65which collectively may confer a positive synergistic effect on survival among patients with CVD. Nevertheless, individualized exercise should be prescribed by an experienced professional and should be based on several factors determined by a patient’s clinical history, exercise stress testing, or functional imaging and echocardiography.
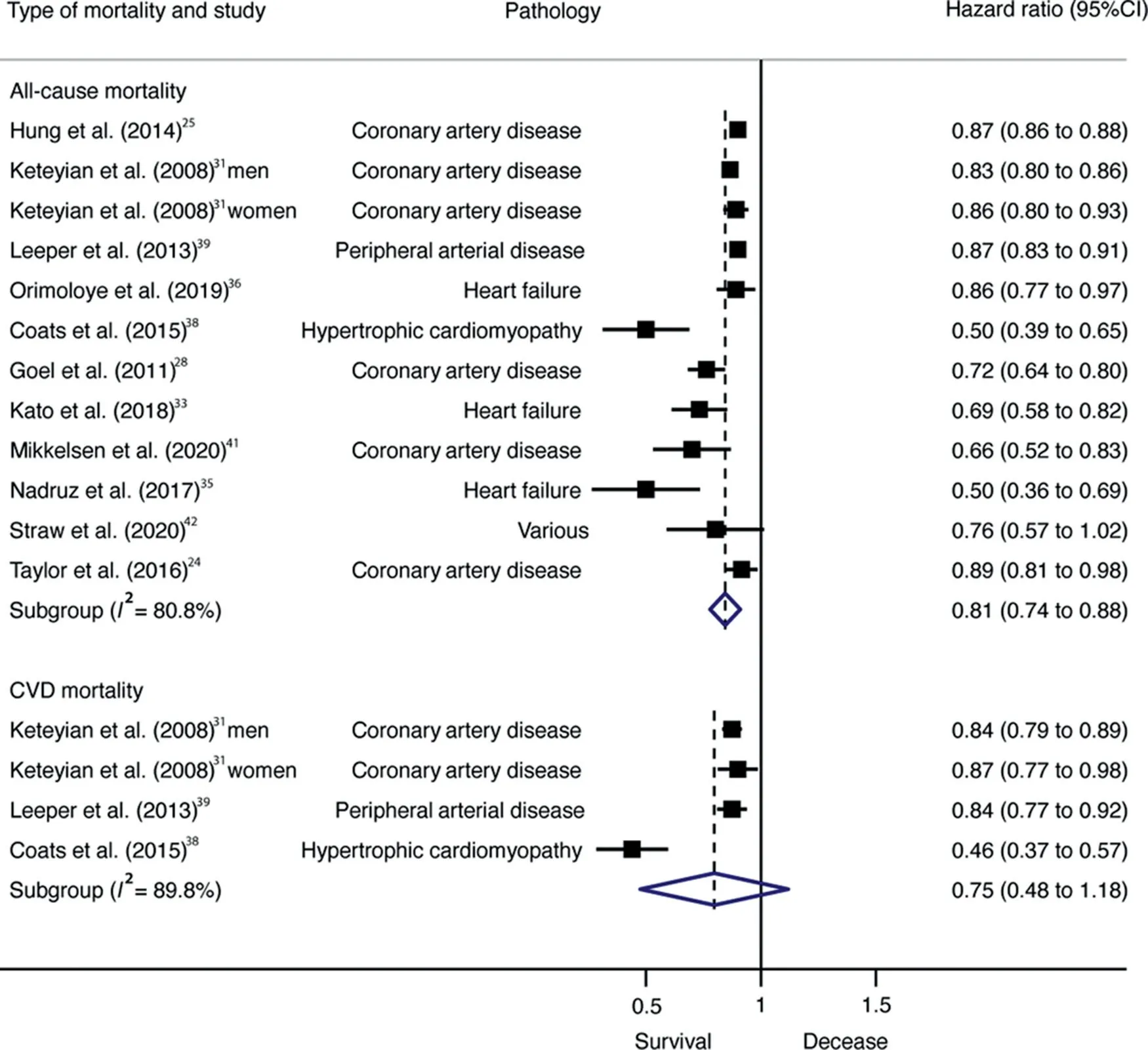
Fig 3. Forest plot showing the hazard ratios of all-cause mortality in patients with CVD per each 1-MET increase in CRF. 95%CI=95% confidence interval;CRF=High cardiorespiratory fitness;CVD=cardiovascular disease;MET=metabolic equivalent.TagedEnd
The generalizability of these results is subject to certain limitations. First, our analysis and interpretation were limited by the availability of studies conducted in adults diagnosed with CVD,which mainly included patients with coronary heart disease and heart failure.Therefore,we could not conclusively determine whether CRF confers a survival benefit in all patients with CVD. Second, most studies measured CRF at baseline with subsequent mortality follow-up and did not address changes in individual levels of fitness over time due to changes in lifestyle habits.Third,it is possible that misclassification bias may have affected our results(e.g.,in cases where we transformed the reported CRF data into MET units or where calls were made in the classification of low vs. high CRF patients, which varied from study to study based on prediction equations or standard cut-off points). Further studies should recognize and implement universal thresholds or cutoff points that distinguish low, moderate, and high CRF categories in patients with CVDs, thereby allowing comparison within studies and improving risk stratification. We believe that evaluating VO2maxas a percentage of age-, body mass index-,and sex-predicted VO2maxequations may infer the contribution of physical fitness in prognosis with more accuracy.66
Several studies in our analysis assessed CRF in patients undergoing an exercise-based cardiac rehabilitation program,23,24,26-31,34,41which may have provided additional health benefits in this population. However, whenever possible,we included data on CRF levels prior to cardiac rehabilitation programs, minimizing the potential influence of these interventions on our results. We therefore advocate caution when interpreting the present findings,as they may be affected by limitations present in the original articles. In the era of precision medicine, there is a need for more sophisticated tools,such as machine learning,modeling,and simulation solutions techniques,which could help to better predict the association between CRF and clinical outcomes.
5. Conclusion
Our findings support the relevance and use of CRF as a powerful and useful prognostic indicator for mortality in patients with CVD. More focus on physical activity in this population should be explicitly promoted with an aim to increase CRF.Further research is needed to determine whether exercise-based strategies according to risk category lead to improvements in risk of mortality; this could be achieved through the design and implementation of large-scale randomized controlled trials in different populations of patients diagnosed with CVD.This may help to determine how best to use physical activity recommendations and exercise therapy to reduce mortality risk in patients with CVD.
Data availability statement
The data that support the findings of this review are available upon reasonable request from the corresponding author(Antonio Garc´ıa-Hermoso).
Acknowledgments
AGH is a Miguel Servet Fellow at the Instituto de Salud Carlos III (CP18/0150). RRV is funded in part by a Postdoctoral Fellowship (Resolution ID 420/2019) from the Universidad P´ublica de Navarra.
Authors’contributions
AGH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, AGH is also responsible for the data analysis;AGH and YE contributed to the conception,methodology,formal analysis,investigation,wrote the first draft of the article and reviewed and edited it,MI and RRV supervised the study data collection,contributed to data analysis,and contributed to the article preparation;JN and JC reviewed and edited the article.All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.06.004.
 Journal of Sport and Health Science2021年6期
Journal of Sport and Health Science2021年6期
- Journal of Sport and Health Science的其它文章
- The association of grip strength with cardiovascular diseases and all-cause mortality in people with hypertension:Findings from the Prospective Urban Rural Epidemiology China Study
- The relationships between step count and all-cause mortality and cardiovascular events:A dose-response meta-analysis
- IGF1-PI3K-induced physiological cardiac hypertrophy:Implications for new heart failure therapies,biomarkers,and predicting cardiotoxicity
- The epigenetic landscape of exercise in cardiac health and disease
- Exploring the impact of COVID-19 on the movement behaviors of children and youth:A scoping review of evidence after the first year
- No independent associations between physical activity and clinical outcomes among hospitalized patients with moderate to severe COVID-19
