Exploring the anti-diabetic effects and the underlying mechanisms of ethyl acetate extract from Sophora flavescens by integrating network pharmacology and pharmacological evaluation
Yan Yang,Yi Liu,Yan-Ping Gao,Kai-Rui Zhao,Zhao-Cheng Li,Yun Luo,Lei Chen*
1Engineering & Technology Research Center for Chines Materia Medical Quality of Guangdong Province, School of Traditional Chinese Medicine, Guangdong Pharmaceutical University,Guangzhou 510006,China.2School of Chinese Medicine,Southern Medical University,Guangzhou 510515,China.
Abstract Background: Sophora flavescens, a traditional Chinese herb medicine, has been used to prevent and cure type 2 diabetes mellitus (T2DM) both in folk medicine and medical institutions.Modern pharmacological studies have also demonstrated that the flavonoids obtained from Sophora flavescens ethyl acetate extract (SFE) exhibited potential anti-diabetic activity.Our previous study elucidated that SFE exerts anti-T2DM effects by regulating the host-microbial metabolic axis.In the present study, we further explored the pharmacodynamic effect and the potential targets of the anti-T2DM activity of SFE by integrating network pharmacology and pharmacological evaluations. Methods: The diabetic rat model was created by streptozotocin and oral administration with SFE for 8 weeks.Then, the T2DM-related index was estimated to assess the interventional effect of SFE.Network pharmacology was applied to identify the likely targets and the pathways modulated by SFE components.Furthermore, Western blotting was applied to verify the prediction. Results: Pharmacological evaluation in vivo revealed that SFE could markedly improve the blood glucose, serum insulin secretion, insulin resistance, liver glycogen synthesis, and the liver tissue structure in T2DM rats.Through network pharmacology analysis, 101 active compounds of SFE and 114 targets belonging to 128 pathways were identified.The insulin, TNF, IL-6, and PI3K/Akt pathway may be the key targets and pathway.Based on the results of network pharmacology analysis, IRS/PI3K/Akt and IKK/NF-κB/TNF pathways were selected for further validation.Subsequently, experimental results of Western blotting confirmed that SFE may exert anti-T2DM effects by modulating the IRS/PI3K/Akt and IKK/NF-κB/TNF pathways.Conclusion: SFE may protect the T2DM rats by relieving the insulin resistance and inflammation through regulating the IRS/PI3K/Akt and IKK/NF-κB/TNF pathways.The results of the present study would improve the comprehension of the pharmacological basis of SFE against T2DM and provide a theoretical basis for the clinical use of Sophora flavescens.
Keywords: Sophora flavescens; insulin resistance; inflammation; network pharmacology;molecular mechanism
Background
Diabetes mellitus is a complex chronic metabolic disease that is characterized by persistent hyperglycaemia and has a high prevalence worldwide, with type 2 diabetes mellitus (T2DM) accounting for at least 90% of all cases [1, 2].Insulin resistance (IR) and inflammation are the major pathogenesis of T2DM [3, 4].Several studies reported that T2DM that is continuously accompanied by a chronic inflammatory environment can aggravate the development of IR[5-7].Inflammatory factors such as tumour necrosis factor (TNF)-α,interleukin-6 (IL-6), interferon-γ, and interleukin-1 can inhibit the signal transduction of insulin and thus induce the IR of T2DM.In addition, inflammatory factors in the major metabolic tissues (such as liver, muscle, and adipose tissues) can induce the dysfunction of these tissues and exacerbate the symptoms of T2DM, resulting in a vicious cycle [4].Therefore, the improvement of insulin sensitivity and chronic inflammation is considered an important therapeutic method for T2DM.
Sophora flavescens, also named “Kushen” (Figure 1), has been first listed in the ancient book ofShennong’s Classic of Materia Medica(written by Shennong in Han Dynasty, 25 C.E.-220 C.E.).As one of traditional Chinese medicines,Sophora flavescenshas wide application in the treatment of dysentery, haematochezia, leukorrhea, eczema,jaundice, scabies, and skin itching [8, 9].In 1998 and 1999, the researches of Huang and Shi showed that Kushen could reduce the blood glucose level of T2DM rats and relieve diabetic cataract, and also been used to treat T2DM in folk medicine in China [10, 11].Moreover, modern pharmacological studies have revealed that flavonoids derived fromSophora flavescensexhibit obvious antidiabetic activities [12-17].Recently, Yang et al.reported that the flavonoids obtained fromSophora flavescensethyl acetate extract (SFE)inhibited the development of T2DM in KK-Ay mice [18]; however, the study did not investigate the treatment mechanism.Our previous study elucidated the anti-T2DM therapeutic effect and the mechanism of SFE on the host-microbial metabolic axis based on the relationship between metabolic biomarkers and gut bacteria [19], but the main targets and pathways related to this mechanism are not clear and need to be further explored.
Figure 1 The whole plant of Sophora flavescens and its medicinal root
Network pharmacology is a systematic and holistic research method that combines systems biology, multi-directional pharmacology, and bioinformatics [20, 21].This approach can explicate the underlying complex mechanisms of drugs at the molecular level through a network based on public databases [22, 23].Therefore,multi-component and multi-target interaction information can be predicted by network pharmacological analysis, with obvious advantages and wide applications in traditional Chinese medicine[24-26].
In the present study, we explored whether SFE can improve IR and inflammation and contribute towards restraining the development of T2DM.For this purpose, network pharmacology was applied to predict the T2DM-related targets and western blotting was performed to verify this prediction.
Materials and methods
Drugs and reagents
Streptozotocin (Lot number: S110910) was purchased from Sigma Aldrich (St.Louis, Missouri, USA).RIPA lysis buffer (Lot number:BB-3201-1) was supplied by BestBio Science (Nanjing, China).The bicinchoninic acid protein concentration determination kit (Lot number: P0010S) was procured from Beyotime Biotechnology(Shanghai, China).The Pierce™ECL Western blotting substrate (Lot number: 32209) was obtained from Thermo Fisher Technology Ltd.(Shanghai, China).The primary and horseradish peroxidase-tagged secondary antibodies were supplied by the Affinity Biosciences(Cincinnati, Ohio, USA).The immobilon polyvinylidene difluoride membrane (Lot number: ISEQ00010) was bought from Merck Millipore (Darmstadt, Hesse,Germany).
Insulin receptor substrate-1 (IRS-1) antibody (Lot number: AF6273),phosphatidylinositol 3-kinase (PI3K) antibody (Lot number: AF6241),phospho-phosphatidylinositol 3-kinase(p-PI3K) antibody(Lot number:AF3241), phospho-protein kinase (p-AKT) antibody (Lot number:AF0016), glucose transporter type 4 (GLUT4) antibody (Lot number:AF5386), nuclear transcription factor-kappa B (NF-κB) antibody (Lot number: AF5006), phospho-nuclear transcription factor-kappa B(p-NF-κB) antibody (Lot number: AF2006), phospho-I-kappa-B-kinase alpha (p-IKK-α) antibody (Lot number: AF3013),phospho-I-kappa-B-kinase beta (p-IKK-β) antibody (Lot number:AF3010), I-kappa-B (IκB) antibody (Lot number: AF5002),phospho-I-kappa-B (p-IκB) antibody (Lot number: AF2002), TNF antibody (Lot number: AF7014), IL-6 antibody (Lot number: DF6087)and beta-actin (β-ACTIN) antibody (Lot number: AF7018) were supplied by Affinity Biosciences (Cincinnati, Ohio, USA).Protein kinase (AKT) antibody (Lot number: AF1789) was produced by Beyotime Biotechnology (Shanghai, China).Goat Anti-Rabbit IgG(H+L) horseradish peroxidase antibody (Lot number: S0001) was obtained from Affinity Biosciences (Cincinnati, Ohio, USA).
Preparation of SFE
Slices of drySophora flavescensroot were obtained from The Good Agricultural Practice Planting Base in Changzhi (Shanxi, China) and authenticated by Dr.Lei Chen at the Guangdong Pharmaceutical University of Chinese Medicine College (Guangdong, China).The voucher specimen (No.20181352) has been deposited at the College of Traditional Chinese Materia Medical, Guangdong Pharmaceutical University, China.
The sample preparation method was referred from our previous study [27].Briefly, slices of “Kushen” were extracted thrice for 2 hours through reflux extraction with 90% ethanol.The extracts were evaporated, and the residue was dissolved in water (1: 8, v/v) and then filtered.The aqueous solution was further extracted four times with ethyl acetate (1: 1, v/v).After removal of the ethyl acetate portion by using a rotary evaporator, lyophilisation was performed to obtain the SFE.The yield of extracts was 4%.The total flavonoids in the SFE were quantified through ultraviolet spectrophotometry.In brief, a batch of SFE with 3 samples was weighted, and the contents were determined according to the steps detailed in the Supplementary Materials and Methods.The quantification of total flavonoid compounds in SFE.According to our previous research [27],UHPLC-MS was performed to detect the flavonoid compounds in the SFE [19].The conditions of UHPLC-MS are listed in the Supplementary Materials and Methods.
Animals
Male Sprague-Da wley rats aged 8-10 weeks were supplied by the Laboratory Animal Centre of the Southern Medical University(Guangzhou, China; quality certificate number: SYXK2012-0125).All the animals were raised and tested under a controlled environment at 22 ± 2 °C room temperature and 55% ± 5% humidity and maintained under a 12-h light/dark cycle.All the rats were provided a normal diet and free access to water.All experimental procedures were approved by the Animal Ethics Committee of the Guangdong Pharmaceutical University (No.GDPU2016067, 01/01/2017).
Animal rearing and experimental scheme
After a week of acclimation, all the rats, except thecontrol group rats,were fed with a high-fat diet(15% sucrose,70% normal chow diet,9%lard, 5% yolk powder, and 1% cholesterol) for 4 consecutive weeks.Next, the rats that were fed a high-fat diet were intraperitoneally injected with 35 mg/kg streptozotocin to induce T2DM, and an equivalent dose of saline was provided to the control group.The rats induced by streptozotocin, with fasting blood glucose (FBG) levels >16.7, were considered to be diabetic.The T2DM rats were randomly assigned to the following 4 groups: high-dose SFE group (75 mg/kg/day), low-dose SFE group (37.5 mg/kg/day), metformin group(22.5 mg/kg/day), and the model group.The daily intake of Sophora flavescens root slices for adults recommended by the Chinese Pharmacopoeia is 4.5-9.0 g.The extraction rate of SFE was 9.2% and the conversion rate of rat/human (the dose of rat/ the dose of human)was 6.3.Therefore, 37.5 mg and 75 mg were selected for our study.During the experiment, the body weight of the animals was tested every 3 days, and the FBG was tested every week after an 8 h of fasting.
After 8 weeks of treatment, all the animals were starved overnight.The blood samples of the rats were collected from the abdominal aorta after anesthetizing them with chloral hydrate.The liver tissues were dissected out and rinsed with cold saline.One part of the tissues was stored at −80 °C and another was immersed in 4% paraformaldehyde for further histopathologic assay.
Serum insulin, liver glycogen,and histopathologic assays
A commercially available ELISA kit (purchased from Jiancheng Biotechnology, Nanjing, China) was used to determine the fasting insulin (FINS) level in the serum, referring to the detailed steps in Supplementary Materials and Methods.The homeostatic model assessment for IR (HOMA-IR) was calculated according to the following formula (1) [28].
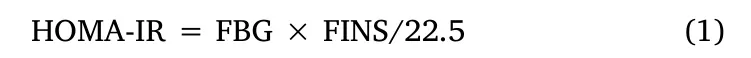
The liver glycogen level was determined through the colorimetric assay, as per the manufacturer’s instructions (Jiancheng Biotechnology), by using the anthracenone method [29].The detailed steps were shown in Supplementary Materials and Methods.
The liver tissues were immersed in 4% paraformaldehyde for 48 h and then dehydrated by ethanol, embedded in paraffin,and sliced into 3-μm thickness sections.Finally, the sections were stained with haematoxylin and eosin and observed under a light microscope to observe pathological changes in the liver.
Network pharmacological analysis
Collection of targets for SFE and T2DM.According to the laboratory prophase accumulation and published articles, 126 flavonoid compounds of SFE were obtained.These compounds were imported into the SwissADME database (http://www.swissadme.ch/index.php)to acquire the parameters of Lipinski’s rule, which was then assigned as the criteria to evaluate the drug-likeness and bioavailability of the chemical compounds [30, 31].The compounds that met with the standard of Lipinski’s rule were employed for further analysis.The SFE-related targets were obtained from the PharmMapper database(http://www.lilab-ecust.cn/pharmmapper/), with a Z score > 1;BATMAN database (http://bionet.ncpsb.org/batman-tcm/), with a score > 20; Swiss Target Prediction database(http://www.swisstargetprediction.ch/), with a probability > 0.5;and all targets in TCMSP (http://lsp.nwu.edu.cn/tcmsp.php).The T2DM-related targets were collected from Drugbank(https://www.drugbank.ca/), OMIM (https://omim.org/), DisGeNET(http://www.disgenet.org/), and Therapeutic Target Database(http://bidd.nus.edu.sg/group/ cjttd/) database.All target names were standardised by limiting the species to “Homo sapiens” in the UniProt database(https://www.uniprot.org/).
Functional enrichment analysis.Gene ontology analysis was implemented to explain the biological processes of the common targets of SFE and T2DM that includes the cellular component,molecular function, and biological process.These common targets were imported into the DAVID Bioinformatics website(https://david.ncifcrf.gov/) for functional enrichment.The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway data were obtained from the STRING database (https://string-db.org/).The KEGG pathways withP< 0.01 were considered as the key pathways for the anti-T2DM effect of SFE, and they were depicted in a bubble diagram by using R software 3.6.0.
Network construction and analysis.The STRING database helps predict the information about protein-protein interaction (PPI), of which the data have been experimentally proven [32].The common targets of SFE and T2DM were placed into the database to obtain their interaction information and the combined score.The PPI and compound-target-pathway networks were constructed using Cytoscape 3.7.2.In the generated networks, their interaction data were obtained with reference to the network topological analysis by using Cytoscape 3.7.2, which included closeness centrality, between centrality, degree, and average shortest path length.
Western blotting
The liver tissues (100 mg) were homogenised in cold RIPA buffer(BestBio, Shanghai, China) supplemented with phosphatase inhibitor and protease inhibitor cocktail, and then homogenized at 4 °C,followed by centrifugation at 12,000 rpm for 15 min and collection of the supernatant.The total protein content was measured by using the BCA protein assay kit.The prepared sample was separated by 8%sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro transferred onto the polyvinylidene difluoride membrane.Then, the membranes were incubated with primary antibody (1:1000)at 4 °C overnight after blocking with 5% non-fat milk for 1 h.After that, the membranes were incubated with a second antibody (1:10000; horseradish peroxidase-conjugated affinipure) for 2 hours.Finally, an enhanced ECL chemiluminescence detection kit was implemented to visualise the protein bands.
Data analysis
Graphpad Prism 7.0 was used for statistical analyses.All data are expressed as mean ± standard deviation (SD).One-way analysis of variance was performed to assess differences between the groups.AP-value of < 0.05 was considered statistically significant.
Results
Flavonoid compounds of SFE
The total flavonoid compounds in SFE were quantified by ultraviolet spectrophotometry in three parallel measurements.The average content of flavonoids was 61.28% and that of relative standard deviation was 2.45%.In addition, 27 flavonoid compounds in SFE were unambiguously identified by comparing their retention time and fragment ions in the MS2 spectra relative to the reference standards.The total ion current chromatogram and the structures of the 27 flavonoid compounds in SFE are presented in Supplementary Figure 1&2 and Supplementary Table 1.These 27 components represent the most important therapeutic contents belonging to the flavonoid of SFE and possess good drug-likeness and bioavailability indicators, which can be considered as the core potential active compounds.
Effects of SFE on the body weight and the relative liver weight in T2DM rats
During the experiment, the model group rats demonstrated gradual emaciation compared to the control rats (P< 0.001).The weights of the treated T2DM rats showed a slowly increasing trend, while those of the control group showed a continued increase (Table 1).These results indicated that high-dose SFE could increase the weights of T2DM rats.However, the results of the low-dose group were not statistically significantly different.In addition, the model group exhibited a higher relative liver weight ((liver weight/body weight)%)than the control group, and SFE was significantly decreased, such that the high-dose group showed a more significant reduction than the low-dose group (Table 1).These results suggest that SFE can protect rats from T2DM by acting directly on the liver.

Table 1 Effects of SFE treatment on the body weight and relative liver weight of the rats(n = 6)
Effects of SFE on FBG and FINS in T2DM rats
After 8 weeks of treatment, the FBG and FINS of each rat were measured.As shown in Figure 2A, when compared with the model group, the FBG levels of the high-dose group rats showed an obvious decrease, although it was still higher than those of the control group rats.These results suggest that SFE exerts a certain inhibitory effect on the high FBG levels in T2DM rats.
The FINS level was significantly decreased in the model group compared with that in the control group.The oral administration of high-dose SFE for 8 weeks in T2DM rats could significantly increase the FINS level (Figure 2B).Consistently, treatment with SFE could partially decrease HOMA-IR when compared with the untreated T2DM rats (Figure 2C).Based on these results, we speculate that SFE may treat T2DM by stimulating insulin secretion and improving IR.
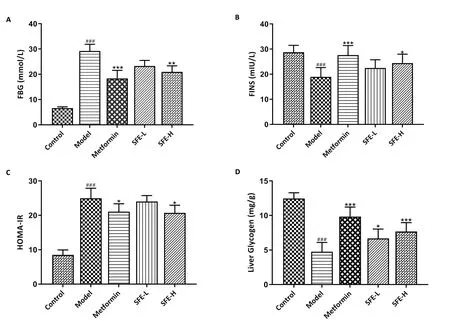
Figure 2 The effects of SFE on FBG, FINS, HOMA-IR, and liver glycogen (n = 6) in rats.A, FBG level; B, FINS level; C, homeostatic model assessment for IR; D, liver glycogen.*P < 0.05,**P < 0.01,***P < 0.001 vs.model group;##P < 0.01 vs.control group.SFE-L, Sophora flavescens ethyl acetate extract low-dose;SFE-H,Sophora flavescens ethyl acetate extract high-dose;FBG,fasting blood glucose;FINS,fasting insulin;HOMA-IR,homeostatic model assessment for insulin resistance.
Effects of SFE on liver glycogen synthesis
As is already well-known, the liver plays a vital role in glucose homeostasis.Insulin decreases the blood glucose level mainly by converting the surplus glucose into glycogen and storing it in the liver.Therefore, glycogen synthesis in the liver is closely related to insulin secretion and IR [33, 34].As shown in Figure 2D, compared with the liver glycogen synthesis in the control rats, that in the T2DM rats was reduced significantly (P< 0.001).The treatment of T2DM rats with SFE could significantly improve the liver glycogen level (P< 0.001).Thus, the high-dose SFE was found to be more effective than the low-dose SFE.
Effects of SFE on the pathology of the liver in T2DM rats
As shown in Figure 3, some obvious liver tissue structure alterations were observed in the T2DM rats.When compared with that in the control group, the structure of the hepatic lobule cells was disorganised, the cells were swollen and deformed, and the lipid accumulation was obvious; moreover, the large and small fat vacuoles occupied much of the hepatocyte cytoplasm in the T2DM rats.After oral administration with SFE for 8 weeks, the alterations were significantly alleviated.The abnormal liver structure was obviously relieved,and the lipid droplet accumulation was significantly reduced.Moreover, the improvement in the high-dose group was found to be significantly better than that in the low-dose group.
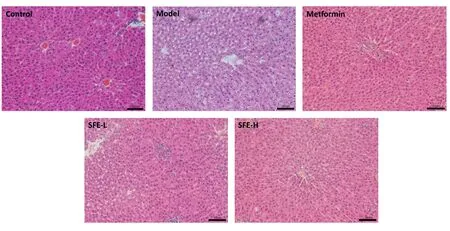
Figure 3 Effects of SFE on histomorphological changes in the liver (scale bar represented 50 µm).SFE-L, Sophora flavescens ethyl acetate extract low-dose; SFE-H, Sophora flavescens ethyl acetate extract high-dose.
Results of the network pharmacology analysis
Intersection of SFE targets with T2DM-related genes.Based on the bioinformatics analysis, 101 flavonoids of SFE passed the five key characteristics (molecular weight < 500, Hydrogen-bond donor < 5,Hydrogen-bond acceptor < 10, MLogP < 5, and Rotatable bonds <10).Meanwhile, 27 of the 101 compounds were identified by UHPLC-MS and the reference standards, which were considered as the key potential active ingredient (Supplementary Figure 1&2 and Supplementary Table 1).Screening the Swiss Target Prediction,BATMAN, PharmMapper, and TCMSP databases yielded 1,110 targets corresponding to 101 active ingredients in SFE.From the Therapeutic Target Database, OMIM, DisGeNET, and Drugbank databases, 364 T2DM-related targets were obtained.Among these, 114 targets were shared in SFE and T2DM.
Functional enrichment.Gene ontology analysis describes the function of 114 shared targets from three aspects: biological process,cellular component, and molecular function.The biological process mainly involves the positive regulation of transcription from transcription, DNA templated, RNA polymerase II promoter, negative regulation of immune cytokine secretion, angiogenesis, and oxidation-reduction process (Figure 4A, Supplementary Table 2).The cellular component is related to the extracellular space, blood microparticle, extracellular region, receptor complex, and apical plasma membrane (Figure 4B, Supplementary Table 3).The molecular function mainly include drug binding, steroid hormone receptor activity, heme binding, growth factor activity, cytokine activity,sequence-specific DNA binding, and RNA polymerase II transcription factor activity (Figure 4C, Supplementary Table 4).These data suggest that SFE may regulate and control multiple physiological processes to treat T2DM.
The interaction of target proteins and KEGG enrichment analysis.Through the network topological analysis of the 114 shared targets of SFE and T2DM, 50 major targets were selected to construct the PPI network by using the STRING database and Cytoscape 3.7.2 (Figure 5A).The targets insulin, TNF,and IL-6 showed a high degree and close connection with the shared genes.They were closely related to the processes of IR and inflammation, which may be the key targets for the treatment of T2DM.
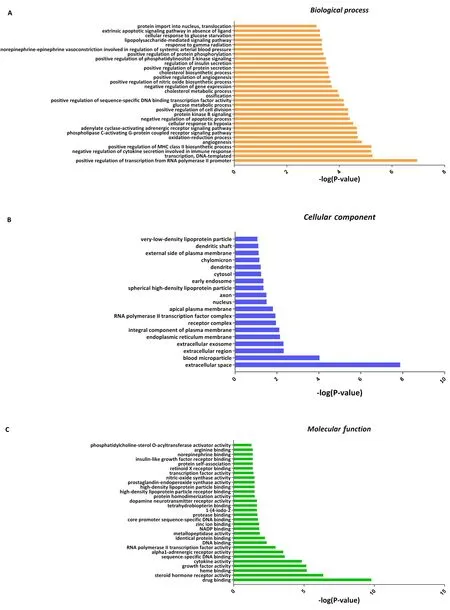
Figure 4 Gene ontology enrichment analysis.A, biological process; B, cellular component;C,molecular function.
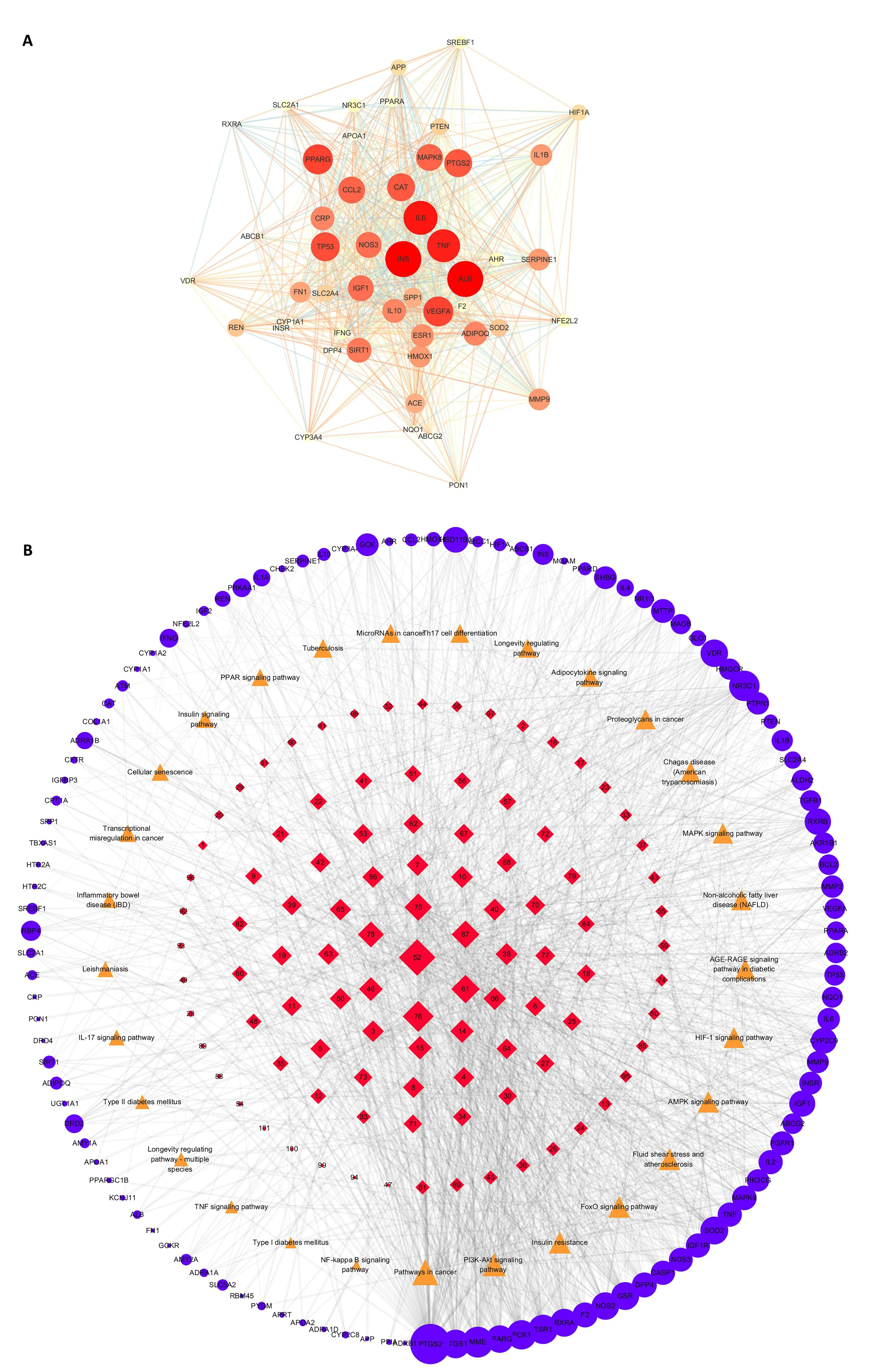
Figure 5 Protein-protein interaction network of the major targets(A) and“compound-target-pathway”network(B).A,the size and color of the nodes represent the value of degree, the larger the nodes, the greater the degree and the darker the color, the greater the degree; B, red diamonds represent components, orange triangles represent pathways, and blue circles represent targets, the size of nodes represents the value of degree, the larger the nodes, the greater the degree.
In the KEGG analysis (Table 2, Figure 6), a total of 159 pathways were obtained, of which 128 pathways were statistically significant (P< 0.01).According to the enriched score of the pathways, the following functional clusters stood out: for the PI3K/AKT pathway,the degree value was 20;for IR,the degree value was 17;for FoxO,the degree value was 16; and for AMPK signaling pathway, the degree value was 15, which are all strong indicators.The analyses indicate that the PI3K/AKT signaling pathway may be the key pathway of SFE for improving IR.Subsequently, we applied Cytoscape 3.7.2 to construct the “compound-target-pathway” network.The results in Figure 5B indicate that SFE may play a synthetical role in the treatment of T2DM by influencing multiple targets and pathways.Among them, the PI3K/Akt signaling pathway may play a key role in the anti-T2DM activity of SFE.
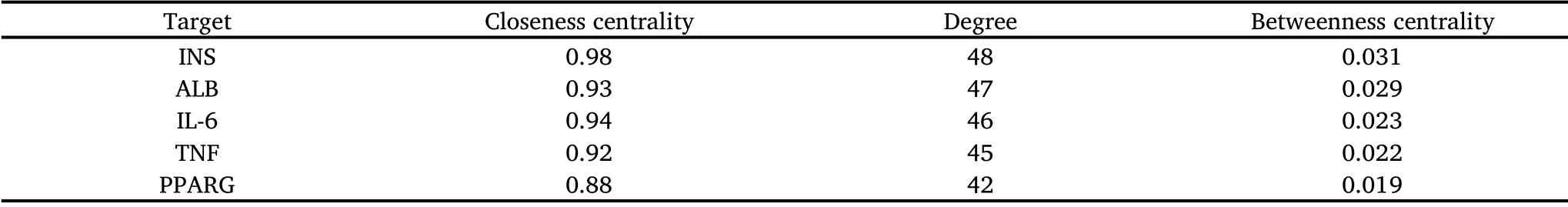
Table 2 Network topological analysis of the potential targets by Cytoscape
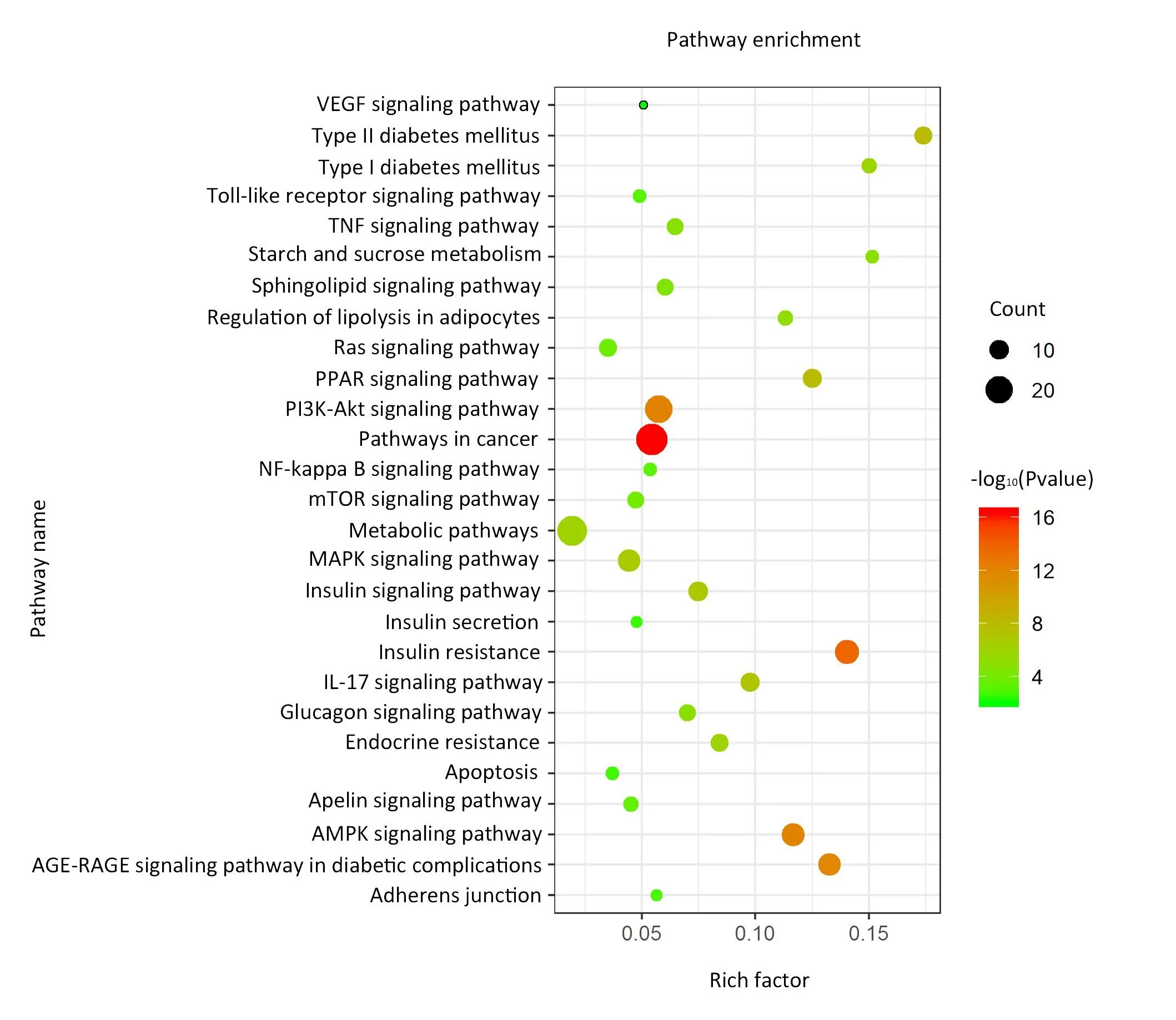
Figure 6 Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis.The vertical axis represents the pathway name, the horizontal axis represents the rich factor.“Count” is the number of screened targets attributed to the pathway.The larger the Count value, the more enriched targets the pathway contains.
The KEGG pathway and PPI network analyses indicated that the PI3K/Akt signaling pathway and inflammation cytokines (such as TNF and IL-6) were the key mechanisms of the anti-T2DM activity of SFE.The PI3K/AKT pathway is a major insulin transduction pathway,where insulin plays the function of regulating the blood glucose level,mainly through the PI3K/Akt signaling pathway [35, 36].The IKK/NF-κB/TNF pathway is a classic inflammatory signaling pathway[37].Inflammatory cytokines TNF-α and IL-6 may contribute to IR through I-kappa-B-kinase (IKK)-mediated serine phosphorylation of IRS-1, which reduces tyrosine phosphorylation of IRS-1 and inhibits the activation of PI3K/Akt and insulin action [38].Consequently, the IRS/PI3K/AKT and IKK/NF-κB/TNF pathways were selected for further validation.
SFE regulates IR and inflammation in the liver
To confirm the network pharmacology prediction, western blotting was applied to determine the protein expression level.The results obtained are presented in Figure 7.The expression levels of IRS-1,p-PI3K, PI3K, p-PI3K/PI3K, p-AKT, AKT, p-AKT/AKT, and GLUT4 protein in the model group were found to be significantly lower than those in the control group.Treatment with SFE for 8 weeks increased the expression levels of IRS-1, PI3K, p-PI3K, Akt, p-Akt, and GLUT4 protein compared with those in the model group, and the efficacy of the high-dose SFE was found to be more obvious.
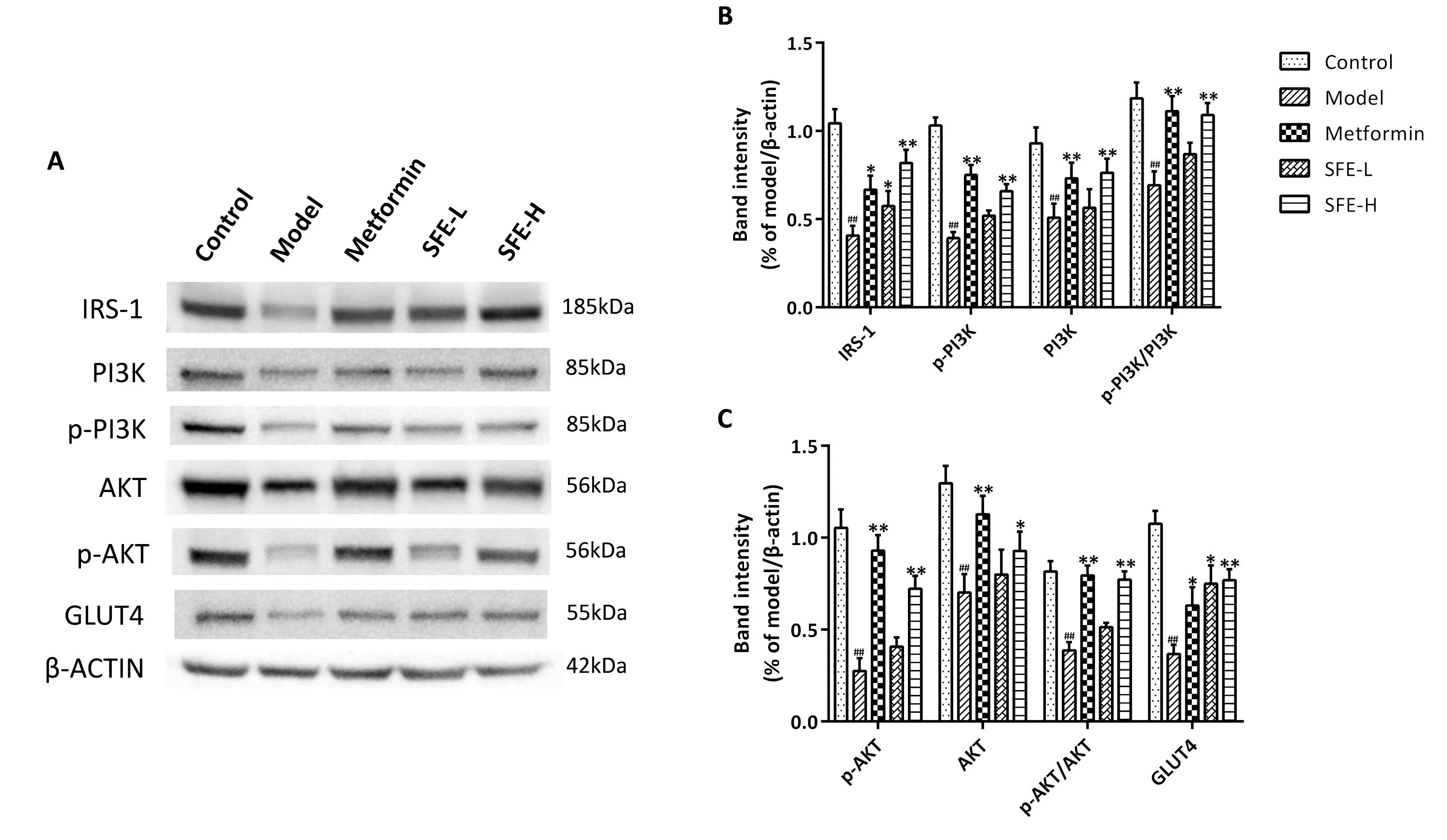
Figure 7 Western blot analysis of the proteins.(A) Representative bands of IRS-1, p-PI3K, PI3K, p-AKT, AKT and GLUT4 were shown.Quantitative presentation of expression of (B) IRS-1, p-PI3K, PI3K, p-PI3K/PI3K, and (C) p-AKT, AKT, p-AKT/AKT, GLUT4.Data was shown as mean ± standard deviation (n = 3).*P < 0.05,**P < 0.01, vs.model group;##P < 0.01 vs.control group.IRS-1, insulin receptor substrate-1;p-PI3K, phospho-phosphatidylinositol 3-kinase; PI3K, phosphatidylinositol 3-kinase; p-AKT, phospho-protein kinase; AKT, protein kinase; GLUT4,glucose transporter type 4; β-ACTIN, beta-actin.
For the IKK/NF-κB/TNF pathway, the p-NF-κB, p-NF-κB/NF-κB,p-IK-α, p-IKK-β, p-IκB, p-IκB/IκB, TNF, and IL-6 levels were significantly increased in the model group compared with those in the control group.SFE treatment significantly reduced the expressions of p-NF-κB,p-IKK-α, p-IKK-β, p-IκB,TNF, and IL-6 in the liver compared with those in the model group; thus, the therapeutic effect of the high-dose group was better than that of the low-dose group(Figure 8).
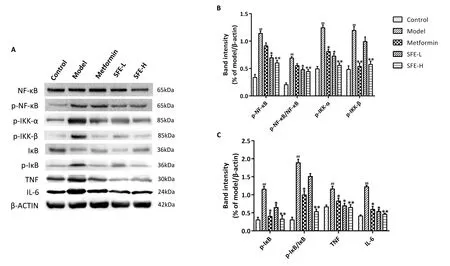
Figure 8 Western blot analysis of the proteins.(A) Representative bands of NF-κB, p-NF-κB, p-IKK-α, p-IKK-β, IκB, p-IκB, TNF and IL-6 were shown.Quantitative presentation of expression of (B) p-NF-κB, p-NF-κB/NF-κB, p-IKK-α, p-IKK-β, and (C) p-IκB, p-IκB/IκB, TNF, IL-6.Data was shown as mean ± standard deviation (n = 3).*P < 0.05,**P < 0.01, vs.model group;##P < 0.01 vs.control group.NF-κB, nuclear transcription factor-kappa B; p-NF-κB, phospho-nuclear transcription factor-kappa B; p-IKK-α, phospho-I-kappa-B-kinase alpha; p-IKK-β,phospho-I-kappa-B-kinase beta; IκB, I-kappa-B;p-IκB, phospho-I-kappa-B; TNF, tumour necrosis factor; IL-6, interleukin-6; β-ACTIN, beta-actin.
Discussion
In this study, we successfully established a T2DM rat model, and the T2DM rats were characterized by an increase in the FBG and liver index and a decrease in the body weight and serum insulin and liver glycogen content.Moreover, lipid accumulation was obvious in the liver, and the structure of the hepatic lobule cells was disorganised.As expected, SFE treatment could significantly increase the body weight,FINS, and liver glycogen level, reduce the FBG and the relative liver weight, with improvement in the liver morphological damage and lipid accumulation, thereby alleviating the symptoms of T2DM rats.
However, the underlying molecular mechanisms of SFE treating T2DM remain unclear.Therefore, a systematic bioinformatics method was applied in this study to predict the sophisticated mechanisms of SFE.As a result, 101 flavonoid compounds of SFE that met the standard of Lipinski’s rule were used for predicting the potential targets for the anti-T2DM effect of SFE.Meanwhile, 27 of the 101 flavonoid compounds representing the most important therapeutic contents and with good drug-likeness and bioavailability indicators in SFE were unambiguously identified by comparing the retention time and fragment ions in the MS2 spectra with the reference standards.Based on the results of network pharmacology prediction, 101 flavonoid compounds of SFE may regulate 114 targets and 128 pathways that were shared with T2DM.The targets insulin, TNF, and IL-6 located at the central position were primarily enriched by PPI analysis.They were found to be closely related to inflammation and IR of T2DM.In the KEGG pathway analysis, the pathways with a high degree and low p-value were significantly enriched, including PI3K/AKT, IR, FoxO, and AMPK signaling pathways, which are all related to the glucose and lipid metabolism and IR.These results strongly support the use of SFE for improving IR and inflammation of T2DM.The PI3K/AKT pathway and insulin, TNF, and IL-6 may be the key pathway and targets of the anti-T2DM activity of SFE.Therefore,we selected the IRS/PI3K/AKT and IKK/NF-κB/TNF pathways in the present study.
The PI3K/Akt pathway is a central part of insulin metabolism,where insulin plays the function of regulating the blood glucose levels,mainly through the PI3K/Akt signaling pathway [35, 36].Activated insulin receptor induces the phosphorylation of IRS-1/2 and then activates PI3K; the activated PI3K can then catalyse the production of Akt through second messengers, phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-(3,4,5)-trisphosphate.Activated Akt exerts metabolic biological effects by regulating the downstream molecules GLUT4, thereby regulating the blood glucose level [35, 39].
Previous studies have indicated that chronic inflammation can enhance IR [40, 41].NF-κB is the key factor involved in the inflammatory signaling pathways,as well as plays an important role in the activation and regulation of inflammatory factors.IκB is an inhibitor of NF-κB, and the dissociation of IκB can promote the phosphorylation of NF-κB.Activated NF-κB can enhance the production of inflammatory cytokines such as IL-6 and TNF [37].These inflammatory cytokines may contribute to IR through IKK-mediating serine phosphorylation of IRS-1, which in turn reduces tyrosine phosphorylation of IRS-1, thereby restraining the downstream signaling and insulin action and generating a vicious cycle [38].According to the results of bioinformatics analysis and the connection between these two pathways, the IRS/PI3K/AKT and IKK/NF-κB/TNF pathways were selected for further validation.Consistent with our hypothesis, the results of western blotting revealed that SFE could treat T2DM rats by upregulating the expression of the IRS-1/PI3K/AKT signaling pathways and downregulating the expression of the IKK/NF-κB/TNF signaling pathways in the liver.
In our recent study, we elucidated the influence of SFE on the gut microbiota and host metabolism in the treatment of T2DM.We found that SFE may regulate the amino acid, glucose, and lipid metabolism by modulating the gut microbiota of T2DM rats and thereby ameliorate the IR and metabolic disorders of T2DM [19].In this study,we revealed that SFE alleviates the IR and inflammation of T2DM rats mainly through the regulation of the IRS1/PI3K/AKT and IKK/NF-κB/TNF signaling pathways.In conclusion, SFE may play a comprehensive role in the development of T2DM by influencing multiple metabolic pathways, including the host-microbial metabolic axis,PI3K/AKT, and inflammatory pathways.
Conclusion
Our study suggests that SFE could ameliorate the symptoms of T2DM by relieving the IR and inflammation through the regulation of the IRS/PI3K/AKT and IKK/NF-κB/TNF pathways.This study thereby provides a theoretical basis for the clinical application ofSophora flavescens.Meanwhile, our research indicated that the combination of bioinformatics and molecular biology can provide a strategy for the exploration of the internal mechanism of traditional Chinese medicine.
 Traditional Medicine Research2022年1期
Traditional Medicine Research2022年1期
- Traditional Medicine Research的其它文章
- The artificial intelligence watcher predicts cancer risk by facial features
- Evaluation of bioactive flavonoids in Citri Reticulatae Pericarpium from different regions and its association with antioxidant and α-glucosidase inhibitory activities
- Effects of Shenling Baizhu powder on endoplasmic reticulum stress related signaling pathway in liver tissues of nonalcoholic fatty liver disease rats
- Artificial neural network techniques to predict the moisture ratio content during hot air drying and vacuum drying of Radix isatidis extract
- Safety of Lycium barbarum L.:more information needed
- Mechanisms and status of research on the protective effects of traditional Chinese medicine against ischemic brain injury
