Evaluation of bioactive flavonoids in Citri Reticulatae Pericarpium from different regions and its association with antioxidant and α-glucosidase inhibitory activities
Na Liu,Jia-Chen Sun,Wen-Na Yang,Di Liang,Lan-Ping Guo,Xia Li*,Wen-Yuan Gao*
1Tianjin Key Laboratory for Modern Drug Delivery&High-Efficiency,School of Pharmaceutical Science and Technology,Tianjin University,Tianjin 300193,China.2School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China.3National Resource Center for Chinese Materia Medica,Academy of Chinese Medical Sciences,Beijing 100700,China.
Abstract Background: To explore the differences of 14 batches of Citri Reticulatae Pericarpium from different regions. Methods: The main aim of this study was to develop a high-performance liquid chromatography method - photodiode array detection for determining the contents of five flavonoids and the chromatographic fingerprints of Citri Reticulatae Pericarpium from different regions.The α-glucosidase inhibitory activities and antioxidant properties of Citri Reticulatae Pericarpium, based on free-radical scavenging assays against 1,1-diphenyl-2-picrylhydrazyl, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), and hydroxyl radicals, were estimated and compared. Results: Among the tested compounds, the content of hesperidin (13.386-68.235 mg/g) was the highest and that of hesperitin(0.045-0.277 mg/g) was the lowest.In comparing different sources of Citri Reticulatae Pericarpium, the contents of narirutin in Citri Reticulatae Pericarpium from Guangdong(0.824-0.851 mg/g) and Sichuan (1.069-1.204 mg/g) provinces of China were lower than in other provinces.In contrast, nobiletin (8.429-12.237 mg/g) and tangeretin (3.947-4.613 mg/g)were most abundant in Guangdong sources of Citri Reticulatae Pericarpium, followed by samples from Sichuan Province (nobiletin: 6.761-7.658 mg/g; tangeretin: 3.422-3.933 mg/g).Correlation analysis showed that nobiletin and tangeretin were the main contributors to the antioxidant capacity, and narirutin was the main active component inhibiting the α-glucosidase activity of Citri Reticulatae Pericarpium. Conclusion: This work revealed that the intrinsic quality of Citri Reticulatae Pericarpium was affected by different growing regions, which provides a scientific basis for controlling the quality of Citri Reticulatae Pericarpium and rationally developing and utilizing Citri Reticulatae Pericarpium.
Keywords: Citri Reticulatae Pericarpium; flavonoids; HPLC-PDA; antioxidant activity;α-glucosidase inhibitory activity
Background
Citri Reticulatae Pericarpium(CRP, Chenpi in Chinese), the dry mature peel ofCitrus reticulateBlanco or its cultivated varieties, has been extensively used as a well-known traditional Chinese medicine, food,and dietary supplement in China (Figure 1) [1].Many CRP cultivars are distributed in different areas of China, such as Guangdong Province, Sichuan Province, Zhejiang Province, Fujian Province,Hunan Province, and others [2].Guangchenpi (Citrus reticulata“Chachi”), Chuanchenpi (Citrus reticulata“Dahongpao”), Zhechenpi(Citrus reticulata“Unshiu”), and Jianchenpi (Citrus reticulata“Tangerina”) are officially recorded in theChinese Pharmacopoeia[3].Among the main CRP cultivars, the pericarp ofCitrus reticulata“Chachi” (Guangchenpi) from Xinhui District (Guangdong Province),is regarded as the best national product because of its excellent quality[4].
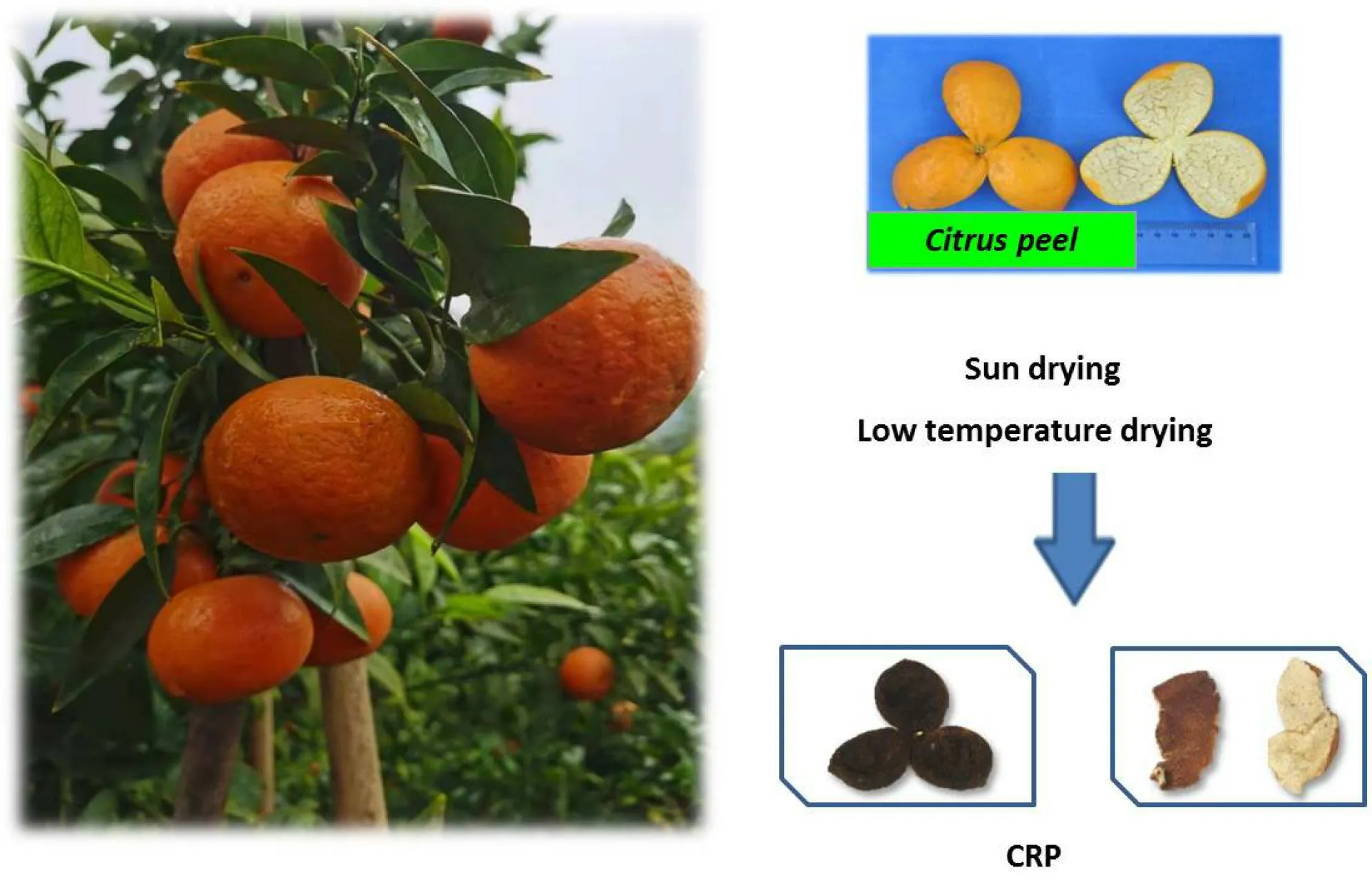
Figure 1 The citrus plants and its medicinal cite CRP.CRP, Citri Reticulatae Pericarpium.
According to traditional Chinese medicine theory, CRP was initially recorded inShennong’s Herbal Classic(unknown author), which was written during the Eastern Han Dynasty (25 C.E.-220 C.E.).CRP is mainly considered to act as a regulator of Qi (Qi is the subtle substance in the human body and is constantly moving.) and to boost digestion [5].In the Northern and Southern Dynasties, the CRP function of relieving cough and diuresis was recorded in theMiscellaneous Records of Famous Physicians[6], which was written by Hongjing Tao between 220 C.E.and 450 C.E.Subsequent herbal works began to summarize the effect of CRP in regulating Qi,relieving cough,and resolving phlegm, such asSupplement to Materia Medica(written by Zangqi Chen, 739 C.E.) andMedicinal Theory(written by Quan Zhen, 541 C.E.-643 C.E.) [7, 8].In the Ming Dynasty(1368 C.E.-1644 C.E.) and Qing Dynasty (1636 C.E.-1912 C.E.), with the in-depth understanding of the functions of traditional medicine, the efficacy of CRP was finally determined.With the development of modern medicine, CRP has been used as a Qi-regulating herb to treat respiratory and digestive diseases, such as nausea, vomiting,indigestion, coughs, and expectoration [9].Modern pharmacological studies indicate that CRP extracts exhibit profound anti-inflammatory,anticancer, and antioxidant activities, as well as a hypoglycemic effect[9-11].
Phytochemical studies demonstrated that the chemical components of CRP are flavonoids, volatile oils, alkaloids, and limonoids.Flavonoids have been proved to be the main compounds in CRP, and these are generally categorized into two groups: flavanone glycosides(flavoneC-glycosides and flavoneO-glycosides) and polymethoxyflavones[1].Hesperidin, a flavoneO-glycoside,is used as a chemical reference for CRP quality control in theChinese Pharmacopeiabecause of its high content.polymethoxyflavones are a unique class of flavonoids that contain more than two methoxyl groups in their chemical structure.At present, tangeretin, nobiletin,and 3,5,6,7,8,3',4'-heptamethoxyflavone are the most widely studied polymethoxyflavones.These compounds are more active than flavonoid glycosides but are generally present as minor components in CRP [12, 13].Many studies have shown that the flavonoid contents and antioxidant capacity of CRP vary according to the cultivars and growing regions [14].However, information about the effects of growing conditions on important bioactivities, such as hypoglycemic effect, is limited.
High-performance liquid chromatography (HPLC) fingerprint map determination is considered to be a reliable method for evaluating the quality of traditional Chinese medicine.The similarities and differences can be described by similarity results,which can be used to analyze a large number of samples.Moreover, fingerprint analysis has been internationally recognized in the evaluation and quality determination of traditional Chinese medicine [1, 15].The chemical composition and biological activities of CRP samples may differ significantly depending on different cultivars and growing environments.In the present study, to systematically evaluate the quality of CRP from different sources, 14 samples from seven different growing regions were collected, and a satisfactory HPLC method with photodiode array (PDA) detection was developed for the simultaneous quantitative determination of five components (narirutin, hesperidin,hesperitin, nobiletin, and tangeretin) in CRP.A fingerprint map of CRP from different origins was established to evaluate the similarity and difference between samples.In addition, the antioxidant and α-glucosidase inhibitory activities of the CRP samples were compared.This study provides a feasible and reliable method to evaluate the quality of CRP and provides detailed information for further study of CRP in food and pharmaceutical industries.
Materials and methods
Materials and chemicals
Fourteen batches of CRP werecollected from seven provinces(Guangdong,Sichuan,Fujian, Anhui, Zhejiang,Shandong,and Hunan)in China.Information on the samples is listed in Supplementary Table S1.
Narirutin, hesperidin, hesperitin, nobiletin, and tangeretin were purchased as HPLC-grade chemicals (purity ≥98.0%) from Dalian Meilun Biotechnology Co., Ltd.(Dalian, China).α-Glucosidase was purchased from Solarbio Life Science Co., Ltd.(Beijing, China).4-Nitrophenyl-α-D-glucopyranoside was obtained from Tianjin Heowns Biochemical Technology Co., Ltd.(Tianjin, China), and acarbose was obtained from Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd.(Hangzhou, China).Milli-Q water was obtained via a Millipore system (Millipore, Bedford, MA, USA).HPLC-grade acetonitrile was purchased from Concord Technology Co.,Ltd.(Tianjin, China).HPLC-grade formic acid was obtained from Aladdin Biochemical Technology (Shanghai, China).2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was obtained from Dalian Meilun Biotechnology Co., Ltd.(Dalian, China) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from Biotop Biochemical Technology Co., Ltd.(Beijing, China).All the other reagents used in this study were of analytical grade.
HPLC-PDA analytical conditions
A Shimadzu LC-2030C HPLC system was used for quantitative analysis(Shimadzu, Kyoto, Japan).The system was equipped with a binary pump, automatic sampler, and PDA detector.The experimental data were collected and processed with LabSolutions software (Shimadzu,Kyoto, Japan).Separation was achieved using a Kromasil C18(4.6 ×250 mm, 100 A, 5 µm) chromatography column.The mobile phase was composed of solvent A (1% formic acid aqueous solution) and solvent B (acetonitrile) and was eluted in a gradient program: 0-50 min with 17% B,50-55 min with 17%-26%B,55-75 min with 26% B,75-80 min with 26%-42% B, 80-110 min with 42% B, 110-115 min with 42%-100% B, 115-125 min with 100% B.The flow rate was 1 mL/min, and the sample injection volume was 5 μL.The detection wavelength was set at 283 nm for narirutin,hesperidin,and hesperitin,and 330 nm for nobiletin and tangeretin.The chromatographic column temperature was 25 °C.
HPLC-mass spectrometry (MS) system
Analysis was conducted using an Agilent 1200 HPLC system (Agilent,Santa Clara, CA, USA) connected to an electrospray ionization tandem triple quadrupole mass spectrometer (ESI-QQQ-MS, Bruker Daltonics,Billerica, MA, USA).The chromatographic conditions were the same as described for HPLC-PDA analysis.The mass scan range wasm/z100-1,000 in positive mode and negative mode.The drying gas (N2)flow rate and drying gas temperature were set at 8.0 L/min and 320°C.The nebulizer pressure, capillary voltage, and fragmentor voltage were set to 40 psig (1 psig = 6894.76 pa), 3,000 V, and 175 V,respectively.Bruker Compass Data Analysis software version 4.3(Bruker Daltonics, Bremen, Germany) was used for data acquisition and analysis.
Solution preparation
CRP was ground into homogeneous powder (passed through a 60-mesh sieve).Sample powder (0.2 g) was accurately weighed and extracted with 5 mL of 100% methanol in an ultrasonic water bath at 45 °C for 40 min.The extraction solution was centrifuged at 1,000 g for 10 min to collect the supernatant.Each extraction was repeated three times.The supernatants were combined and concentrated under reduced pressure.The residue was reconstituted in 5 mL of methanol as the test solution.
All reference standards were accurately weighed and dissolved in methanol to give separate standard solutions.A mixed standard solution of appropriate concentration was obtained by mixing known volumes of the respective standard stock solutions.
All the solutions were stored at 4 °C until analysis and filtered through a 0.45-µm membrane filter before direct injection into the HPLC system.
Method validation
Sensitivity evaluation.The standard solutions were sequentially diluted to appropriate concentrations, and all the solutions were analyzed by HPLC-PDA.The limit of detection (LOD) was calculated as a signal-to-noise ratio (S/N) of 3, while the limit of quantification(LOQ) was calculated as a S/N of 10.
Linear regression equation and standard curve.The standard solution was sequentially diluted to the appropriate concentrations,and the resulting solutions were analyzed by HPLC-PDA.Linear regression analysis was performed with the integral value of different concentrations and peak areas of the compound, and the standard curve was obtained.
Evaluation of precision, repeatability, and stability.The same standard solution was repeatedly analyzed (six times) according to the analytical conditions mentioned above.The peak area of each compound was recorded, and the relative standard deviation (RSD)was calculated to evaluate the precision of the analytical method.For the test of repeatability, six replicates of the same sample solution were prepared independently for analysis.To confirm the stability,the same sample solutions were analyzed by repeated analysis at times of 0, 2, 4, 8, 12, and 24 h.The calculated RSD values were used to assess the repeatability and stability.
Accuracy evaluation.Recovery tests were performed for the quantified components to further evaluate the accuracy of the method.Known amounts of the five standards were added into a 2.0-g powder sample of six batches of the same samples, and then extracted and analyzed with the same procedures.The recovery rate was calculated as follows: Recovery rate (%) = (Discovered amount −Original amount)/Added amount × 100.
Establishment of chromatographic fingerprints
HPLC-PDA analytical conditions.The analytical conditions for HPLC-PDA were the same as those described above.
Solution preparation.The methods of solution preparation were the same as those mentioned above.
Establishment of chromatographic fingerprints.Different batches of CRP solutions were analyzed by HPLC according to the abovementioned chromatographic conditions, and all chromatographic fingerprints were recorded.The chromatographic fingerprint data were imported into the Similarity Evaluation System for Chromatographic Fingerprint of traditional Chinese medicine(2012) for similarity calculation.After selecting the reference chromatogram for multi-point calibration and automatically matching each peak, the mean method was used to generate superimposed chromatographic fingerprints.
Antioxidant activity assay
DPPH free-radical scavenging activity.The DPPH free-radical scavenging capacity of CRP solution was performed as described by Song et al.[16] with slight modification.Briefly, 200-μL aliquots of different sample solutions (2, 4,6, 8, 10,and 12 mg/mL) were reacted with 200 μL of DPPH solution(0.1 mmol/L,dissolved in methanol) for 30 min in darkness, and the absorbance was measured at 519 nm.The reaction mixtures without DPPH were used as a control and vitamin C was used as positive control.The results were presented as IC50values(concentration scavenging 50% of DPPH radicals).The DPPH free-radical scavenging activity was calculated according to the following equation:
Scavenging rate(%) = (A0−(A1−A2))/A0× 100
whereA0is the absorbance without sample,A1is the absorbance of DPPH solution with the sample, andA2is the absorbance without DPPH solution.
ABTS free-radical scavenging activity.The ABTS free-radical scavenging capacity of CRP solution was measured according to the method of Guo et al.[17] with appropriate modifications.Briefly,ABTS diammonium salt solution (7.4 mmol/L) was reacted with K2S2O8solution (2.6 mmol/L) in darkness for 12 h at room temperature to give the ABTS+•solution.Before use, the ABTS+•solution was diluted with methanol to an absorbance of 0.7 ± 0.02 at 734 nm.Subsequently, 800 μL of ABTS+•working solution was mixed with 200 μL of sample solution (1, 2.5, 5, 10, and 20 mg/mL), and the absorbance was measured at 734 nm.Methanol and vitamin C were used as a control and positive control, respectively.The results were presented as IC50values.The ABTS free-radical scavenging activity was calculated according to the following equation:
Scavenging rate(%) = (A0−(A1−A2))/A0× 100
whereA0is the absorbance without sample,A1is the absorbance of ABTS+•solution with the sample, andA2is the absorbance without ABTS+•solution.
Hydroxyl radical scavenging assay.The hydroxyl radical scavenging assay was based on the method of Gu et al.[18] with some modifications.Briefly, 0.7 mL of sample(2.86, 5.71, 11.43, 14.29, and 20 mg/mL) was mixed with 0.2 mL of FeSO4(9 mmol/L) and 0.2 mL of salicylic acid (9 mmol/L in ethanol) before 0.1 mL of H2O2(8.8 mmol/L) was added to start the reaction.After incubation for 1 h in a water bath at 37°C,the absorbance was measured at 510 nm.Distilled water was used instead of H2O2to measure the background absorbance and VC (vitamin C) was employed as positive control.Three replicate analyses were performed for each sample.The results were presented as IC50values.The hydroxyl radical scavenging rate was calculated according to the following equation:
Scavenging rate(%) = (A0−(A1−A2))/A0×100
whereA0is the absorbance without sample,A1is the absorbance of the sample, andA2is the background absorbance without H2O2.
α-Glucosidase inhibitory assay
The α-glucosidase inhibitory assay was determined following the method of Miao et al.[19] with minor modifications.Briefly, sample solutions (50 μL) of different concentrations were mixed with 50 μL of α-glucosidase (0.1225 U/mL) in 50 μL of 0.1 mol/L phosphate buffer solution (pH 6.8).After incubation at 37 °C for 10 min, 100 μL of 4-nitrophenyl-α-D-glucopyranoside (5 mM) was added to the mixture and the temperature was maintained at 37°C for 20 min.The reaction was terminated by adding 100 μL of 0.2 M sodium carbonate solution,and the absorbance was measured at 405 nm.Phosphate buffer solution was used instead of α-glucosidase to measure the background absorbance and acarbose was used as positive control.The results were presented as IC50values.The inhibitory rate was calculated according to the following equation:
Inhibition rate(%) =(A0−(A1−A2))/A0× 100
whereA0is the absorbance of the blank sample,A1is the absorbance of the test sample, andA2is the absorbance of the test sample without enzyme.
Statistical analysis
All experiments were performed in triplicate.The data were expressed as the mean ± standard deviation (SD).Statistical analysis was conducted using a one-way analysis of variance (SPSS version 20.0;SPSS, Chicago, IL, USA) and Duncan’s test (P< 0.05).The chromatographic fingerprints of 14 batches of CRP were established via the Similarity Evaluation System for Chromatographic Fingerprint of traditional Chinese medicine (2012).A correlation matrix analysis was performed and Pearson correlation coefficients (r) were used to analyze relationships between the contents of the five compounds and the antioxidant and α-glucosidase inhibitory activities.
Results
Optimization of HPLC-PDA conditions
Chromatographic conditions are very important for the separation efficiency of compounds in CRP.To develop a satisfactory HPLC-PDA method for analysis of CRP, the mobile phase compositions, gradient elution programs, detection wavelengths, flow rates, and injection volumes were investigated.The peak shape and separation efficiency of the five compounds were optimal when the mobile phase consisting of acetonitrile-water (with 1% formic acid) was used for gradient elution.At the same time, detection wavelength is also a crucial factor affecting the accurate quantitative analysis of the five compounds in CRP.According to the results of HPLC-PDA analysis, the maximum absorption wavelengths and chemical structures of the five ingredient compounds are shown in Supplementary Table S2.The detection wavelength was set at 280 nm for narirutin,hesperidin,and hesperitin,and 330 nm for nobiletin and tangeretin, which was consistent with previously reported results [13].Flow rate, injection volume, and chromatographic column temperature were set as 1 mL/min, 5 μL,and 25 °C, respectively.Representative HPLC-PDA chromatograms of standard substances and a sample solution are shown in Figure 2.
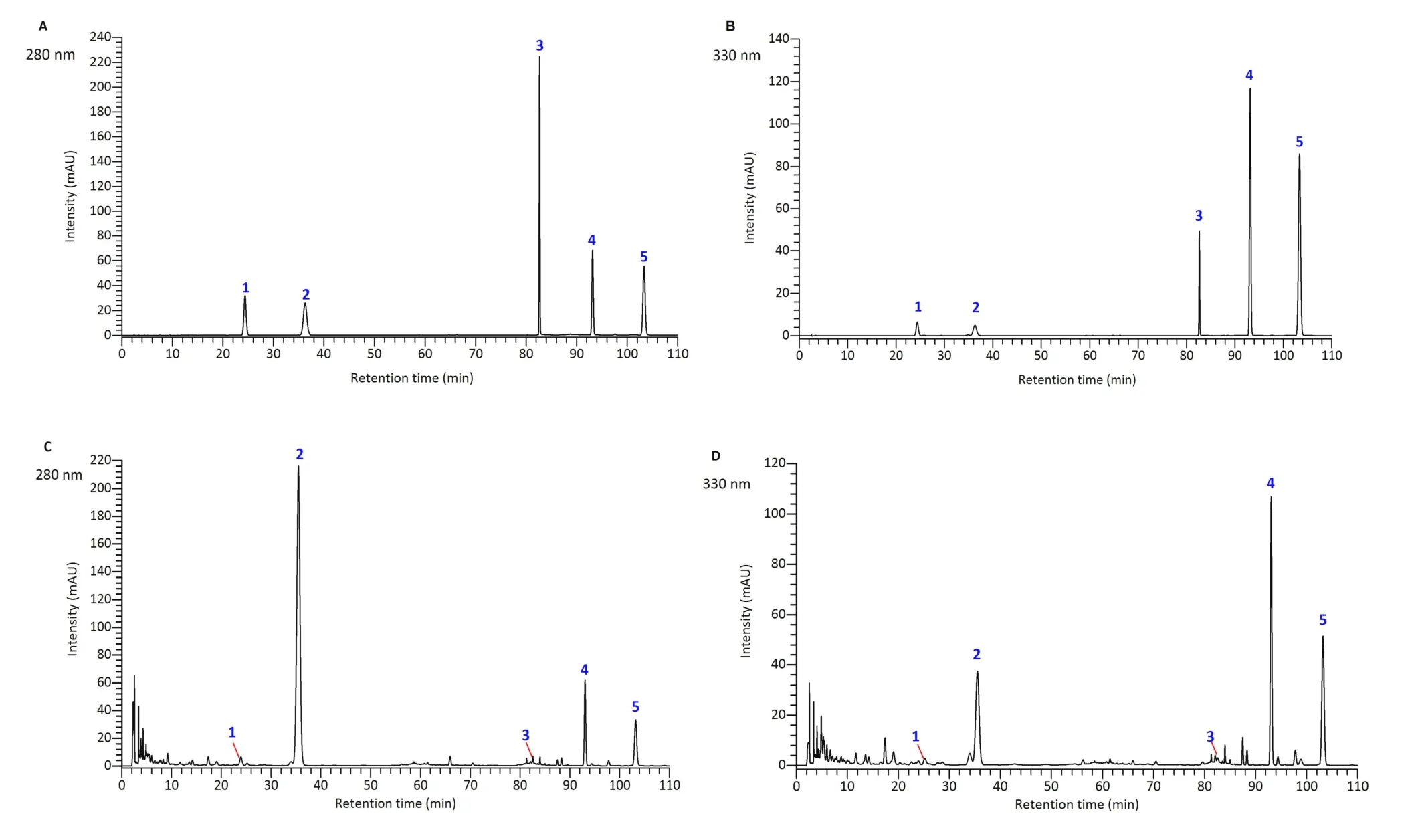
Figure 2 Representative HPLC-PDA chromatograms of CRP.A, detection wavelength set at 280 nm for standard mixture; B, detection wavelength set at 330 nm for standard mixture; C, detection wavelength set at 280 nm for sample solution; D, detection wavelength set at 330 nm for sample solution.1- narirutin, 2- hesperidin, 3- hesperitin, 4- nobiletin and 5- tangeretin.CRP, Citri Reticulatae Pericarpium; HPLC-PDA,high-performance liquid chromatography photodiode array.
Optimization of HPLC-MS conditions
HPLC-PDA mainly identifies compounds by comparing retention times with those of standard substances.However, the retention times of some ingredients are very similar, and it is not accurate to identify components by retention time alone.Therefore, we used a more accurate HPLC-MS method to further verify the identification of the five compounds and standard substances.According to the UV spectra,mass spectra fragments, and retention times of the compounds, the five characteristic peaks in the HPLC chromatograms of the CRP extracts were identified by MS.The positive mode was more sensitive and more suitable for the detection of target compounds than the negative mode.The mass spectra of the five analyte compounds in CRP sample solution are shown in Figure 3.According to the mass-to-charge ratio characteristics of the ion peaks, selected ion monitoring was used atm/z581.2 [M+H]+for narirutin,m/z611.1[M+H]+for hesperidin,m/z303.1[M+H]+for hesperitin,m/z403.1[M+H]+for nobiletin, andm/z373.2 [M+H]+for tangeretin.
Method validation
The linearity of all five compounds was tested with the established HPLC-PDA method and their calibration curves are shown in Supplementary Table S3.The linear correlation coefficients for each ingredient were higher than 0.9994 within the test range.The LODs and LOQs were less than 0.23 μg/mL and 0.76 μg/mL, respectively,which indicates good linearity and sensitivity.For the five main compounds of each sample, the RSD values of peak areas were calculated to estimate precision, stability, repeatability, and recovery(Supplementary Table S4).The RSDs were within the range of 0.68%-0.91% for intraday precision and 1.01%-1.47% for interday precision.The repeatability was in the range of 1.08%-2.38%, and the stability was less than 1.49%.The recovery of the method was in the range of 99.23%-101.16% with RSD less than 1.45%.These results indicated that the developed method is suitable for the chemical analysis of CRP.
Quantitative analysis of five components in CRP
The developed HPLC-PDA method was used for the simultaneous determination of five flavonoids in 14 batches of CRP collected from different regions.The quantitative results based on an external standard method using calibration curves are summarized in Table 1.The contents of the five compounds in sample extracts from different geographical regions were inconsistent, which indicated that the growing conditions may influence the quality of CRP.Across all CRP samples, the content of hesperidin was 13.386-68.235 mg/g, which was the highest among the tested compounds (Table 1).Meanwhile,the contents of narirutin in CRP from Guangdong (0.824-0.851 mg/g)and Sichuan (1.069-1.204 mg/g) provinces were lower than in other provinces.In contrast, nobiletin (8.429-12.237 mg/mL) and tangeretin (3.947-4.613 mg/mL) were most abundant in Guangdong sources of CRP,followed by samples from Sichuan Province(nobiletin:6.761-7.658 mg/mL and tangeretin: 3.422-3.933 mg/mL).These results are in line with the results of Chen et al.[14]and Luo et al.[2].Among the five compounds of interest, hesperitin showed the lowest content in all samples, while samples from Guangdong Province contained more hesperitin than samples from other provinces.
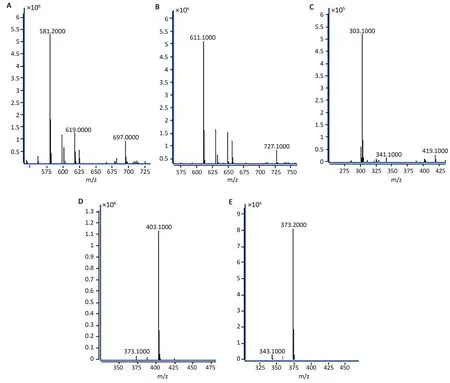
Figure 3 Full-scan mass spectra of five flavonoids in CRP.The selected ions corresponding to compounds A-E were monitored at m/z 581.2,611.1, 303.1, 403.1 and 373.2, respectively.CRP, Citri Reticulatae Pericarpium.
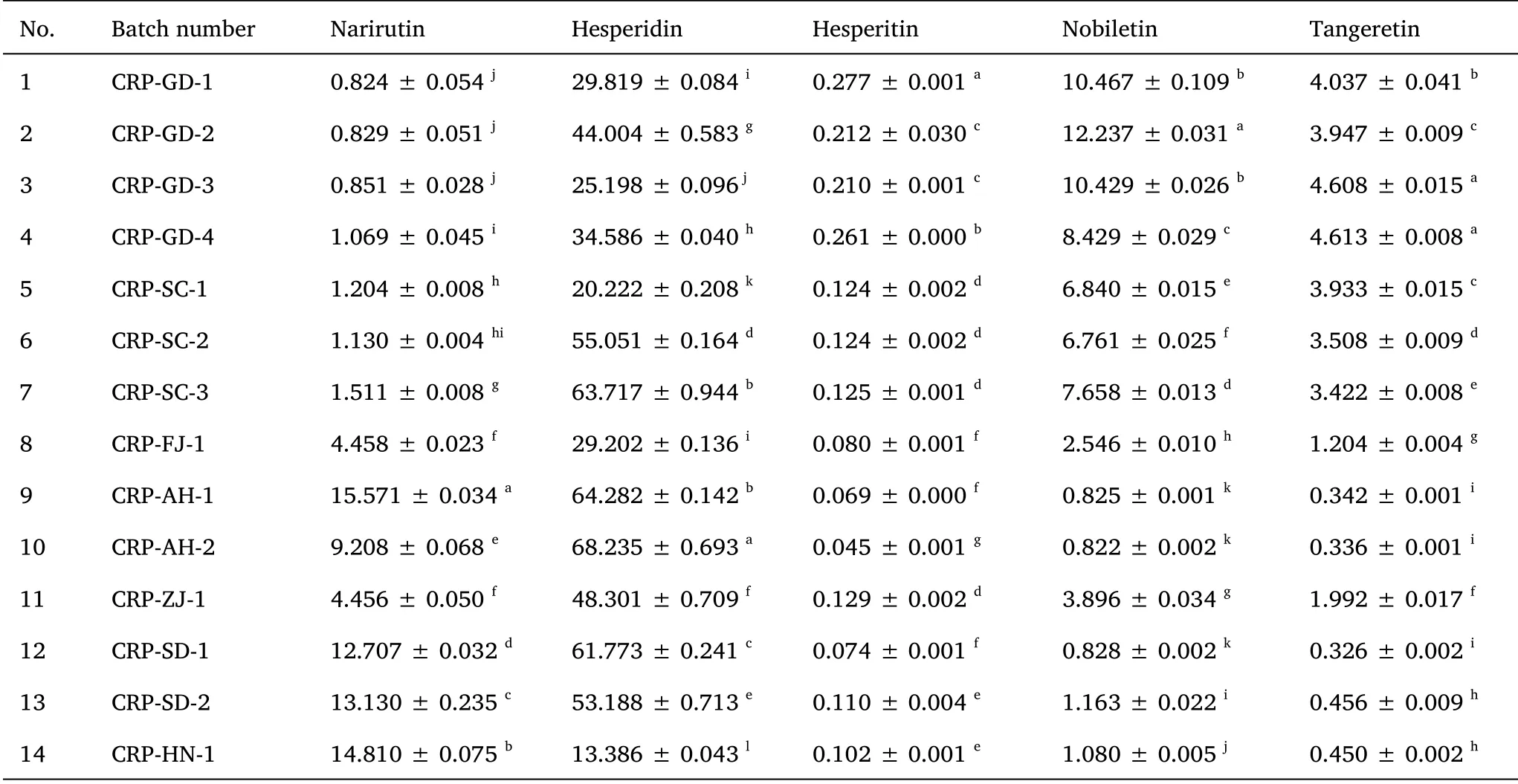
Table 1 Contents (mg/g dry weight) of five bioactive flavonoids in different CRP samples (mean ±SD, n =3)
Hierarchical cluster analysis is a statistical method for finding relatively homogeneous groups of cases based on measured characteristics [20].In this study, based on similarity or dissimilarities of the contents of five compounds in 14 CRP samples, hierarchical cluster analysis was conducted to divide datasets into clusters.The similarity was calculated by the square of hierarchical distance and the Euclidean distance.The degree of correlation between samples depends on the distance in the tree, and the shortest distance represents the strongest relationship [15].The dendrogram of the 14 CRP samples is shown in Figure 4.CRP samples were apparently classified as four main groups.Cluster 1 included samples collected from Sichuan Province, while Cluster 2 was mainly composed of samples collected from Guangdong and Sichuan provinces.Although the contents of five flavonoids in CRP from Guangdong Province are distinguishing,they could be classified into one cluster.In other words,the chemical compounds of CRP from Guangdong Province are similar.Cluster 3 consisted of samples collected from Fujian, Zhejiang, and Hunan provinces, which indicates that these samples were similar.Cluster 4 included samples collected from Anhui and Shandong provinces.The results provide a reference for the selection of appropriate CRP with the desired contents of ingredients, and for the selection of alternate cultivars in the absence of the required varieties.Based on the contents of the five compounds of interest, CRP-SC-1 showed similarity with the CRP from Guangdong Province.

Figure 4 Hierarchical cluster analysis of the contents of five compounds in CRP from different regions.The Arabic numerals 1,2, 3 and 4 in x-axis represent the number of CRP from different regions of China; the Arabic numerals on the y-axis represent the distance, and the shortest distance represents the highest relationship.CRP, Citri Reticulatae Pericarpium.
Establishment of chromatographic fingerprints
Besides multi-component quantitative analysis, fingerprint analysis is another good method for quality control of traditional Chinese medicine because it can systematically reflect the ingredients of the medicinal materials [21].The chromatographic fingerprints of CRP from different regions are shown in Supplementary Figure S1, and the similarity values are displayed in Supplementary Table S5.All the samples were compared with the corresponding reference samples based on their calculated similarity results.The similarity results of CRP were in the range of 0.758-0.958.The similarity values of the samples from Guangdong (0.899-0.958) and Sichuan provinces(0.896-0.929) were higher than those of the other provinces(0.758-0.857), which indicates that the chemical profiles of the samples from Guangdong and Sichuan provinces were similar to that of the reference fingerprint.In addition, it was speculated that the CRP collected in Guangdong Province is similar to that collected in Sichuan Province.Overall, the heterogeneity of similarity indicates that different regions affect the quality of the CRP samples.
Antioxidant activity of CRP samples
Free radicals and active oxygen are harmful to organisms and are implicated in various diseases, such as cancer, inflammation, diabetes,and neurological disorders [22].CRP is a rich source of naturally occurring antioxidants.Flavonoids, as the most abundant compounds in CRP, have been reported to possess a variety of antioxidant activities [23].The antioxidant activity of CRP samples from different regions was evaluated using three different chemical methods, namely DPPH, ABTS, and hydroxyl free-radical scavenging activity.All three chemical methods have been extensively applied to estimate antioxidant activity in both food and biological systems.
The principle of the DPPH assay is based on the reduction of the original purple DPPH radical to the yellow (non-radical) form by hydrogen donation.The decrease of DPPH absorption is equivalent to the intensity of the color change at 519 nm [18].Table 2 shows the scavenging activities of CRP samples against the DPPH radical.The IC50values of samples were in the range of 1.705-3.641 mg/mL.Among them, CRP-SC-3 (IC50= 1.705 mg/mL) and CRP-GD-4 (IC50=2.073 mg/mL) displayed relatively higher DPPH free-radical scavenging capacities with lower IC50values, followed by CRP-SC-1(IC50= 2.348 mg/mL) and CRP-SC-2 (IC50= 2.463 mg/mL).CRP-HN-1 (IC50= 3.641 mg/mL) and CRP-FJ-1 (IC50= 3.519 mg/mL)exhibited low DPPH free-radical scavenging activity.The ABTS assay is based on the transfer of electrons from antioxidant compounds to ABTS radicals [24].
In Table 2, the ABTS assay IC50of CRP was in the range of 0.495-0.931 mg/mL.CRP-GD-2 (IC50= 0.495 mg/mL) showed the highest ABTS free-radical scavenging activity, while the lowest (IC50= 0.931 mg/mL) was found in samples from Linyi Shandong.Hydroxyl radical is the most reactive oxygen radical and can result in serious damage, so scavenging of hydroxyl radicals is essential for protecting life systems [25].The results are shown in Table 2; the hydroxyl radical scavenging assay IC50of CRP was in the range of 4.864-22.258 mg/mL.The scavenging effects of CRP-GD-4 (IC50=4.864 mg/mL) on hydroxyl radical were much higher than that of other batches of CRP.
All CRP samples showed antioxidant activity, and the levels of antioxidant activity were significantly different because of different growth environments.Comprehensive analysis of the antioxidant activity in DPPH, ABTS, and hydroxyl radical assays showed that the samples produced in Guangdong and Sichuan provinces had relatively higher antioxidant activities, which is likely related to the contents of chemical compounds in the samples.
α-Glucosidase inhibitory activity of CRP samples
α-Glucosidase is a key enzyme that catalyzes the final step in the process of carbohydrate digestion.The inhibition of α-glucosidase can delay the digestion and absorption of carbohydrates, thereby inhibiting postprandial hyperglycemia, which is considered to be a new method to control type II diabetes [26, 27].In this study, the α-glucosidase inhibitory activities of CRP samples from different regions were examined.
The α-glucosidase inhibitory activities of all CRP samples are shown in Table 2, and significant differences (P< 0.05) were observed among the 14 batches of CRP samples.All CRP samples showed a hypoglycemic effect, and the IC50value was in the range of 0.252-1.581 mg/mL.CRP-AH-1 with the lowest IC50value (IC50=0.252 mg/mL) exhibited the greatest α-glucosidase inhibitory activity,while the IC50value obtained for CRP-ZJ-1 was the highest of all of the samples tested at 1.581 mg/mL.CRP-AH-2 also showed good α-glucosidase inhibitory activity with an IC50value of 0.678 mg/mL.In general, the in vitro hypoglycemic effect of CRP extract from Anhui Province was far more effective than other sample extracts.
Correlation analysis
Correlation analysis was conducted to reveal the correlation between chemical composition and biological activities of samples.Pearson correlation was conducted to measure the correlation between variables.Pearson correlation coefficient (r) was calculated using bivariate correlation analysis, and its absolute value was between 0 and 1 [19, 26].In this study, the correlations between narirutin,hesperidin, hesperitin, nobiletin, tangeretin, and antioxidant and α-glucosidase inhibitory activities were evaluated by Pearson correlation coefficient, where “−” and “+” were used to indicate negative and positive correlations, respectively.The results are presented in Table 3.
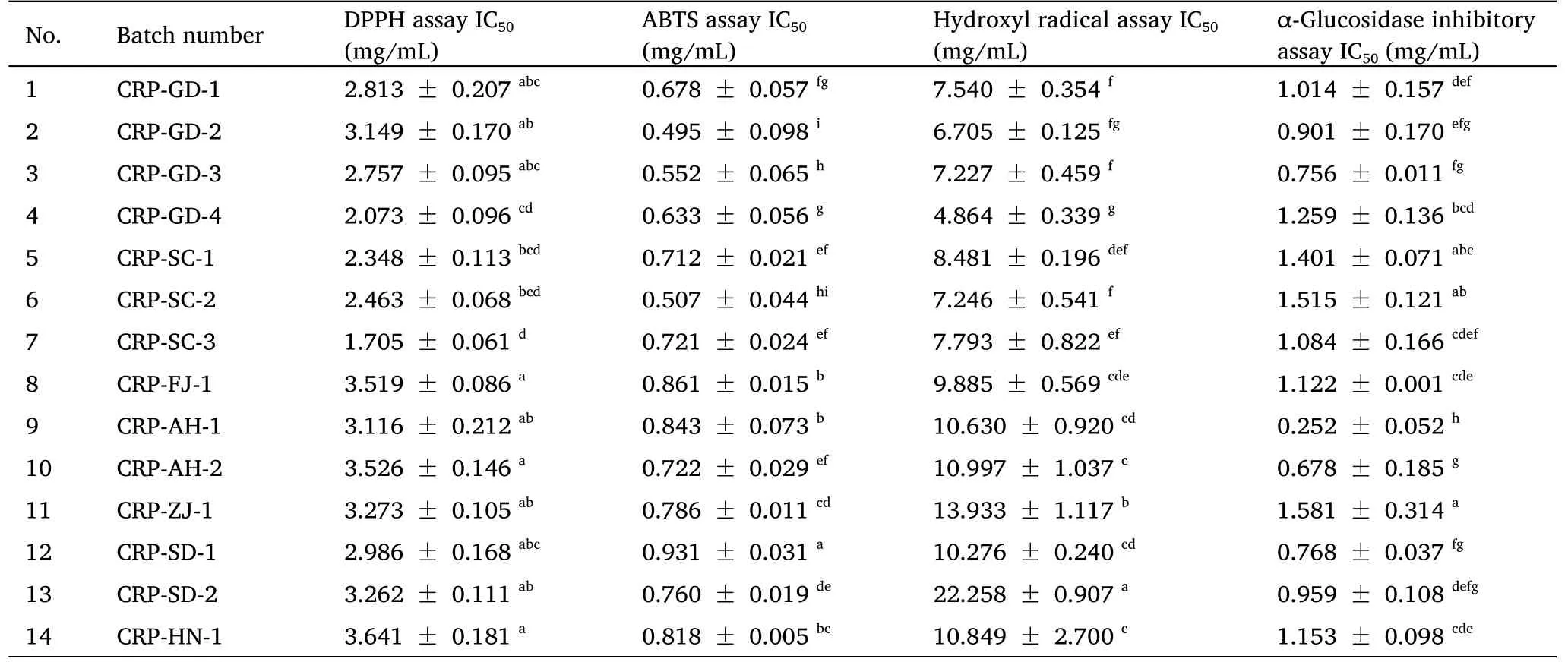
Table 2 Results of activity assays of extracts from CRP
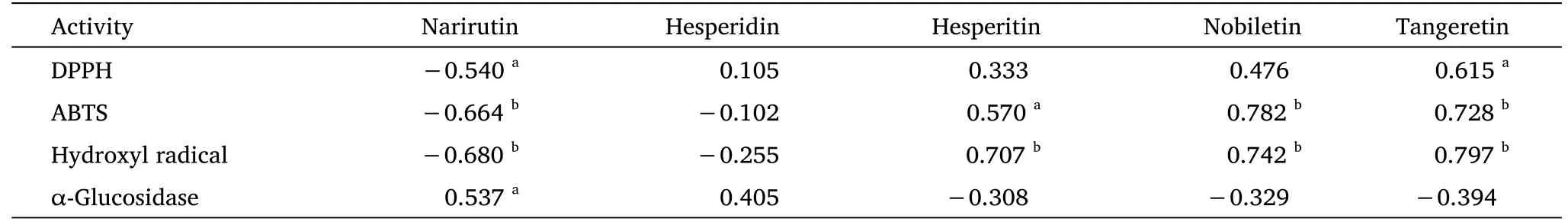
Table 3 Pearson’s correlation coefficients (r) of the five flavonoids and bioactivities
Our results showed positive correlation between the contents of hesperidin, hesperitin, nobiletin, and tangeretin, and a negative correlation between the contents of narirutin and the 1/IC50values of DPPH scavenging activity.Moreover, the 1/IC50values for DPPH scavenging were weakly correlated with the contents of hesperidin (r=0.105), hesperitin (r=0.333), and nobiletin(r=0.476).However,there was a strong correlation between the 1/IC50value for DPPH scavenging and the content of tangeretin (r= 0.615;P< 0.05).In terms of ABTS radical scavenging capacity, a strong positive correlation was found for nobiletin (r= 0.782) and tangeretin (r=0.728) at theP< 0.01 level.The hydroxyl radical scavenging activity was also positively correlated with the content of hesperitin (r=0.707), nobiletin (r= 0.742), and tangeretin (r= 0.797) at theP<0.01 level.Overall, the correlation between the contents of the five flavonoids and antioxidant activity showed that nobiletin and tangeretin are the main contributors to the antioxidant capacity of CRP.
For the α-glucosidase inhibitory assays, the Pearson correlation coefficients (r) for narirutin, hesperidin, hesperitin, nobiletin, and tangeretin were 0.537, 0.405, −0.307, −0.329, and −0.394,respectively.The 1/IC50values of α-glucosidase inhibitory activity showed strong significant correlation with the content of narirutin (P< 0.05).Therefore, narirutin is the main active component of CRP in inhibiting α-glucosidase activity and plays an important role in antihyperglycemia activity, which has not been reported before.Our findings indicated that CRP from Guangdong and Sichuan provinces contained higher contents of nobiletin and tangeretin, which makes them suitable for the treatment of diseases caused by oxidative stress,while CRP-AH-1 with high narirutin content can be used as a hypoglycemic agent.
Discussion
The quality standard of CRP is included in theChinese Pharmacopoeia.In theChinese Pharmacopeia(2015 edition) [28], the appearance characteristics of Guangchenpi are described separately, which confirms the importance of Guangchenpi as a geoherb.However,other test items do not distinguish Guangchenpi from other Chenpi.In addition, the content of hesperidin in all Chenpi and Guangchenpi should not be less than 3.5%.Research data show that the content of hesperidin in some Guangchenpi does not reach 3.5%, so the geoherb has been tested as “inferior medicine”, which is unreasonable.Therefore, the quality evaluation of Chenpi has been greatly revised in theChinese Pharmacopoeia(2020 edition) [29].In addition to the obvious appearance difference between Guangchenpi and Chenpi,identification of Guangchenpi through thin-layer chromatography was also established.More importantly, a method for the determination of multiple components in Guangchenpi was established.In theChinese Pharmacopoeia(2015 edition), hesperidin is described as the only quality-control marker in Chenpi.The main pharmacological effects of hesperidin are antibacterial and anti-inflammatory activities,cardiovascular disease prevention, and smooth muscle function [13].Guangchenpi also contains abundant polymethoxyflavones, mainly including nobiletin, and tangeretin.Its anticancer,cholesterol-lowering, antioxidation, and anti-inflammatory effects were confirmed by pharmacological experiments [30].In our study,an efficient method was successfully established for the determination of five flavonoids (narirutin, hesperidin, hesperitin, nobiletin,tangeretin).Narirutin and hesperidin are flavanone-O-glycosides.According to our results, the contents of these two components in Guangchenpi were not dominant compared with other sources of Chenpi, which is consistent with literature results [14].However,hesperitin, which is a flavanone aglycone, was higher in Guangchenpi than in other Chenpi, which is a previously unreported result.Levels of nobiletin and tangeretin, which are polymethoxyflavones, were high in the four batches of Guangchenpi, which is consistent with theChinese Pharmacopoeia.In general, according to our experimental results and the literature, when compared with Chenpi, the content of narirutin in Guangchenpi was lower, but the contents of hesperitin,nobiletin, and tangeretin were higher.These traits provide a theoretical basis for distinguishing Guangchenpi and Chenpi.
Conclusion
In this study, an efficient and sensitive HPLC-PDA analytical method was successfully established for the determination of five flavonoids(narirutin, hesperidin, hesperitin, nobiletin, tangeretin) in CRP from different regions, and the chromatographic fingerprints were established.This work showed that different origins have significant effects on the intrinsic quality of CRP.Examination of data by hierarchical cluster analysis demonstrated that CRP from Guangdong and Sichuan provinces possessed high contents of nobiletin and tangeretin and were divided into the same group, while CRP samples from other provinces were clustered into one group.In addition,nobiletin and tangeretin were the main contributors to the antioxidant capacity of CRP, and narirutin was the main active component to inhibit the activity of α-glucosidase.The present study deepens the understanding of the relationship between the chemical composition and bioactivities of CRP, which will contribute to its quality control and promote its effective utilization in food and pharmaceutical production.
 Traditional Medicine Research2022年1期
Traditional Medicine Research2022年1期
- Traditional Medicine Research的其它文章
- The artificial intelligence watcher predicts cancer risk by facial features
- Exploring the anti-diabetic effects and the underlying mechanisms of ethyl acetate extract from Sophora flavescens by integrating network pharmacology and pharmacological evaluation
- Effects of Shenling Baizhu powder on endoplasmic reticulum stress related signaling pathway in liver tissues of nonalcoholic fatty liver disease rats
- Artificial neural network techniques to predict the moisture ratio content during hot air drying and vacuum drying of Radix isatidis extract
- Safety of Lycium barbarum L.:more information needed
- Mechanisms and status of research on the protective effects of traditional Chinese medicine against ischemic brain injury
