High performance lithium-sulfur batteries using three-dimensional hierarchical porous carbons to host the sulfur
SHAN Yu-hang, LI Li-bo*, DU Jin-tian, ZHAI Mo
(School of Materials Science and Chemical Engineering, Harbin University of Science and Technology, Harbin 150040, China)
Abstract: Lithium-sulfur batteries are promising for future energy storage because of their high-energy density and low price.However, they have many problems, especially the large volume change during cycling and the shuttle effect of the soluble polysulfides. To solve these problems, a three-dimensional porous carbon (3D-HPC) was investigated as the sulfur host of a lithium-sulfur battery. The 3D-HPC was prepared by a template method using polymethyl methacrylate and zinc oxide as the templates to form mesopores and macropores, respectively. The results showed that the interconnected macroporous channels and abundant large mesopores formed a three-dimensional conductive carbon network which is beneficial for electron/ion transfer and relieves the cathode volume change by the physical limiting effect. The pores alleviate the shuttle effect by the capillary condensation. A 3D-HPC-S composite used as the cathode has excellent electrochemical properties. The first discharge specific capacity of the 3D-HPC-S is 1 314.6 mAh g−1 at 0.2 C with a sulfur loading of 70%. After 100 cycles, the capacity retention rate is 69.13%. At 0.5 C, the capacity retention rate after 200 cycles is 59.02% and the average coulombic efficiency is 98.16%.
Key words: Three-dimensionally structure;Hierarchical porous carbon;Macropore;Lithium-sulfur battery
1 Introduction
Energy storage devices have developed rapidly in recent years[1–10]. Sulfur is a high-activity material in lithium-sulfur batteries, which is of low price and abundance in the earth[11–13]. Lithium-sulfur-battery has a high specific capaticy (1 675 mAh g−1) corresponding to a high energy density (2 567 Wh kg−1),which is promising as new secondary batteries[14–16].However, lithium-sulfur batteries have the following serious disadvantages. The electrical conductivities of elemental sulfur and sulfide (Li2S2and Li2S) are poor.The density difference in S and Li2S causes the volume change of the anode material greatly. The intermediate products (Li2Sn, 4≤n≤8) dissolve in the electrolyte, causing the shuttle effect and the irreversible loss of capacity[17–21]. Many advanced materials have been prepared to overcome the shortcomings of lithium-sulfur batteries, such as polymer materials(e.g. polyaniline[22]and polypyrrole (PPy)[23,24]) and carbon materials (e.g. grapheme[25,26], carbon nanotube[27]and carbon nanofiber[28–30]). After structured and functionalized further, the carbon materials as the host of sulfur are ideal materials for the cathode. Porous carbon material has good electrical conductivity,strong inertia and a large specific surface area for hosting sulfur. Many researchers reported that the highly conductive Li-S composite cathodes were designed to solve the problem of the low utilization rate of active substance and to reduce the shuttle effect[31–40]. 3D-porous carbon materials can encapsulate sulfur and promote electrochemical conversion between sulfur and the lithium polysulfides[41]. The 3D-porous carbons fabricated by soft and hard template methods are commonly used to control their size and morphology[42]. A pore-forming agent in the template eventually decomposed, extracted or etched to obtain the 3D morphology. Jang et al.[43]used a simple one-step vapor deposition method to prepare the mesoporous carbon using polyacrylonitrile as the carbon source and silica colloidal particles as the template. Deng et al.[44]reported the preparation of the 3D graphene-based carbon materials using a series of sodium salts (NaCl, Na2CO3, Na2SiO3) as templates.Cheng et al.[45]prepared a graphene-based nitrogendoped 3D porous material by KOH activation. Gu et al.[46]prepared a 3D carbon material with a high specific surface area of 1 568.2 m2g−1from agaric hydrosol, which provided fast transfer pathways for electron/ion. Wang et al.[47]used the ketjen black and carbon nanotubes to make the cathode materials with a 3D structure to solve the shuttle problem of polysulfides. They coated polypyrrole on C/S composite materials by an in-situ gas polymerization method.
Cellulose acetate (CA) is a renewable material and there are few reports on the preparation of lithium-sulfur batteries with the cellulose acetate as a carbon source. Xu et al.[48]prepared CA from discarded cigarette holders and then combined it with graphene to prepare porous carbons. Graphene wraps around CA to form a stable network structure. The cathode prepared by this method was charged and discharged over 1 000 cycles at 0.5 C, and the average capacity of each cycle only attenuated by 0.04%. Herein, conductive carbon materials with the hierarchical pore structure were prepared by a template method. Specifically, CA was used as the carbon source, PMMA(polymethyl methacrylate) and ZnO (zinc oxide) were used as templates. The decomposition of PMMA occurred during carbonization, and the ZnO was removed by pickling after carbonization. The 3D hierarchical porous carbon (3D-HPC) materials that had interconnected macroporous channels and abundant large-sized mesopores formed a 3D conductive carbon network to promote the electron and lithium-ion transfer.
2 Materials and methods
2.1 Preparation of 3D-HPC
All chemicals such as CA, PMMA and ZnO were analytical grades and directly used without any purification. First, 2.0 g CA and 0.5 g PMMA were dissolved in dimethylacetamide (DMAC) and stirred for 12 h. Then, the 2.0 g (in 3D-HPC-1) or 0.67 g (in 3DHPC-2) ZnO nanoparticles were put into the above solution and stirred for 12 h. The mixture was cast on a glass plate and dried in a vacuum drying oven at 100 °C for 1.5 h. After that, the precursor was carbonized at 475 °C for 1 h and then heated up to 850 °C for 3 h in nitrogen atmosphere with a heating rate of 5 °C min−1. After cooled, it was washed with 20%HCl and the ZnO was removed by the ultrasonic treatment for 30 min. Finally, the carbon material was cleaned with deionized water and ethanol, and dried at 60 °C for 24 h to get 3D-HPCs.
2.2 Preparation of 3D-HPC-S
The 3D-HPC-1 and 3D-HPC-2 were mixed with sulfur at mass ratios of 30∶70 and 45∶55, respectively. Then the composite materials were mixed in an agate mortar for 1 h. The nearly uniform mixtures of carbon-sulfur were further annealed at 155 °C for 2 h to get 3D-HPC-S composite materials. The obtained composite materials with the different components were denoted as 3D-HPC-1-S and 3D-HPC-2-S.
2.3 Characterization
Amorphous carbon was revealed by X′PertPRO X-ray diffractometer using Cu Kα radiation, and the 2θrange varied from 10° to 90° at room temperature.The scanning electron microscopy (SEM) FEISirion200 was used to characterize the morphology of 3D-HPCs and 3D-HPC-S and their microstructures were further observed by transmission electron microscopy (TEM) JEM-2100. The specific surface areas of the materials were calculated by the Barrett-Emmettteller (BET) method (QuantachromeASiQwin Station 2). The X-ray photoelectron spectroscopy (XPS) was conducted to reveal the bonding mechanism of the sulfur and the carbon through PHI15700 from American physical electronics.
2.4 Electrochemical measurements
The 3D-HPC-S composite materials (80 wt%)were used to prepare the cathode slurry after mixed with AB (10 wt%) as the conductive agent and PVDF(10 wt%) as the binder in N-methyl-2-pyrrolidone.Then the above mixture slurry was stirred for 24 h and dried at 60 °C for more than 24 h to get cathode material. After that, the cathode material was cut into a circle with a diameter of 14 mm by a Tablet Punching Machine. LIR2025 coin batteries were assembled in the glove box under the protection of argon. Celgard 2500 membrane was used as a separator and lithium foil (diameter of 18 mm, the thickness of 1 mm) as the anode. The electrolyte was consisted of 1 mol L−1lithium bis(Trifluoromethanesulfonic) imide (LiTFSI) in a mixture of 1,3-dioxolane (DOL) and dimethoxymethane (DME) (1∶1,v/v) with addition of 1 wt%LiNO3. The coin battery was tested on the land-CT2001A battery test system. The galvanostatic charge-discharge tests were operated at the voltage range from 1.5 to 3 V at room temperature. Cyclic voltammetry (CV) was characterized on the CHI760E electrochemical station with a scanning rate of 1 mV s−1and the voltage range of 3–1.5 V. Electrochemical impedance spectroscopy (EIS) was performed on the CHI760E electrochemical station with 0.01–105Hz under room temperature.
3 Results and discussion
The 3D-HPCs were produced by the template method. In carbonization, when the temperature was increased to 475 °C and kept for 1 h, PMMA (b.p.105 °C) decomposed and the products were vaporized in situ from the matrix to form mesopores. Then,the ZnO was removed by pickling in 20% HCl to get macropores after carbonization to obtain the 3D-HPC materials as described in Fig. 1.
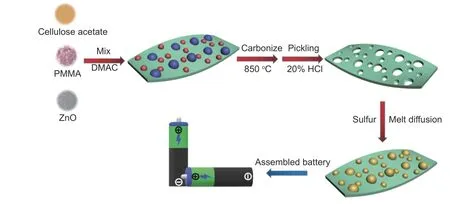
Fig. 1 Flow chart and schematic diagram illustrating the preparation process of 3D-HPC-S composite.
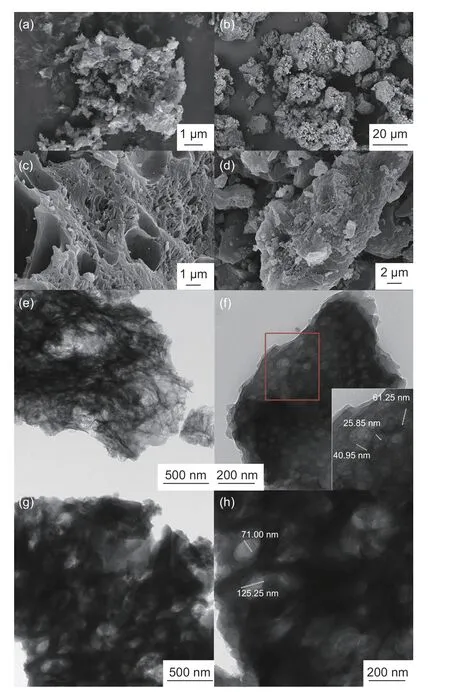
Fig. 2 SEM images of (a) 3D-HPC-1, (b) 3D-HPC-1-S, (c) 3D-HPC-2, (d)3D-HPC-2-S, (e) TEM images of 3D-HPC-1 (10000 ×), (f) 3D-HPC-1(20000 ×), (g) 3D-HPC-2 (10000 ×), (h) 3D-HPC-2(20000 ×).
The SEM images of the 3D-HPC-1 and 3D-HPC-2 are shown in Fig. 2a and 2c, respectively. The pictures clearly show that the 3D-HPC-2 material has a large number of macropores that are distributed on the surface of the carbon layer. In contrast to the 3DHPC-2, the 3D-HPC-1 material is made up of a smallsize aggregated carbon particles. Because of the high content of the template, the particle sizes of 3D porous carbon are reduced. Compared with the 3DHPC-2-S, the 3D-HPC-1-S is also consisted of the small-size aggregated C/S composite particles as shown in Fig. 2b and 2d. The cathode that is made up of small-size particles in the 3D porous carbon can improve the interface area between the cathode(Fig. 2f and 2h) and the electrolyte, increasing the degree of contact between the cathode active substance and the electrolyte[48]. The clear mesoporous morphology is also described by TEM (Fig. 2e-2h), which further proves that the 3D-HPC-1 and 3D-HPC-2 are 3D hierarchical macroporous, mesoporous and microporous structure. Fig. 2e-2f demonstrate that the 3DHPC-1 material has a lamellar 3D structure with the interconnecting macropores and large size mesopores.The good conductive networks provide an excellent host for the sulfur element. The large pores can effectively alleviate the expansion of the cathode during the cycle process. It can prevent the deactivation of active substances and the short circuit of the battery caused by the collapse of the cathode structure, thus improving the battery life. TEM images of the 3DHPC-2 material in Fig. 2g-2h show that 3D-HPC-2 is a thick 3D structure with a large number of macropores and mesopores. Compared with 3D-HPC-2, the 3D-HPC-1 material is thinner and its pore size distribution is more narrow. The interconnected 3D porous structure provides a good medium for electron/ion transfer and more active sites for sulfur, which is conducive to the redox kinetics in charging and discharging. Abundant mesopores provide the large specific surface area and pore volume, which adsorb active substances through the capillary condensation and produce a physical limiting effect on active substances and polysulfides. They prevents the polysulfides from dissolving in the electrolyte and relieves the capacity loss caused by the shuttle effect.
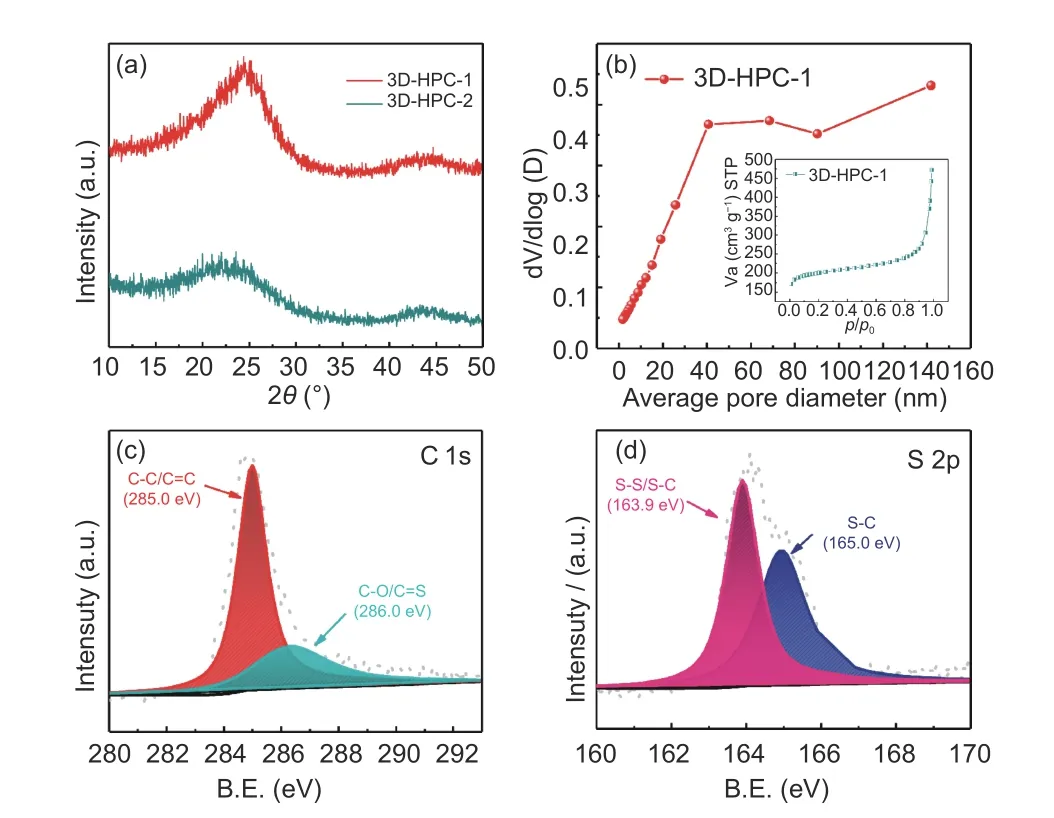
Fig. 3 (a) XRD patterns of 3D-HPC-1 and 3D-HPC-2, (b) the pore size distribution and adsorption isotherm of 3D-HPC-1 and the XPS narrow (c)C element and (d) S element spectra of 3D-HPC-1-S.
Fig. 3a shows the XRD patterns of 3D-HPC-1 and 3D-HPC-2. The XRD peaks of 3D-HPC-1 at 2θ=22.4° and 43.28°, the peaks of 3D-HPC-2 at 2θ=21.73° and 42.94° are broad and assigned to the characteristic peaks of the amorphous carbon[26]. This indicates that the 3D porous carbons are not fully graphitized during the heat treatment. Comparing the peaks of 3D-HPC-1 with 3D-HPC-2, it is found that the peak area and strength of 3D-HPC-1 are larger and stronger than that of the 3D-HPC-2, so the graphitization degree of 3D-HPC-1 material is higher than that of the 3D-HPC-2, which is more conductive to electron/ion transfer. Fig. 3b shows the pore size distribution and adsorption-desorption isotherms of 3D-HPC-1 material. By BET calculation, the specific surface area of 3D-HPC-1 material is 694.70 m2g−1and the total pore volume of 3D-HPC-1 material is 0.60 cm3g−1. It can be seen from the pore size distribution that there are mesopores and macropores at 20-140 nm in the material. The curve belongs to the Ⅱ adsorption isotherm that the monolayer adsorption occurs when the relative pressure is low. When the monolayer adsorption reaches saturation, the adsorbing capacity remains stable with the increase of relative pressure.With increasing the relative pressure continuously, the multilayer adsorption begins to happen. At the saturated vapor pressure, the number of adsorption layers indefinitely increases. It indicates that the pore structure type of the material is mesoporous and macroporous. The results of the BET test are consistent with SEM characterization results. The carbon and sulfur XPS narrow spectra of 3D-HPC-1 are shown in Fig. 3c-3d. It can be seen from Fig. 3c that the peaks at 285.0 eV and 286.4 eV are assigned to C―C/C=C and C―O/C―S, respectively[49]. The XPS spectrum of S 2p is shown in Fig. 3d. The peaks at 163.9 eV and 165.0 eV correspond to S―S/S―C and S―C, respectively[50]. The results show that the material contains a C―S chemical bond, which is produced by the active sulfur bonding to the carbon. The redox kinetics in charging and discharging processes is accelerated by the combination of sulfur anion and lithiumion[49].
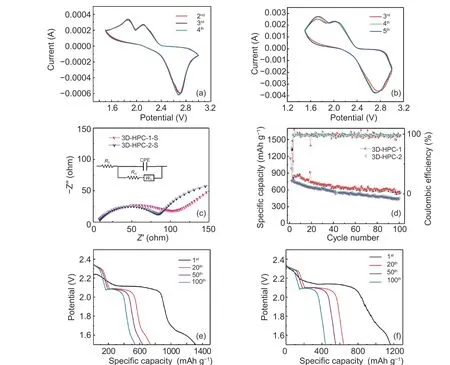
Fig. 4 CV of (a) 3D-HPC-1-S and (b) 3D-HPC-2-S cathodes between 3.0 V and 1.5 V with a scan rate of 1 mV s−1, (c) EIS spectra of the 3D-HPC-1-S and 3D-HPC-2-S cathodes before the cycle, (d) long-term cycling stabilities of the 3D-HPC-1-S cathode and 3D-HPC-2-S cathode over 100 cycles, charge-discharge profiles of (e) 3D-HPC-1 and (f) 3D-HPC-2 cathode at 0.5 C for 1st, 20th, 50th and 100th cycles.
As shown in Fig. 4, the CV curves of the 3DHPC-1-S and 3D-HPC-2-S show that the two typical reduction peaks are 2.10 V and 1.90 V, respectively.The sulfur cathode has a multi-step reaction during the discharge process. Elemental sulfur is reduced to high-order polysulfides (Li2Sn, 4≤n≤8) at the peaks of 2.10 V and 1.90 V, which is manifested as Li2S2and Li2S, respectively. In the subsequent oxidation scanning process, a peak can be observed at 2.70 V, which is the inverse process of the corresponding reduction reaction, that is oxidation of Li2S2/Li2S to elemental sulfur[39]. As the number of cycles increases, the cyclic voltammetry curves have almost coincided in Fig. 4a and Fig. 4b. The redox peaks of 3D-HPC-1-S are sharper and its peak area is larger than 3D-HPC-2-S, so 3D-HPC-1 cathode has better cycling performance.
Before the cycle, the EIS diagrams of the 3DHPC in Fig. 4c and Fig. 4d consist of a semicircle in the middle to the high-frequency region and a diagonal line in the low-frequency region. According to the circuit fitting, the intercept between the high-frequency semicircle and the real axisZ' is related to the impedanceR0of the electrolyte. The impedance in the high-frequency region reflects by the electrode reaction kinetics (charge transferRct) control and the impedance in the low-frequency region reflects the lithium-ion diffusion control. TheR0of 3D-HPC-1-S(8.31 Ω) is almost equal to that of 3D-HPC-2-S(8.65 Ω), and the charge transfer resistanceRctof 3DHPC-2-S (83.87 Ω) is slightly lower than that of 3DHPC-1-S (103.11 Ω) because the sulfur content of 3DHPC-1-S is up to 70% while the sulfur content of 3DHPC-2-S is only 55%. The conductivity of the sulfur is not very good so theRctof 3D-HPC-1-S with a higher sulfur content is higher. However, the 3DHPC-1 material has a better hierarchical pore structure with better interconnections, which can provide more active sites for transferring electrons/ions of the active substances. Therefore, 3D-HPC-1-S material has a higher specific capacity. The lower resistance corresponds to the XPS test results. The good chemical bonding between carbon and sulfur accelerates the transfer of electrons/ions and effectively reduces the resistance, thus ensuring the conductivity and good charge-discharge performance of the cathode.Fig. 4d describes the coulomb efficiency and charge-discharge trends of 3D-HPC-1-S and 3D-HPC-2-S at 0.2 C. The first discharge specific capacities of 3D-HPC-1-S and 3D-HPC-2-S are 1 314.6 and 1 163 mAh g−1, respectively. After the first discharge,the specific discharge capacity has obvious attenuation. The SEI is generated between the electrode/electrolyte interface after the first discharge. After 100 cycles, the specific discharge capacities of 3D-HPC-1-S and 3D-HPC-2-S are 556.9 and 441.2 mAh g−1, respectively. The capacity retention rates from the second cycle to the 100 cycles of 3D-HPC-1-S and 3D-HPC-2-S are 69.13% and 57.96%, respectively.The 3D-HPC-1-S presents an obvious better electrochemical performance than 3D-HPC-2-S. Fig. 4e and Fig. 4f show the discharge curves of the 3D-HPC-1 and 3D-HPC-2 cathodes for 1st, 20th, 50thand 100thcycles with a cutoff voltage window of 2.5–1.5 V. The discharge curve is consistent with that of typical lithium-sulfur batteries. During the reduction process,there are two reduction platforms corresponding to the transformation of elemental sulfur to long-chain polysulfide (Li2Sn, 4≤n≤8) and long-chain polysulfides into insoluble products (Li2Sn, 1≤n≤2).
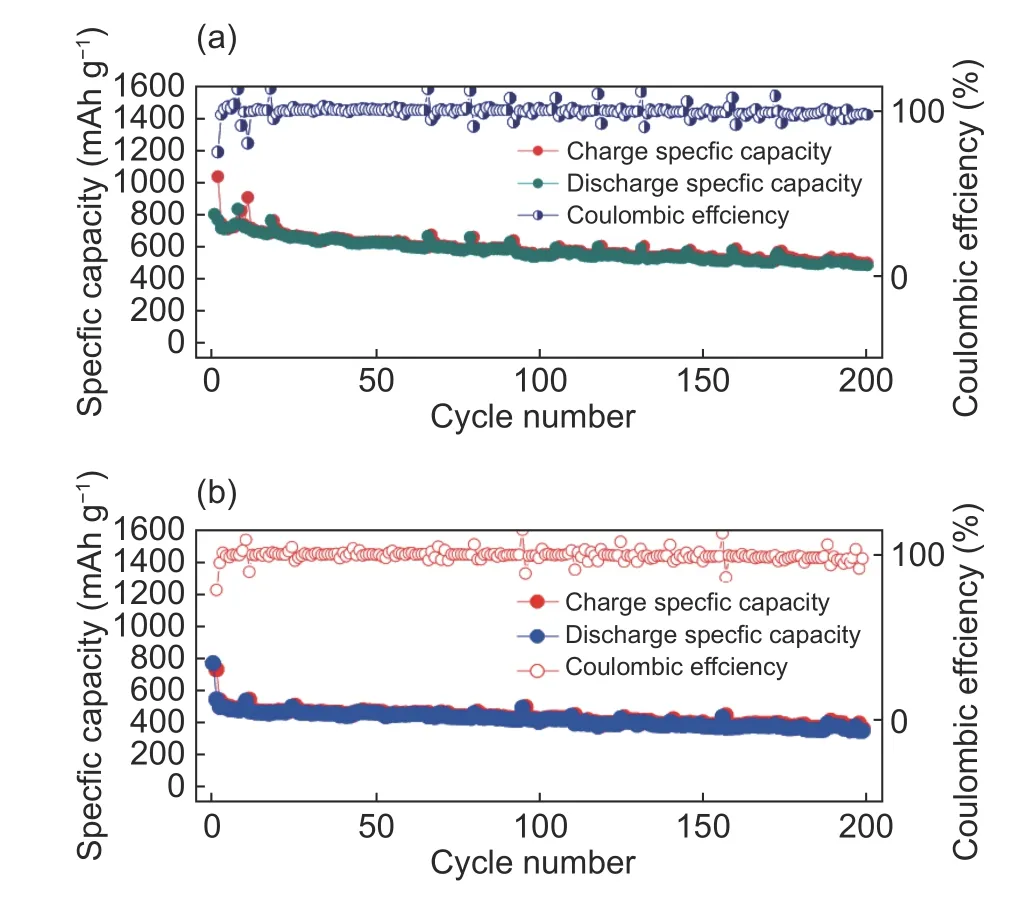
Fig. 5 (a) Long-term cycling performance of 3D-HPC-1-S cathode with a sulfur loading of 0.327 6 mg cm−2 in practical environment and (b) longterm cycling performance of 3D-HPC-1-S cathode with a sulfur loading of 0.582 3 mg cm−2 in practical environment.
Long-term cycling capability of the 3D-HPC-1-S cathode in practical environment is evaluated at 0.5 C for 200 cycles. As shown in Fig. 5a and Fig. 5b, the 3D-HPC-1-S cathode with a sulfur loading of 0.327 6 mg cm−2delivers an initial discharge capacity of 780.8 mAh g−1and maintains at 460.8 mAh g−1after 200 cycles. The 3D-HPC-1-S cathode with a sulfur loading of 0.582 3 mg cm−2delivers an initial discharge capacity of 787.3 mAh g−1and maintains at 386.0 mAh g−1after 200 cycles. The corresponding capacity retention rates are 59.02% and 49.03%, respectively. After increasing the sulfur load, the capacity retention rate slightly decreases. The corresponding average coulomb efficiencies are 98.16% and 97.96% after the second cycle, respectively. The regular fluctuation of the curve during the cycle is caused by the temperature difference between day and night in the practical environment. After increasing the sulfur loading, the average coulomb efficiency remains almost constant. Fig. 5 demonstrates that the whole charge and discharge cycle is very stable without serious attenuation. 3D-HPC-1 cathode is a 3D interconnected porous material, which effectively allows the rapid entry of lithium-ions through the electrolyte into the pores to combine with the active materials.Abundant mesopores provide a large specific surface area and a pore volume, which can effectively inhibit the dissolution and diffusion of polysulfides, hold back the shuttle of polysulfides, and promote the electrochemical properties of electrode materials. The macropores can solve the problem of the cathode expansion of Li-S battery and prolong the battery life in the practical environment.
4 Conclusion
The conductive carbon materials with the 3D hierarchical porous structure were prepared by the template method using PMMA and ZnO as the templates and the cellulose acetate as the carbon source.The SEM and TEM results show that 3D-HPC materials have multiple interconnected pores, which can provide an excellent host for elemental sulfur. The electrochemical test show that 3D-HPC materials can load sulfur up to 70%. The performance of the cathode had been obviously improved with the increase of the content of template agents. The 3D-HPC-1-S cathode exhibits a high specific capacity of 1 314.6 mAh g−1at 0.2 C and the average coulombic efficiency is 98.16% of 200 cycles at 0.5 C in the practical environment. In this paper, the lithium-sulfur battery cathode with the 3D hierarchical pores was prepared by a simple method and a clean raw material. This strategy can promote the commercialization of lithium-sulfur batteries in the future.
Acknowledgement
This work was supported financially by the National Natural Science Foundation of China (No.21706043).
- 新型炭材料的其它文章
- 基于碳化钽涂层改性碳基材料的研究进展
- Two-dimensional layer materials for highly efficient molecular sensing based on surface-enhanced Raman scattering
- Coating a Na3V2(PO4)3 cathode material with carbon to improve its sodium storage
- Preparation of a N-P co-doped waste cotton fabric-based activated carbon for supercapacitor electrodes
- High-surface-area porous carbons produced by the mild KOH activation of a chitosan hydrochar and their CO2 capture
- Preparation of a porous carbon from Enteromorpha prolifera with excellent electrochemical properties

