Preparation of a N-P co-doped waste cotton fabric-based activated carbon for supercapacitor electrodes
HUANG Ling, WANG Shuai, ZHANG Yu, HUANG Xiang-hong,PENG Jun-jun*, YANG Feng
(Hubei Key Laboratory of Biomass Fibers and Eco-dyeing & Finishing, College of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan 430200, China)
Abstract: Transforming waste resources into energy storage materials is a new way to convert them into value-added products and help solve the problems of energy shortage and environmental pollution. A nitrogen-phosphorus co-doped activated carbon was synthesized from waste cotton fabric by combining carbonization and activation in ammonium polyphosphate and a molten salt system (ZnCl2 and KCl with a molar ratio of 52∶48). The morphology, microstructure and composition of the activated carbon were characterized by SEM, nitrogen adsorption, Raman spectroscopy and XPS. Cyclic voltammetry and galvanostatic charge/discharge were used to test the supercapacitor performance of the activated carbon. Results show that the co-doped activated carbon had a specific surface area of 751 m2·g−1, a specific capacitance of 423 F·g−1 at a current density of 0.25 A·g−1, and a capacitance retention rate of 88.9% after 5 000 cycles at a current density of 5 A·g−1. The energy density was 28.67 Wh·kg−1 at a power density of 200 W·kg−1 for a symmetrical supercapacitor using the activated carbon.
Key words: Waste cotton fabric;Activated carbon;Nitrogen/phosphorous co-dopant;Supercapacitor
1 Introduction
As an important part of current energy storage devices, supercapacitors have excellent power density,high rate capability and stable cycle performance, so that they have been widely used in new energy vehicles, backup power supply and other energy storage systems. Carbon materials are the most commonly used electrode materials for electrochemical double layer capacitors (EDLCs), including activated carbon, graphene, carbon nanotubes and mesoporous carbon[1–4]. Among them, active carbon is the most widely used electrode material because of its abundant source and low price. However, the low energy density of activated carbon (<2-10 Wh·kg−1) has not been able to meet the demand of high-performance energy devices[5], thus greatly limits the development of supercapacitors. Therefore, improving the energy density of activated carbon materials has become one of the important research directions of supercapacitor materials.
In recent years, carbon materials doped with heterogeneous elements have become an effective method to improve the performance of carbon-based supercapacitors. In particular, the doping of nitrogen or phosphorus in carbon materials has attracted extensive attention[6,7]. For example, Huang[8]introduced nitrogen atoms into carbon skeleton, and achieved the capacitance of 720 F·g−1, and the energy density of 38.5 Wh·kg−1. The main reason is that the doping of nitrogen improves the interface properties of carbon materials, promotes the ionic conductivity, and produces some pseudocapacitance. Moreover, the introduction of phosphorus atoms can improve the defect of carbon materials, broaden the voltage window, and improve the wettability of carbon materials[9]. These effects can make the performance of carbon based-supercapacitors significantly improved. Nitrogen and phosphorus elements co-doped carbon materials have been proven to perform superior electrochemical performance in supercapacitor[10,11], thus becoming a new approach to improve the performance of carbon-based supercapacitors.
Conversion of waste biomass into carbon materials have attracted enormous attention in the field of supercapacitors, this is because that it can reduce environmental pollution, conserve resources, and produce some valuable energy materials. For example,grapefruit peel[12], rice husk[13,14], peanut husk[15], pine husk[16]and other wastes were studied by researchers through different carbonization and activation technologies. Also, in combination with doping nitrogen and phosphorus elements, these activated carbon materials exhibited good performance for supercapacitor[17,18]. Recently, our research group has used waste wool as the precursor to prepare the activated carbon material with doped nitrogen element through onestep molten salt carbonization and activation method,which provides a new strategy of converting waste textile resources into supercapacitor materials[19]. Cotton fabric is the general waste resource in human clothing[20]. It is very significant to convert waste cotton fabric into high-performance carbon materials for supercapacitor application. Although some researchers have begun to carry out this work[21,22], there is no relevant report on how to use nitrogen and phosphorus co-doping technology for producing waste cotton fabric based carbon materials. Molten salts have been regarded as templates and porogen to form porous structure for carbon materials, which has attracted more attention by researchers[23,24]. ZnCl2/KCl molten salt is a kind of composite molten salt, which enables to low melting point temperature of molten salt and benefit carbonization process of precursors. Meanwhile, ZnCl2can act a good activator in pore forming process, which has been paid much attention in the preparation of conventional activated carbon materials[25].
In this paper, the waste cotton fabric-based activated carbon materials co-doped with nitrogen and phosphorus element were prepared by one-step carbonization methods in ZnCl2/KCl molten salt. The waste cotton cloth was used as the precursor, and ammonium polyphosphate (APP) acted as the raw material for simultaneously co-doping nitrogen and phosphorus. The structure, morphology, composition and pore size of the activated carbon were characterized,and the properties of their supercapacitor were studied.
2 Experimental
2.1 Reagents and instruments
Waste cotton cloth, provided by dyeing and finishing laboratory of Wuhan Textile University. Zinc chloride, A.R., provided by Shanghai Lingfeng Reagent Co., Ltd.. Potassium chloride, A.R., provided by State Pharmaceutical Group Chemical Reagent Co.,Ltd.. Ammonium polyphosphate (APP), A.R., provided by Shandong Yousuo Chemical Technology Co.,Ltd.
The morphology of products were characterized by scanning electron microscope (SEM, Zeiss, Germany). The crystalline structure of products were tested by D/max-2200/PC type X-ray diffractometer(XRD, Rigaku, Japan). The composition of products were analyzed by Finder Vista type Raman spectrometer (Raman, Beijing zhuolianguang instrument company) and Escalab 250XI type X-ray photoelectron spectrometer (XPS, Thermo Fisher Scientific Inc., USA). The specific surface area and pore size of products were tested by Tristar ii3020 type nitrogen desorption instrument (Micromeritics, USA).
2.2 Preparation of waste cotton fabric-based activated carbon
Firstly, the waste cotton cloth were cut into a few pieces of fabric sheets, and washed with water, then dried in an air dry oven at 110 °C. Secondly, the cotton fabric, ammonium polyphosphate and ZnCl2/KCl(ZnCl2/KCl=52/48, mol ratio) with a mass ratio of 1∶1∶6 were mixed together. Thirdly, the mixture was put in a 100 mL beaker with 30 mL of ultrapure water, and magnetically stirred for 30 min, and dried in air dry oven at 120 °C. Then, the whole mixture was transferred into a corundum crucible for carbonization process. During the molten salt treatment, carbonization and activation process were carried out by one-step in N2atmosphere at a temperature rise rate of 5 °C·min−1. The target temperature was 800 °C, and lasted for 2 h. After natural cooling, the products were washed with 1 mol·L−1hydrochloric acid solution, and then washed with anhydrous ethanol and ultrapure water, until the pH value of the solution reached 7. At last, the products were dried in a vacuum oven at 80 °C. The samples obtained from waste cotton fabric treated by molten salt and APP were named CF-2. For comparison, the sample directly carbonized at 800 °C without molten salt was named CF-0, and the sample processed in 800 °C in ZnCl2/KCl molten salt without APP was named CF-1.
2.3 Electrochemical measurement
All the electrochemical measurements were carried out by Princeton (Princeton, PARSTAT Multi-Channel) workstation. The working electrode was composed of active material, conductive agent (acetylene black) and binder (PTFE) with a mass ratio of 7∶2∶1, which was pressed into a thin film by a roll squeezer. Then, the film was dried in a vacuum oven at 70 °C to 12 h, and cut into a few pieces of sheet with area of 1×1 cm2by scissors. After weighing, the film was pressed on the nickel foam to form an electrode. The amount of carbon mass of working electrode was about 3 mg cm−2. In the three-electrode test system, the electrolyte was 6 mol·L−1KOH, the counter electrode was platinum plate, and the reference electrode was Hg/HgO electrode. Cyclic voltammetry (CV) and constant current charge-discharge(GCD) methods were applied in the voltage range of−0.7-0.3 V.

WhereCis the specific areal capacitance (F·g−1),Iis the discharge current (A), Δtis the discharge time(s),mis the mass of the active substance on the working electrode (g), and ΔVis the voltage range of the discharge process.
In the two-electrode test system, 1 mol·L−1Na2SO4is used as the electrolyte, and two identical electrodes are used as working electrode and counter electrode, respectively.
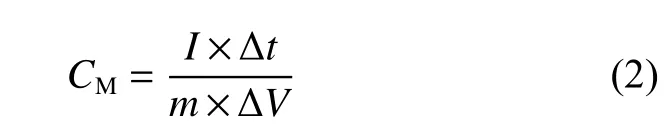
WhereCMis the value of specific capacitance(F·g−1),Iis the discharge current (A), Δtis the discharge time (s),mis the mass of active substance on two electrodes (g), and ΔVis the change range of discharge voltage.
The energy density and power density of the two electrodes are calculated as follows:
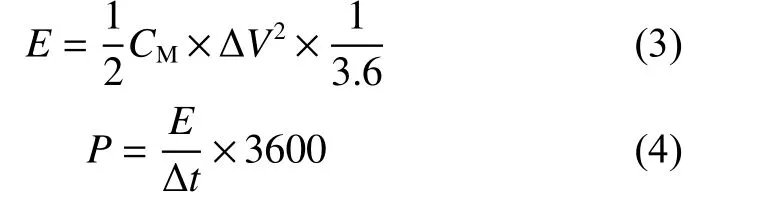
WhereErepresents the energy density (Wh·kg−1)andPrepresents the power density (W·kg−1).
3 Results and discussion
The morphology of waste cotton fabric-based activated carbon is shown in Fig. 1. As shown in Fig. 1(a, b), these samples are obtained from a direct carbonization process. After treated under 800 °C, the material still maintains the fibrous structure. Fig. 1(c,d) show the morphology of the products treated by ZnCl2/KCl molten salt without APP. According to these microstructure images, it can be found that the products are very fluffy, and the fibrous structure has been damaged. Also, it can be seen from the magnification images in Fig. 1(d) that there are some obvious pores in this material. This is because that cotton fabric has undergone some processes of gasification, dehydrogenation, aromatization and pore formation under the treatment of ZnCl2/KCl molten salt[25]. In Fig. 1(e, f), these samples are prepared under ZnCl2/KCl molten salt with the addition of APP. The synergistic effect of molten salt and ammonium polyphosphate on cotton fabric results in formation of porous carbon materials with co-doped nitrogen and phosphorous element. However, the morphology of these products are almost the same as those from Fig. 1(c,d),indicating that the main role of APP in molten salt medium is to introduce heteroatoms into carbon matrix.
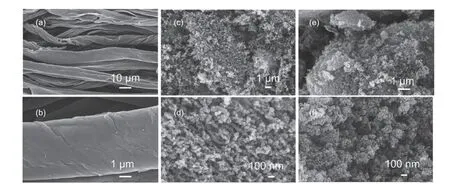
Fig. 1 SEM images of samples (a, b) CF-0, (c, d) CF-1 and (e, f) CF-2.

Fig. 2 (a) XRD and (b) Raman spectra of different waste cottons fabricbased activated carbons.
Fig. 2(a) are the X-ray diffraction (XRD) spectra of the waste cotton fabric-based activated carbons.The two broad peaks with low intensity located at a 2θvalue of 24° and 44° show that the activated carbon materials of CF-0, CF-1 and CF-2 samples are all amorphous carbons[26]. Fig. 2(b) are the Raman spectra of the samples. It can be seen that there are two typical characteristic peaks at 1 343 and 1 585 cm−1,which correspond toDband andGband, respectively.Generally,Dband represents the defect and disorder degree of carbon materials, andGband represents the graphitization degree of carbon materials[27]. TheID/IGvalues of samples CF-0, CF-1 and CF-2 are 1.05, 1.09 and 1.12, respectively. Obviously, theID/IGvalues of CF-2 are the largest, which indicates that the combined effect of ammonium polyphosphate and molten salt makes the most defect for carbon samples. Defect increment is an effective strategy to improve the capacitance of electrode materials. This is because that a few defects on the surface of carbon layer (such as vacancy and edge) can not only increase the Faraday pseudocapacitance, but also enhance the double electric layer capacitance through changing the surface structure of the material[28–30].
The specific surface area and pore size distribution of the samples play an important role in the electrochemical performance of the electrode materials.Fig. 3(a) shows the nitrogen adsorption/desorption isotherms of the samples. According to the classification of IUPAC, the sample CF-1 treated by molten salt carbonization and activation exhibits the mixed type I and IV. It can be seen that the behavior of the type I occurs in low relative pressure, indicating some micropore structures are formed in the activation process. However, in the high relative pressure range, a hysteresis loop appears in the nitrogen adsorption and desorption process, which contributes to the behavior of type IV, and indicates that there are mesopores in the material.
The adsorption/desorption isotherm type of sample CF-2 is similar to that of sample CF-1.Fig. 3(b) shows the pore size distribution of samples CF-0 and CF-1 calculated by BJH method. It can be seen that the pore volume of the two samples are very small in the mesoporous range, mainly less than 6 nm.However, CF-2 (Fig. 3(c)) has the largest pore volume in the mesoporous range, and the main pore size distribution is in the range of 20-40 nm. The detail results of the micropore range are shown in Fig. 3(d), and the maximum pore volume is mainly distributed in the range of 0.7 and 1.2 nm. However, the pore volume in the microporous range is relatively small, and the pore volume is about 0.17 cm3·g−1. The BET specific surface area, total pore volume and average pore diameter of the sample are listed in Table 1. The BET specific surface area of CF-1 is 679 m2·g−1, which is nearly double the sample of CF-0 (350 m2·g−1). These results confirm the pore forming role of molten salt. After adding ammonium polyphosphate, the specific surface area of CF-2 can reach 751 m2·g−1, which is close to that of CF-1. According to the analysis of Table 1,the total pore volume of sample CF-2 is 1.37 cm3·g−1,which is much higher than that of sample CF-0 and CF-1.
In order to prove the successful doping of nitrogen and phosphorus element, X-ray photoelectron spectroscopy (XPS) was used to analyze CF-2 sample.Fig. 4(a) shows that there are 4 elements C, N, O and P in the XPS full spectrum. According to the test, the atomic percentages of C, N, O and P element are calculated to be 92.63%, 3.43%, 3.73% and 0.21%. The content of nitrogen in the whole sample is also determined by an elemental analyzer, and the mass percentage is 4.26%. The ash content of the sample isabout 0.12% by ash test, indicating other impurities are very little in the sample. The mass content of phosphorus is 0.05% tested by ICP-OES analysis.Fig. 4(b) shows the N1s peak of the sample, which can be deconvolved into 4 peaks, corresponding to pyridine nitrogen (N-6, 398.6 eV), pyrrole nitrogen(N-5, 400.2 eV), graphite nitrogen (N-Q, 401.3 eV)and nitrogen oxides (N-X, 403.0 eV). Among them,the presence of N-Q can increase the conductivity of carbon materials, and the occurrence of N-5 and N-6 in the pores of carbon materials can facilitate the ion transfer and provide active sites to increase the pseudocapacitance[31–33]. Fig. 4(c) demonstrates the XPS spectrum of C1s, which can be divided into four fitting peaks, contributing to three functional groups of C―C(284.8 eV), C―O or C―P(285.9 eV) and C=O or C―N(287.5 eV), respectively. Fig. 4(d)shows the XPS spectrum ofP2p, and its intensity is very weak. It can be also divided into three fitting peaks, which correspond to P―O (134.4 eV), P―N(133.8 eV) and P―C(132.7 eV) functional groups[34]. The existence of P―C functional group probably provides some active sites, benefiting the improvement of the capacitance. All these results have demonstrated that ammonium polyphosphate plays a crucial role in the co-doping nitrogen and phosphorus during molten salt carbonization process.

Table 1 BET surface area and pore structure characterization parameters of all samples.

Fig. 4 (a) XPS spectrum, (b) N1s spectrum, (c) C1s spectrum and (d) P2p spectrum of CF-2.
In order to test the electrochemical performance of the waste cotton fabric-based activated carbon materials, cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) measurements were employed in a three-electrode system. Fig. 5(a) shows the CV curves at the scan rate of 20 mV·s−1. It can be seen that CF-0, CF-1 and CF-2 all exhibit similar rectangular curves, indicating that cotton fabric-based activated carbon has the typical behavior of electric double layer capacitor (EDLC). Among them, CF-2 has the largest rectangular area, implying the most capacitance. This may be related to the effect of co-doping nitrogen and phosphorus element. Fig. 5(c) shows the CV curve of CF-2 electrode in 1 mol·L−1H2SO4solution at different scan rates. Compared with the CV in 6 mol·L−1KOH solution in Fig. 5(b), it can be found that there is a pair of symmetrical redox peaks located at about 0.2 V. With the increase of scan rate, the peak current density also increases. This is due to the electrochemical oxidation-reduction process of nitrogencontaining functional groups formed in carbon matrix in acid solution[35]. Therefore, it can be concluded that nitrogen and phosphorus elements have been well doped in carbon materials.
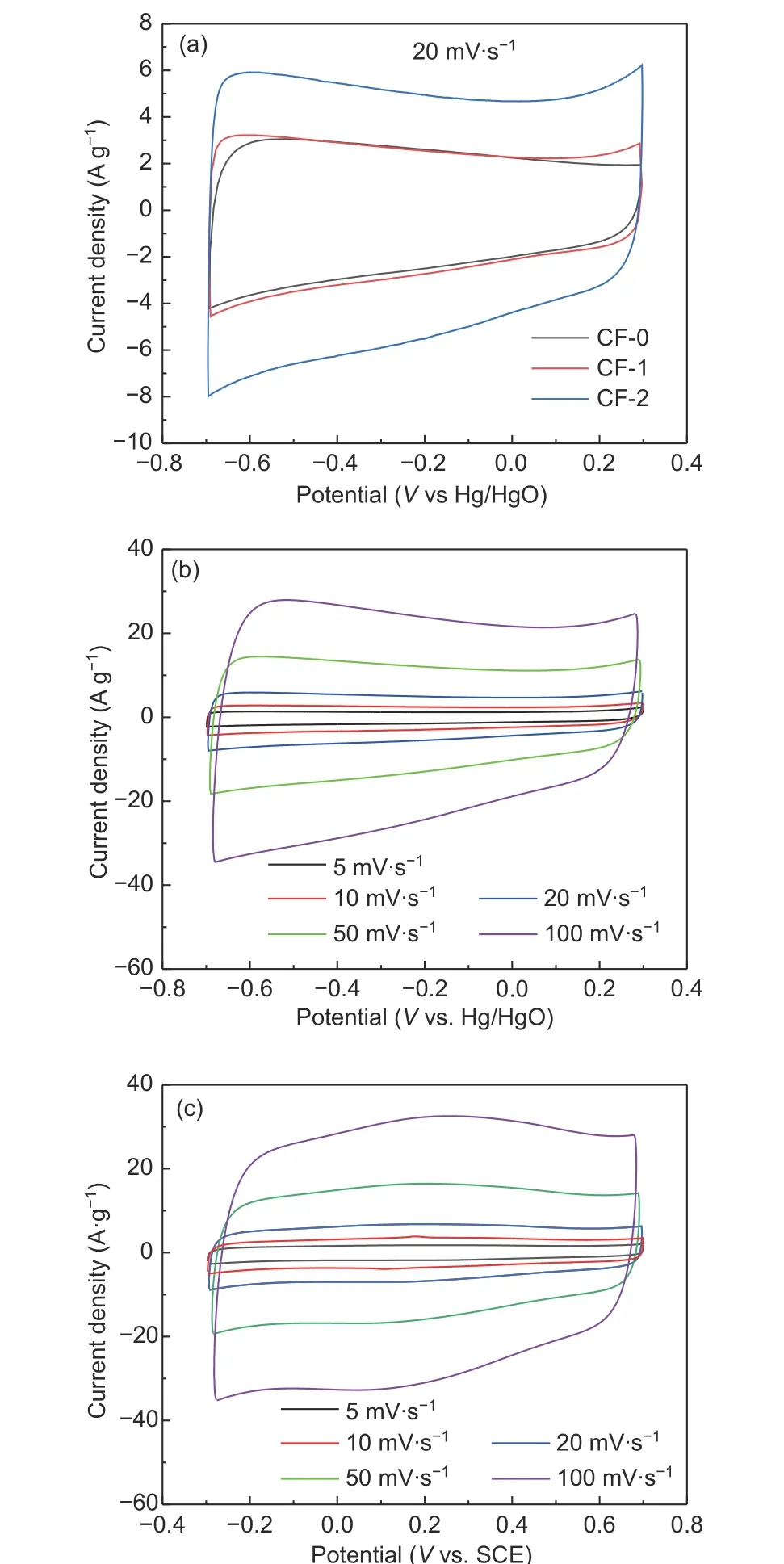
Fig. 5 (a) CV curves of CF-0, CF-1 and CF-2 at 20 mV∙s−1 in 6 moL·L−1 KOH solution; (b) CV curves of CF-2 at different scan rates in 6 moL·L−1 KOH solution; (c) CV curves of CF-2 at different scan rates in 1 moL·L−1 H2SO4 solution.
Fig. 6 (a) shows the GCD curves of CF-0, CF-1 and CF-2 in the voltage range of −0.7– 0.3 V at 1 A·g−1current density. It can be seen that the GCD curves of CF-0 and CF-1 have good isosceles symmetry, which is a typical characteristic of EDLC. While the CF-2 sample has a certain deviation from isosceles symmetry, which should be caused by the pseudocapacitance behavior from the co-doping nitrogen and phosphorus elements. Moreover, CF-2 has the longest charge and discharge time, indicating the largest specific capacitance. This result is consistent with that of cyclic voltammetry. Fig. 6(b) presents the effect of current density on the charge and discharge curves of CF-2. It can be seen that the larger current density is applied, the shorter discharge time is obtained. The discharge specific capacitance of CF-0, CF-1 and CF-2 samples under different current density can be calculated as shown in Fig. 6(c). It is obvious that CF-2 possesses the best capacitance performance compared with CF-0 and CF-1, which has a maximum specific capacitance of 423 F·g−1at 0.25 A·g−1. At a high current density of 10 A·g−1, the specific capacitance can still maintain 270 F·g−1. Fig. 5(f) shows the 5 000 charge-discharge cycle performance of CF-2 at the current density of 5 A·g−1(the inset is the charge-discharge curve of the first 15 cycles). After 5 000 cycles, the specific capacitance can reach 88.9% of the initial capacitance. The reasons on the attenuation of specific capacitance may be related to the following explanation: (1) in the repeated and longtime GCD process, the carbon film materials attached on the current collector are easy to crack or fall off. (2) Some functional groups anchored on the carbon skeleton become invalid due to their instability[35,36].

Fig. 6 (a) GCD curves of CF-0, CF-1 and CF-2 at 1 A∙g−1, (b) GCD curves of CF-2 at different current densities, (c) the specific capacitance of CF-0, CF-1 and CF-2 at different current densities, (d) Cycling performance of CF-2 at 5 A∙g−1.
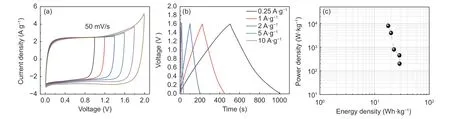
Fig. 7 (a) CV curves of CF-2 at different voltage windows, (b) GCD curves of CF-2 at different current densities, (c) Ragone plot of CF-2// CF-2.
In order to study the practical application of CF-2,a symmetrical supercapacitor (CF-2//CF-2) was assembled to test in neutral electrolyte of 1 mol·L−1Na2SO4. Fig. 7(a) shows the CV curves of CF-2//CF-2 symmetrical capacitor in different voltage windows. It can be seen that CVs keep a good rectangle within a maximum voltage window of 1.6 V. Therefore, the voltage window of GCD measurement can extend from 0 to 1.6 V as shown in Fig. 7(b), which is beneficial to improve energy density. It can be seen that the symmetrical supercapacitor has an ideal EDLC characteristics and good rate discharge performance at different current densities. According to GCD test, the relationship between specific capacitance and current density in two electrode system is calculated. At 0.25 A·g−1, it has the maximum specific capacitance value of 80 F·g−1, then decreases to 50 F·g−1at the current density of 10 A·g−1, representing the retention rate is 62.5%. Fig. 7(c) is the Ragone diagram of CF-2//CF-2 symmetrical supercapacitor. According to the calculation from equation of (2), (3) and (4), the power density of CF-2//CF-2 symmetrical supercapacitor continually decreases with the increment of energy density. When the maximum energy density is up to 28.67 Wh·kg−1, a lowest power density of 200 W·kg−1is obtained. However, the highest power density can reach about 8 000 W·kg−1under an energy density of 17.78 Wh·kg−1.
4 Conclusions
In this paper, N―P Co-doped cotton fabricbased activated carbon materials were prepared by one-step carbonization and activation method in ZnCl2/KCl molten salt medium using waste cotton fabric as precursor and ammonium polyphosphate as doping source. The BET specific surface area of the material is about 751 m2·g−1, and the doping amount of nitrogen and phosphorus is about 3.43% and 0.21%, respectively. In the three-electrode system, the specific capacitance of the carbon materials can reach 423 F·g−1at the current density of 0.25 A·g−1, and it still maintains 88.9% of initial specific capacitance after 5 000 GCD cycles at the current density of 5 A·g−1. A symmetrical supercapacitor (CF-2//CF-2) has a maximum energy density of 28.67 Wh·kg−1under a power density of 200 W·kg−1. This novel method provides a new strategy to construct N/P co-doped carbon materials with high supercapacitor performance from waste textile biomass.
Acknowledgements
The Research Project of Education Ministry of Hubei Province (D2019174), the Innovation Platform Research Funds of Wuhan Textile University(193052), the fund of Hubei Key Laboratory of Biomass Fiber and Ecological Dyeing and Finishing(STRZ201906).
- 新型炭材料的其它文章
- Preparation of a porous carbon from Enteromorpha prolifera with excellent electrochemical properties
- High-surface-area porous carbons produced by the mild KOH activation of a chitosan hydrochar and their CO2 capture
- A DFT study of the effect of stacking on the quantum capacitance of bilayer graphene materials
- 基于碳化钽涂层改性碳基材料的研究进展
- Coating a Na3V2(PO4)3 cathode material with carbon to improve its sodium storage
- Two-dimensional layer materials for highly efficient molecular sensing based on surface-enhanced Raman scattering

