Preparation of a porous carbon from Enteromorpha prolifera with excellent electrochemical properties
LI Shi-jie*, ZHANG Ming-yang, GAO Yan, LI Hui, WANG Qian, ZHANG Lin-hua
(School of Thermal Engineering, Shandong Jianzhu University, Jinan 250101, China)
Abstract: Enteromorpha prolifera (EP) was carbonized, treated by HCl pickling to remove Ca2+ ions to form an "egg-box" structure, and activated by KOH to obtain a porous carbon (PC). The porous texture and electrochemical performance of the PC were compared with one produced without the HCl pickling stage. Results indicate that the HCl treatment leads to the formation of a porous structure with a high specific surface area (SBET), up to 3 283 m2 g−1, with more than 66% of the surface area contributed by mesopores, while the carbon prepared without HCl treatment is microporous. The PC with the HCl treatment had an excellent electrochemical performance when used as the electrode material of a supercapacitor even at high current densities. Its gravimetric capacitance reached 361 F g−1 at a current density of 0.1 A g−1, and the capacitance remained at 323 F g−1 at a current density of 10 A g−1,both of which are higher than obtained using the PC without HCl treatment.
Key words: “Egg-box” structure;Hierarchical porous carbon;Mesopores;Electrochemical property
1 Introduction
Supercapacitors having power and energy densities between electrochemical batteries and conventional capacitors are regarded as promising electrical energy storage devices that provide alternatives for batteries[1–6], and for the applications that require high power density, long cycle life and excellent operational stability[7–10]. However, to meet the high requirements of the future electronic systems such as portable electronics, hybrid electric vehicles, mobile communication and even the national defense and aerospace, substantial performance improvement of the supercapacitors is required through the invention and modification of electrode materials[11–14]. Carbonbased materials, transition metals and conducting polymers are the commonly used electrode materials.Among them, carbon-based materials have become the most widely used ones for supercapacitors owing to their high gravimetric capacitance, long service life and abundant resources[15]. At present, more than 80%of commercial supercapacitors are assembled using carbon-based electrode materials[16], and almost all carbon-containing materials can be used as precursors to prepare activated carbons[17]. Activated carbons with excellent electrochemical property are usually prepared from fossil fuels (coal, petroleum coke, etc.),which makes it inexpensive but harmful to the environment[18]. Biomass-based activated carbons have attracted more and more attentions in recent years due to its low price, renewable sources, sufficient supply and environmentally friendly properties[19,20]. In recent years, many biomass raw materials, such as undaria pinnatifida[21], coconut shell[22], corn kernels[23],wheat straw[24], potato starch[25], rice hull[26], sunflower seed hull[27], have been used as raw materials to prepare activated carbons for supercapacitors. In addition, biomass can also be used for the preparation of porous carbons with tailored pore size distribution and ultra-high rate capability for supercapacitors[28].
Although activated carbons prepared from some local materials of terrestrial plants, such as seed hulls and fruit hulls, exhibit good electrochemical properties, but it is inconvenient to collect these resources,which hinders the development in large-scale industry. Algae are important marine biomass resources,they have the characteristics of high nitrogen content,low ash content and no heavy metal elements as compared with terrestrial plants. Moreover, algae-based activated carbons usually have excellent pore characteristics and electrochemical properties, so they are especially suitable for the preparation of carbon-based electrode materials for supercapacitors.
Generally, the gravimetric capacitance of carbonbased electrode materials increases linearly with increasing the Brunauer-Emmett-Teller surface area(SBET) according to the double layer energy storage principle. However, the gravimetric capacitance of carbon electrodes does not follow a gradual increase trend with theSBETof carbon-based materials[29,30].Therefore, researchers believe that the electrochemical properties of carbon-based supercapacitors are related not only to theSBET, but also to the pore size distribution of carbon-based materials[31]. Previous studies have shown that micropores (< 2 nm in size) contribute most to theSBETof carbon-based materials,provide more adsorption space for electrolyte ions,and supply more double layer capacitance. Mesopores (2–50 nm) are favorable to reduce the resistance of ion transport by providing low resistance transport channels for electrolyte ions to the inner surface of micropores. Macropores (> 50 nm) are beneficial to shorten the transport distance of electrolyte ions by storing a certain amount of electrolyte, thus reducing the resistance of ion transport[32]. Therefore,the carbon electrode materials with ideal pore size distribution should have enough micropores for energy storage, an appropriate amount of mesopores for ion transport, and a small amount of macropores for electrolyte storage. This characteristics of pores can not only ensure the efficient transport of electrolyte ions in the pores, but also provide a sufficient high gravimetric capacitance of carbon-based supercapacitors.Although activated carbons prepared by the KOH activation method generally have abundant micropores that can provide enoughSBETfor adsorbing electrolyte ions, but most of them have poor connectivity and poor ionic transport capacity, which seriously inhibits the efficient transport of electrolyte ions in pores, increasing the series resistance of supercapacitors and adversely affecting the rate performance of carbonbased electrodes[33]. In order to make carbon-based supercapacitors with excellent electrochemical properties in high power applications, algae-based activated carbons must possess high contents of mesopoes with excellent pore connectivity. The template method is usually adopted to prepare activated carbons with a large number of mesopores. The hierarchical porous carbon (HPC) as an eco-friendly electrode material was fabricated from rice husk biochar using the hard template method for electrosorption of ions. The HPC sample achieved a high specific surface area(1 839 m2g−1), large pore volume (1.21 cm3g−1) and 58% of mesopores[34].
In this study, Enteromorpha prolifera (EP), a kind of algae, was selected as raw material to to prepare HPCs by KOH activation, where its carbonized product was leached by hydrochloric acid to remove calcium ions, forming an "egg-box" structure that contributed to the hierarchical pore morphology.
2 Experimental
2.1 Materials
Although EP is not toxic itself, it reproduces very fast, massive reproduction of EP proliferates on the sea surface will form a natural barrier to block sunlight and inhibit the growth of submarine organisms.The chemicals secreted by EP will have adverse effects on some marine organisms, and the dead EP will also consume oxygen in the sea water. Therefore, the outbreak of EP will seriously damage the marine ecosystem. Using EP as a raw material to prepare activated carbons can avoid the outbreak of EP and effectively improve its economic benefits.
The EP used in this study was collected from Weihai, China. The EP was washed thoroughly and then dried for 48 h in a blast drying chamber at 120 ºC. The dried EP was crushed and screened by a fast crusher and a vibrating screen respectively. The material with particle size less than 180 μm was collected and put into a self-sealing bag for subsequent use. Ultimate analysis and proximate analysis of the EP are shown in Table 1.
2.2 Methods
To prepare activated carbons, the crushed and screened EP material was put into a tubular resistance furnace and carbonized at 650 ºC for 120 min in nitro-gen atmosphere at a nitrogen flow rate of 0.5 NL min−1and a heating rate of 10 ºC min−1. After carbonization, the obtained carbon product was put into a nickel crucible and the KOH saturated solution was added according to a mass ratio of KOH to carbonized product of 3∶1. Water was evaporated in the drying oven at 100 ºC and the resulting mixture was activated in an atmosphere muffle furnace. The activation temperature was 800 ºC, the activation time wass 120 min, the heating rate was 10 ºC min−1, the protective atmosphere wass nitrogen, and its flow rate was 1.5 NL min−1. After natural cooling down to ambient temperature under nitrogen atmosphere, the activated carbon was neutralized by 1 mol L−1HCl to pH 7, and then thoroughly washed with deionized water to remove the mineral impurities. The resulting products was dried at 120 °C for 12 h and collected for the subsequent use. The obtained carbon sample was labeled as AC.
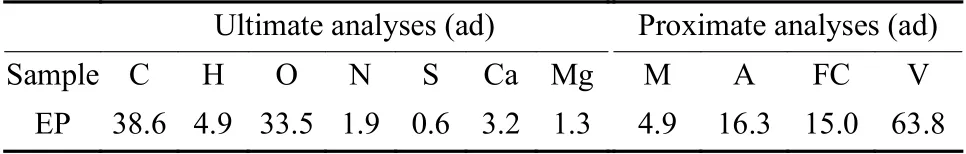
Table 1 Ultimate analysis and proximate analysis of the EP.
To prepare activated carbon with a “Egg-box”morphology, the carbonized EP product was put into 5 mol L−1HCl solution, held in a water bath at 80 °C for 6 h, washed to neutral, dried at 105 °C in a drying oven for 12 h, and then activated by KOH with the same procedure as described above. The prepared activated carbon was labeled as EAC.
To prepare supercapacitors, the electrode slurry was prepared by mixing 80% of AC/EAC, 10% PTFE emulsion and 10% of conductive graphite in appropriate amount of anhydrous ethanol. The slurry was ultrasonically dispersed for 30 min to be fully mixed,then evenly coated on a nickel foam with a diameter of 15.2 mm. The coated nickel foam was placed in a vacuum drying oven and dried at 100 ºC for 12 h to remove excess ethanol on the electrode. After pressed at 12 MPa for 1 min, the dried electrode sheets were assembled into capacitors using a 6 mol L−1KOH solution as the electrolyte.
2.3 Characterization
N2adsorption was carried out at 77 K in an ASAP 2020 (Micromeritics) adsorption instrument.Based on N2adsorption-desorption isotherms, theSBETof AC and EAC were calculated by the BET method,the mesopore and micropore size distributions were determined by BJH and HK method, respectively. To investigate the electrochemical properties of activated carbons, the cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) test and AC impedance measurement were performed on an electrochemical station (CHI760E, CH Instruments).
3 Results and discussion
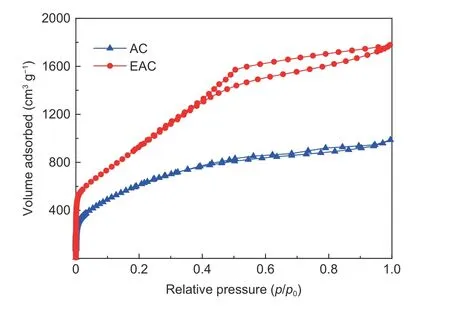
Fig. 1 N2 adsorption-desorption isotherms of AC and EAC.
The pore structure of EP-based activated carbon was characterized by N2adsorption. The N2adsorption-desorption isotherms of AC and EAC are shown in Fig. 1. It can be seen from the figure that the isotherm of AC shows the typical character of the type I,which is characterized by a large amount of N2adsorption in the low pressure range. When the relative pressure is rather low, the adsorption of N2mainly occurs in the micropores, the rapid increase of N2adsorption in this stage indicates that AC has a large number of micropores. With the increase of relative pressure, the N2adsorption increment of AC is almost negligible, a nearly horizontal adsorption platform and a very insignificant hysteresis loop appears in the isotherm, which indicates that AC contains a small number of mesopores. In brief, AC is a typical high microporous carbon material, but it also has a small number of mesopores. Compared with AC, the adsorptiondesorption isotherms of EAC are quite different. The N2adsorption of EAC in low pressure range is still large, but there is no appearance of horizontal platform in relative medium and high pressure ranges of the EAC isotherm. With increasing the relative pressure, the N2adsorption increment of EAC is relatively larger, and the isotherm shows an obvious hysteresis loop and a tail uplift phenomenon. The adsorption isotherm of EAC shows the typical character of the type IV, which indicates that EAC has a wide pore size distribution and a considerable number of mesopores. The pore characteristic of EAC is quite different from that of AC in that the number of mesopores and the range of pore size distribution of EAC are significantly larger. In addition, as can be seen from the adsorption volume of activated carbon in the figure,the N2adsorption capacity of EAC is much higher than that of AC, indicating that the pore volume of EAC is much higher than that of AC.
In order to observe the pore structure of EPbased activated carbon more intuitively, the HK and BJH methods were adopted to calculate the micropore and mesopore size distributions of AC and EAC,respectively, according to the adsorption isotherms of activated carbon. It can be clearly seen from the Fig. 2 that the pore size distribution of AC is relatively narrow, and the pore sizes almost are all within 6 nm. The micropore and mesopore sizes of AC are mainly in 0.5–0.7 nm and 2–4 nm, respectively.Moreover, the number of mesopores decreases gradually with the increase of the pore size. Another obvious character is that the number of micropores is much more than that of mesopores, indicating that AC is a typical high microporous activated carbon. The micropores with diameters of 0.4–1 nm can provide a large amount of inner surface area for the adsorption of electrolyte ions, while the mesopores with diameters more than 2 nm can provide efficient and low resistance transport channels for electrolyte ions[29].Therefore, although AC has enough micropores for energy storage, it does not have an appropriate amount of mesopores for ion transport, which inhibits its electrochemical properties deeply, especially at the high current density. Compared with AC, the number of mesopores in EAC is significantly higher, especially in the range of 2–8 nm in diameter. Significant changes also occurred in the micropore size distribution of EAC, micropores with diameters ranging from 0.5 to 0.6 nm disappear, while the number of micropores with diameters ranging from 0.6 to 1 nm are abundant. The coexistence of micropores and mesopores is beneficial for the efficient transport of electrolyte ions in the pores and the formation of double layer capacitance.
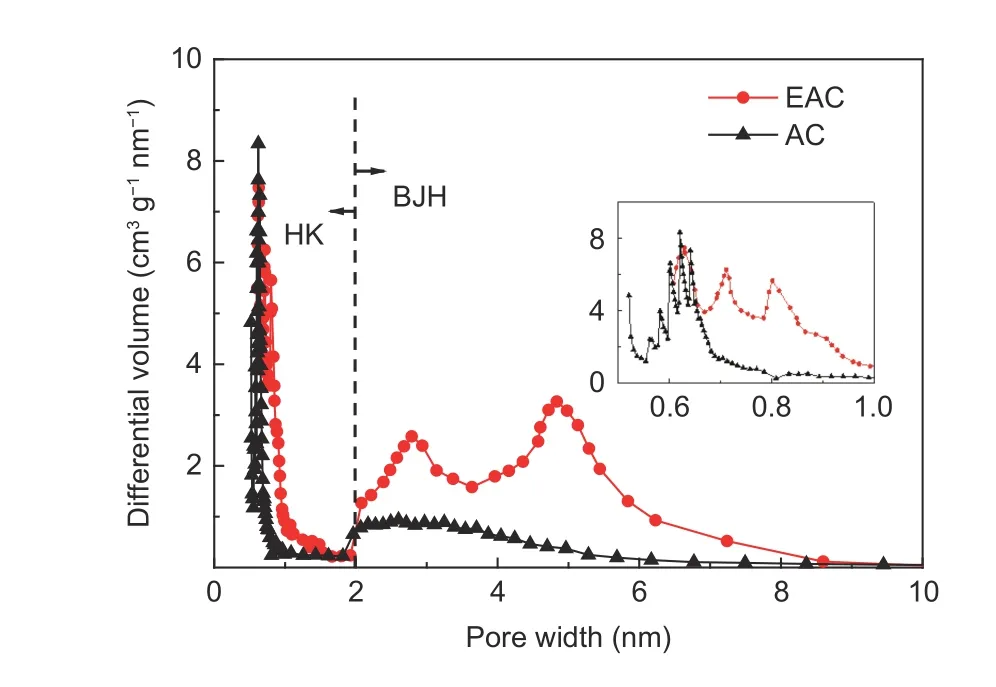
Fig. 2 Pore size distributions of AC and EAC.
The pore texture parameters of AC and EAC are listed in Table 2. As a kind of high microporous activated carbon, theSMicof AC is up to 1 859 m2g−1,while theSMesis only 316 m2g−1, and the value ofSMes/SMicis only 0.17, the number of mesopores is much lower than that of micropores. Compared with AC, theSBETandSMesof EAC are significantly higher,especially theSMesof EAC is 2 178 m2g−1,SMes/SMicis 1.97, indicating a significant percentage of mesopores. TheSMicof EAC is quite low, which is mainly resulted from the disappearance of micropores in the range of 0.5–0.6 nm in size. Even so, EAC still has a large specific surface area contributed from micropores, which can provide enough space for ion adsorption from electrolyte, thus generating a numerous of double layer capacitance. In addition, it can be seen from the pore size distribution of activated carbon that theSMicof EAC is mainly provided by the pores withdiameters of 0.6–1 nm. Compared with the micropore structure of AC, the micropores of EAC have a stronger ability to generate double layer capacitance.TheDMicandDMesof EAC are larger than that of AC,which results in less resistance of EAC pore structure to transport electrolyte ions, and allows more electrolyte ions to pass through at the same time, which is conducive to improving the electrochemical properties of EAC at high current conditions.

Table 2 Characteristics of pores in AC and EAC.
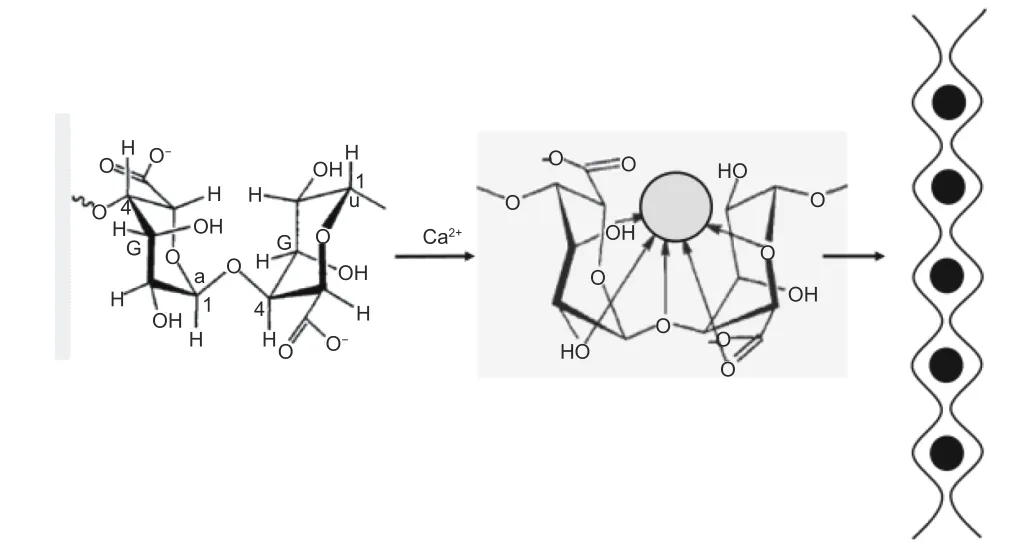
Fig. 3 "Egg-box" structural macromolecular fragments formed by G unit and Ca2+.
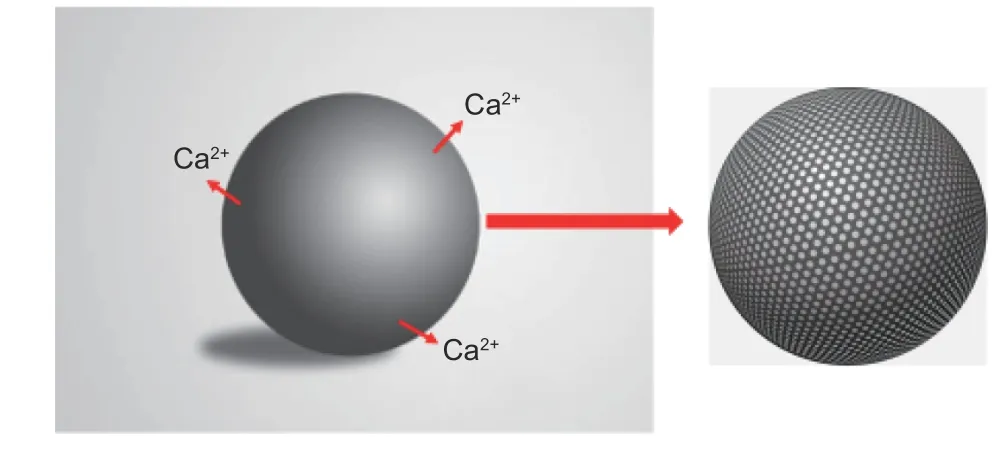
Fig. 4 Principle of pore formation by removing Ca2+ from carbonized products.
As a kind of acid-soluble salt, calcium alginate widely exists in many algae. The G unit in calcium alginate is closely bound with Ca2+ions to form an"egg-box" structure (Fig. 3). The carbonized products of EP with a large number of this "egg-box" structure can be treated with HCl solution to remove Ca2+ions from the "egg-box", thus forming a large number of pores with an “egg-box” structure (Fig. 4). This part of the "egg-box" pore structure can not only provide additional adsorption space for KOH, but also provide efficient transport channels for KOH to enter the raw material, which is beneficial for KOH to enter the raw material, so that the activation reaction can be carried out simultaneously inside and outside the carbonized EP product. The pore structure develops from all directions inside and outside the carbonized EP product,which not only facilitates the formation of pore structure, but also makes the pore structure more uniform and has better connectivity, ultimately leading to better ion transport capacity of EAC in electrolyte. In terms of pore size distribution, the pore structure formed by removal of Ca2+ions in the "egg-box" was further etched during subsequent activation, the pore structure is continuously developed and the average pore size is higher, leading to the collapse of some micropores to form mesopores, resulting in the increase of the mesopore content of EAC.
The surface morphologies of AC and EAC are shown in Fig. 5. The surface of AC is relatively smooth except for some irregular pits formed by carbonization, there is no uniform distribution of regular pits. While the EAC surface has a large number of regular circular pits uniformly distributed, which is resulted from the removal of Ca2+ions in calcium alginate. This indicates that HCl pickling of the carbonized products of EP produces a number of initial pore structures, which play an important role in the development of pore structure of EAC in the subsequent activation of carbon materials. Typical SEM and TEM images (Fig. 6) of the surface of the porous channels,illustrate the existence of a dense distribution of mesopores in EAC.
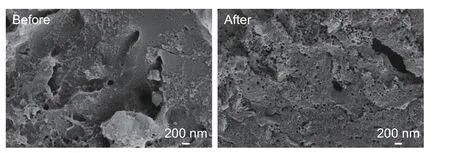
Fig. 5 SEM images of carbonized products of EP before and after HCl pickling.
In order to observe the effect of hydrochloric acid treatment on the element composition of carbonized products, EDS analysis was carried out on the carbonized products before and after pickling, and the results are shown in Fig. 7. As can be seen from the picture, the content of calcium in the carbonized product after pickling is significantly reduced, and the mass fraction of calcium is reduced from 2.57% to 0.19%, indicating that most of the calcium ions in calcium alginate is removed by pickling, and the removal of this part of calcium ions results in the formation of pore structure in the originally occupied space.

Fig. 6 SEM and TEM images of EAC.
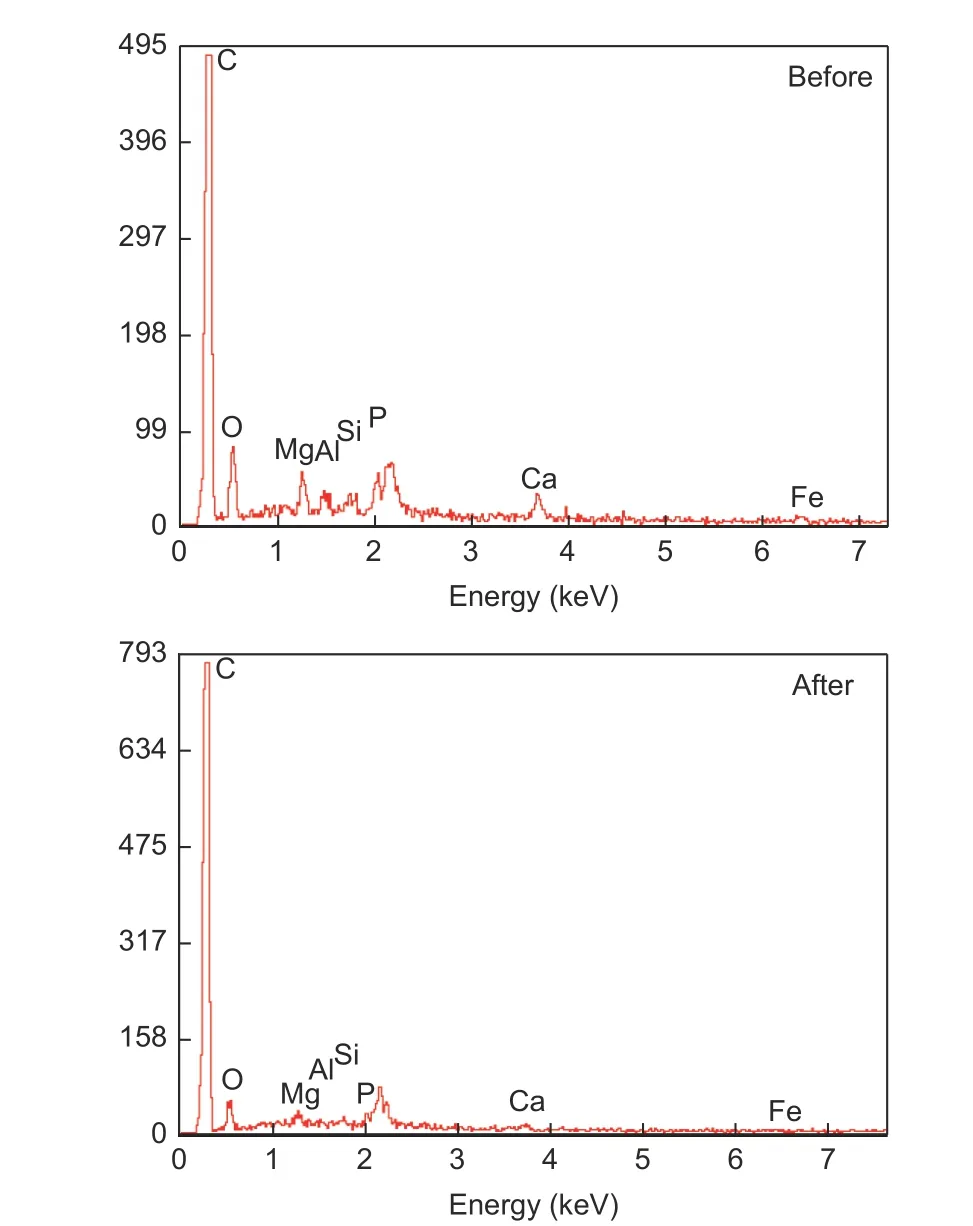
Fig. 7 EDS analysis of carbonization products before and after pickling.
The XRD patterns of AC and EAC are shown in Fig. 8. The XRD patterns for AC and EAC demonstrate two sharp diffraction peaks at 2θ= 23° and 44°,corresponding to (002) and (100) diffraction for carbon. This indicates that AC and EAC have a certain graphite microcrystalline structure[35]. Activated carbons with high specific surface area usually have a relatively poor electrical conductivity due to their abundant pore structures, the existence of graphite microcrystalline can greatly improve their electrical conductivity, which is favorable for the improvement of the electrochemical performance. In addition, it can be found from the XRD patterns that in addition to the diffraction peaks of graphite, there is also the presence of silica crystal peaks (2θ= 29° and 47°). The presence of silicon dioxide will not only reduce the gravimetric capacitance of activated carbon, but also increase the resistance of the supercapacitors, deteriorating the electrochemical stability of the supercapacitor.

Fig. 8 XRD patterns of AC and EAC.
The surface functional groups are associated with the electrochemical performance of the carbon materials by influencing their wettability, polarity and stability[36–40]. In addition, the existence of some surface functional groups can improve the capacitance by generating pseudocapacitance[41]. The FTIR spectra of AC and EAC are shown in Fig. 9. The band is observed around the region of 1 050 cm−1could be attributed to the stretching vibrations of the C―O bonds of esters, alcohols, phenols or ethers[42]. The band at 1 779 cm−1, observed in the spectrum is attributed to the C=O stretching vibration of nonaromatic carboxyl groups with higher intensity in the spectrum resulted from the partial dehydrogenation[43]. The presence of the above two surface functional groups contributes to the improvement of the electrochemical performance of activated carbon in alkaline electrolytes. In addition, the bands at 2 661 cm−1in AC and EAC might be ascribed to the traces of potassium carbonates active centers beside metallic potassium that could be produced at 873 K[44,45]. This indicates that in spite of the extremely prolonged washing, a trace amount of potassium remains chemical bounded inside the pore structure.
Gravimetric capacitance is one of the most important electrochemical property of supercapacitors[46].In order to study the gravimetric capacitances of AC and EAC, the galvanostatic charge-discharge (GCD)curves of supercapacitors at different current densities were measured by the electrochemical workstation. The GCD curves of AC and EAC at the different current densities are shown in Fig. 10. The GCD curves of AC and EAC show good isosceles triangle characteristics, which indicates that the supercapacitors based on EP-based activated carbon have excellent double layer capacitance characteristics and basically no existence of pseudocapacitance[47]. At the current density of 0.5 A g−1, the charging and discharging time of EAC is significantly longer than that of AC, indicating that EAC has larger charge storage capacity and higher gravimetric capacitance. Moreover,the voltage drop of AC is very obvious, but that of EAC is hardly observed. The voltage drop is caused by the equivalent series resistance of supercapacitors,which indicates that EAC has a smaller equivalent series resistance than AC.
It can be observed from Fig. 11 that the CV curves of EAC and AC show excellent rectangular shapes from 0 to 1 V over a wide range of voltage scan rates, indicating an ideal electrical double layer effect of the tested supercapacitors. Moreover, absence of any redox peaks at CV curves implies the absence of pseudocapacitance. When the supercapacitors are measured at a relatively high scan rate of 200 mV s−1, the CV curves still show a rectangular shape, indicating that the EAC and AC electrodes possess relatively smaller equivalent series resistance and strong ion transport ability, leading to the quick charge propagation in carbon electrodes.
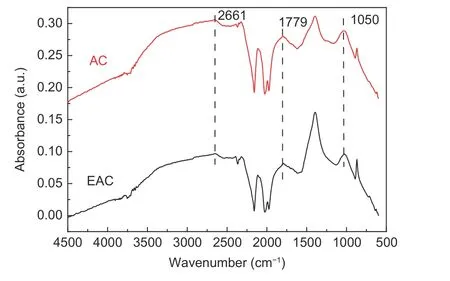
Fig. 9 FT-IR spectra of the AC and EAC.
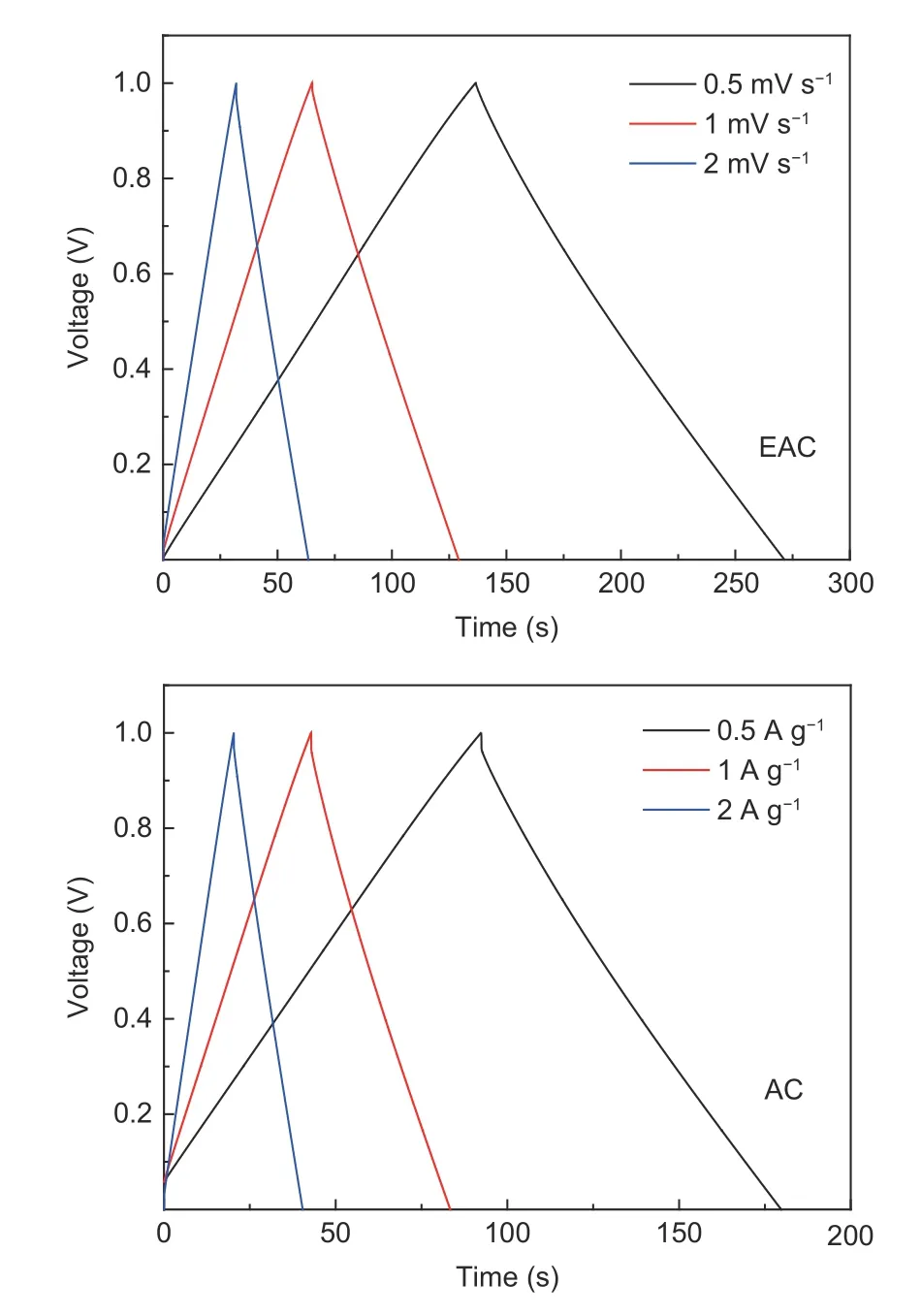
Fig. 10 GCD curves of AC and EAC at the different current densities.
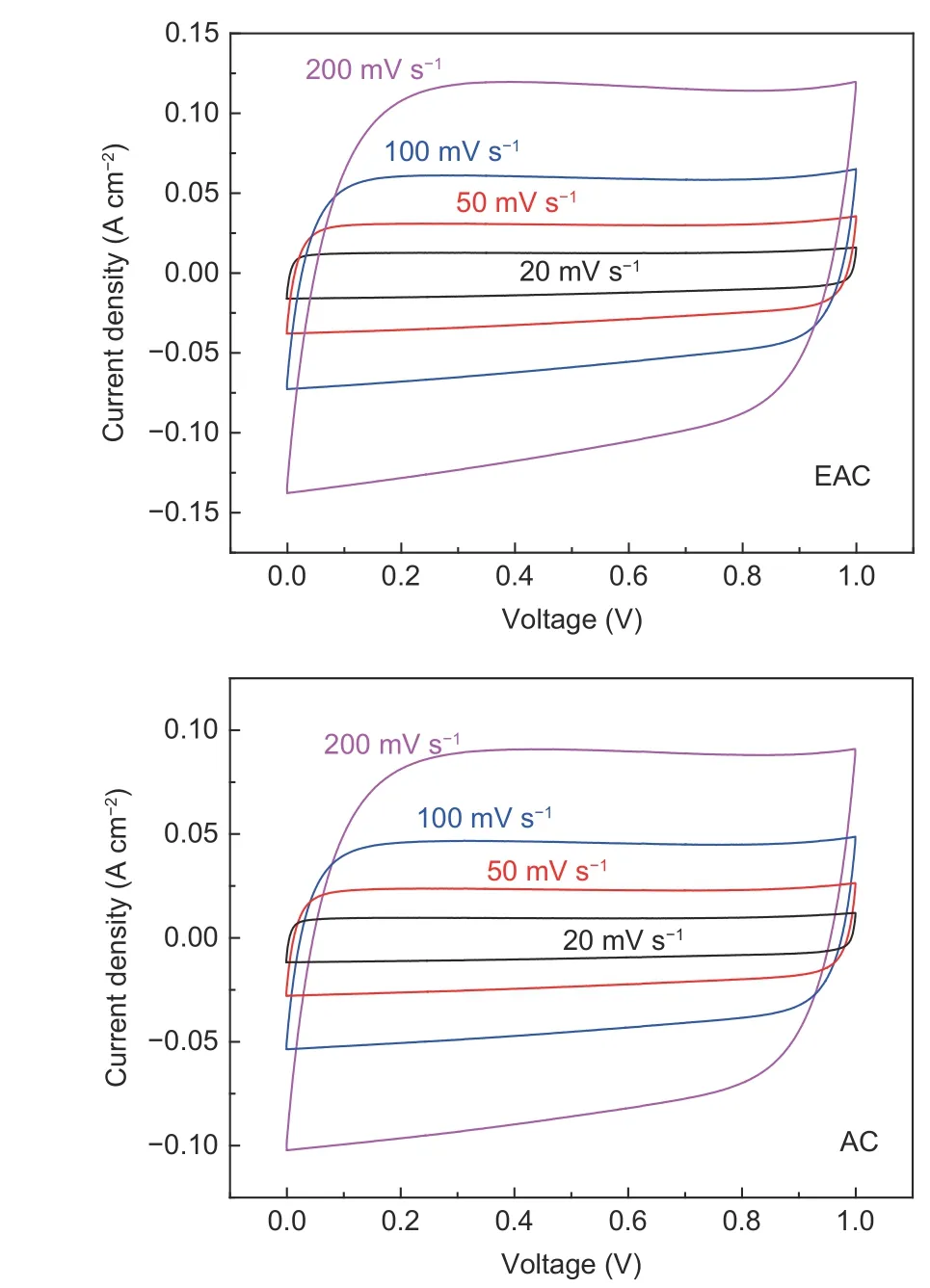
Fig. 11 CV curves of EAC and AC.
The gravimetric capacitances of AC and EAC at different current densities are listed in Table 3. It can be seen from the table that the gravimetric capacitances of EAC are much higher than that of AC at all current densities.
Rate performance is an important requirement for high power applications of supercapacitors[48]. For carbon-based supercapacitors, the transport resistance of electrolyte ions in the pores is an important component of the equivalent series resistance of supercapacitors. Especially when electrolyte ions are transported in the micropores, the transport resistance of ions is very obvious, which increases with the charge-discharge current density, resulting in a negative impact on the rate performance of supercapacitors[49]. The rate performance of AC and EAC are shown in Fig. 12.The gravimetric capacitances of AC and EAC decrease gradually with the increase of charge-discharge current density, but both have high capacitance retention rates. When the current density increases from 0.1 A g−1to 10 A g−1, the gravimetric capacitances of AC and EAC decrease from 253 F g−1and 361 F g−1to 202 F g−1and 323 F g−1and the capacitance retention rates are 79.8% and 89.4%, respectively. Compared with AC, the rate performance of EAC is improved obviously. This is caused by the high mesopore content and average pore size in EAC,
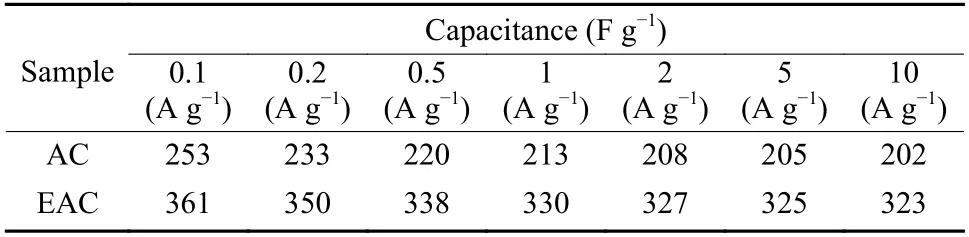
Table 3 Gravimetric capacitance of AC and EAC at different current densities.
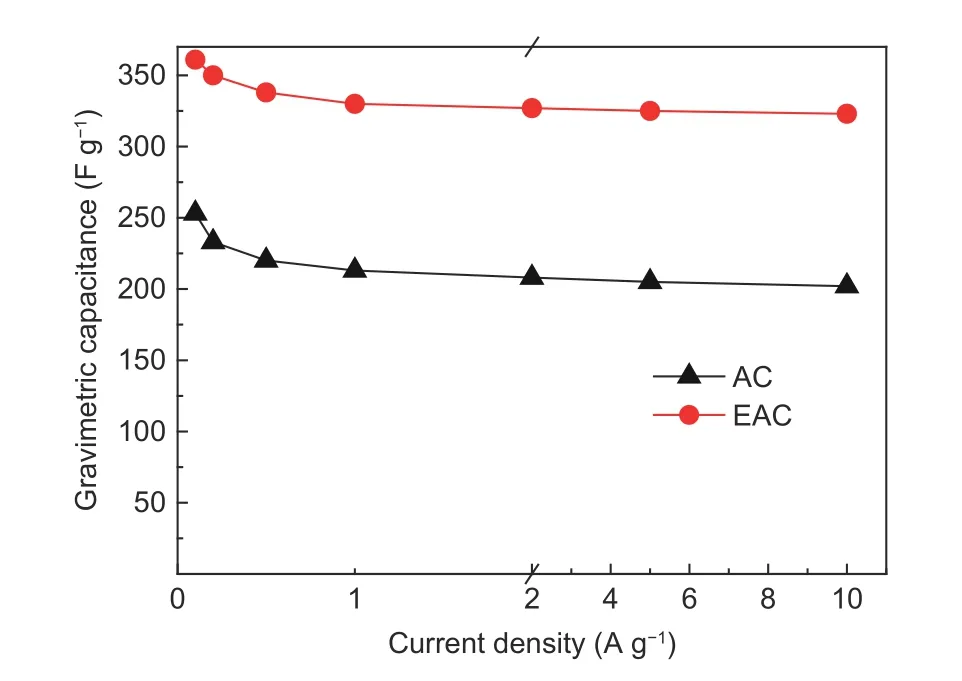
Fig. 12 Rate performance of AC and EAC.
where more electrolyte ions are allowed to pass through the pore cross section at the same time. In addition, the pore structure of EAC develops from both inside and outside of the raw material in the activation process, which improves the connectivity of the pore structure and facilitates the rapid transport of electrolyte ions in the pores. As a result, EAC has better rate performance and can be better applied to high power applications than AC.
The cyclic stability of carbon electrodes is directly related to the service life of supercapacitors[50].Fig. 13 shows the cyclic characteristics of AC and EAC at a current density of 5 A g−1for 10 000 cycles.Since the energy storage in AC and EAC-based supercapacitors are highly dominated by the double layer capacitance, the charge and discharge processes are almost completely reversible. An obvious increase of capacitance retention rate is observed after the HCl pickling pretreatment, the retention values of EAC and AC after 10 000 cycles are 95.4% and 92.2%, respectively, which indicates that EAC has better cycle stability in the electrolyte when it is used as an electrode material. In addition, a negligible capacitance fading is observed after 3 000 cycles.
The Nyquist impedance plots of AC and EAC are shown in Fig. 14. In the Nyquist impedance plots, the equivalent series resistance is the impedance value corresponding to the intersection point of the high frequency arc region and the real axis of the impedance spectrum, which shows that the equivalent series resistance of AC and EAC are very small. The Nyquist impedance plots of the AC and EAC are vertical curves at low frequencies, demonstrating an ideal capacitive behavior of the AC- and EAC-based supercapacitors. Moreover, the EAC impedance curve has a shorter transition domain in the plot than that of AC,suggesting a much smaller charge transfer resistance against the electrolyte penetration into the pores and channels. The equivalent resistance of the electrical double layer supercapacitors can be simplified as shown in Fig. 14(b), whereRsis the series resistance related to the electrolyte impedance,Cdlis capacitor resistance due to electrode/electrolyte interface. Compared with AC, the mesopore content and average pore size of EAC are significantly higher, and the pore connectivity is better, which contribute to the reduction of impedanceRsduring ion transport of electrolyte, resulting in the reduction of series resistance of EAC.
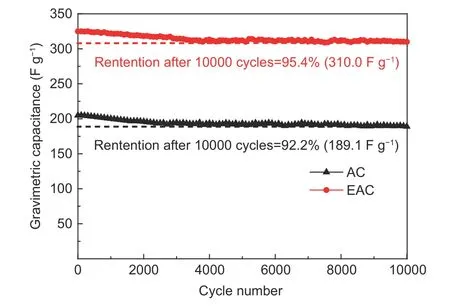
Fig. 13 Cycle performance of AC and EAC at the current density of 5 A g−1.
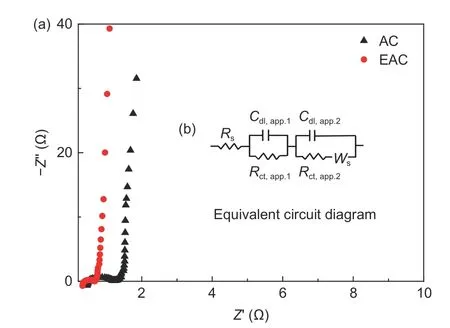
Fig. 14 Nyquist impedance plots of AC- and EAC-based supercapacitors.
4 Conclusion
A hierarchical porous carbon with excellent electrochemical properties was prepared from EP by pretreatment of carbonized EP before KOH activation,leading to the formation of an “egg-box” morphology that is favorable for the formation of a hierarchical porous pore structure. The results show that theSBETandSMesof EAC are significantly higher compared with AC. TheSMesof EAC is 2 178 m2g−1,SMes/SMicis 1.97, while theSMesandSMes/SMicvalues of AC are only 316 m2g−1and 0.17, respectively. This indicates that the mesopore content of EAC is much higher than that of AC. In addition, the micropore size distribution of activated carbon is also changed significantly by the pretreatment. The micropores in EAC with diameters between 0.5 and 0.6 nm basically disappear,but the number of micropores with diameters between 0.6 and 1 nm is significantly higher, and the average pore size is also larger as compared with AC. This change in micropore structure leads to a decrease in theSMicof EAC, but still maintains a large value. As the pore structure of activated carbon has been improved by the pretretment, the gravimetric capacitance and rate performance of EAC are obviously better than that of AC. The gravimetric capacitance of EAC reaches up to 361 F g−1at the current density of 0.1 A g−1, while that of AC is only 253 F g−1.When the current density increases from 0.1 A g−1to 10 A g−1, the capacitance retention values of EAC and AC are 89.4% and 79.8%, respectively. Due to the increase of the mesopore content in EAC and the improvement of pore connectivity, the impedance property of EAC is also improved.
Acknowledgements
This study was supported by the Doctoral Fund of Shandong Jianzhu University (XNBS1838).
- 新型炭材料的其它文章
- High-surface-area porous carbons produced by the mild KOH activation of a chitosan hydrochar and their CO2 capture
- A DFT study of the effect of stacking on the quantum capacitance of bilayer graphene materials
- 基于碳化钽涂层改性碳基材料的研究进展
- Preparation of a N-P co-doped waste cotton fabric-based activated carbon for supercapacitor electrodes
- Coating a Na3V2(PO4)3 cathode material with carbon to improve its sodium storage
- Two-dimensional layer materials for highly efficient molecular sensing based on surface-enhanced Raman scattering

