Coexistence of Two Unique Cu(Ⅱ) Ions in Mononuclear Cu(Ⅱ)Complexes with Furanyl Substituted Triaryltriazoles
GAO Jian‑FeiNIE Rong‑RongFENG ZheZHU Dun‑Ru*,,2
(1College of Chemical Engineering,State Key Laboratory of Materials⁃Oriented Chemical Engineering,Nanjing Tech University,Nanjing 211816,China)
(2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210023,China)
Abstract:Two new mononuclear Cu(Ⅱ) complexes,trans‑[Cu(L1)2(NO3)2]0.5[Cu(L1)2(H2O)2]0.5(NO3)(1)and trans‑[Cu(L2)2(NO3)2]0.5[Cu(L2)2(H2O)2]0.5(NO3)·2CH3OH(2)(L1=3‑(2‑pyridyl)‑4‑phenyl‑5‑(2‑furanyl)‑1,2,4‑triazole,L2=3‑(2‑pyridyl)‑4‑(p‑fluorophenyl)‑5‑(2‑furanyl)‑1,2,4‑triazole),were synthesized and characterized by FT‑IR,elemental analyses,thermogravimetric analyses,single‑crystal X‑ray crystallography and powder X‑ray diffraction.Both complexes 1 and 2 crystallize in triclinic system with space group P1.The crystal structure analysis shows that there are two crys‑tallographically different Cu(Ⅱ) ions in 1 and 2 and each Cu(Ⅱ) ion lies in a distorted octahedral[CuN4O2]environ‑ment.However,in the axial position one Cu(Ⅱ) ion is coordinated with two water molecules,while another Cu(Ⅱ) ion with two nitrate ions.The L1or L2ligand coordinates with Cu(Ⅱ)via one pyridine N atom and one triazole N atom,while the furanyl group does not participate in coordination.In 1 and 2 there are some intermolecular C—H…N,C—H…O,O—H…O hydrogen bonding and C—H…π interactions,linking the mononuclear complex(1 or 2)to form a two‑dimensional network.CCDC:2053558,1;2053559,2.
Keywords:furan;Cu(Ⅱ)complex;crystal structure;triaryltriazole
0 Introduction
1,2,4‑triazole derivatives as a kind of important ni‑trogen‑rich heterocyclic compounds have been widely applied in biological science,coordination chemistry,material chemistry,medicinal chemistry and organic synthetic chemistry because of their unique structures and chemical properties[1‑4].Especially,3,4,5‑ triaryl‑substituted 1,2,4‑triazoles have received extensive at‑tention in coordination chemistry owing to their versa‑tile coordination modes[5‑7].It is worthwhile to note that some Cu(Ⅱ) complexes based on triaryltriazoles can ex‑hibit interesting antimicrobial and anticancer proper‑ties[8‑9].Recently,a series of Cu (Ⅱ) complexes based on triaryltriazoles with pyridyl,quinolyl,thienyl groups have been reported by our group[10‑16].However,2‑furanyl substituted triaryltriazoles have been less explored till now[17‑19].As a continued research on the triaryltri‑azoles,we designed and synthesized two new 2‑furanyl substituted triaryltriazoles:3‑(2‑pyridyl)‑4‑phenyl‑5‑(2‑furanyl)‑1,2,4‑triazole(L1)and 3‑(2‑pyridyl)‑4‑(p‑fluorophenyl)‑5‑(2‑furanyl)‑1,2,4‑triazole(L2)(Scheme 1).Herein,we report the syntheses,crystal structures and thermogravimetric analyses of two mononuclear Cu(Ⅱ)complexes with the synthesized ligands:trans‑[Cu(L1)2(NO3)2]0.5[Cu(L1)2(H2O)2]0.5(NO3) (1) and trans‑[Cu(L2)2(NO3)2]0.5[Cu(L2)2(H2O)2]0.5(NO3)·2CH3OH(2).Notably,it is first observed that two distinct Cu(Ⅱ)ions coexist in these mononuclear Cu(Ⅱ)complexes with furanyl substituted triaryltriazoles[20].
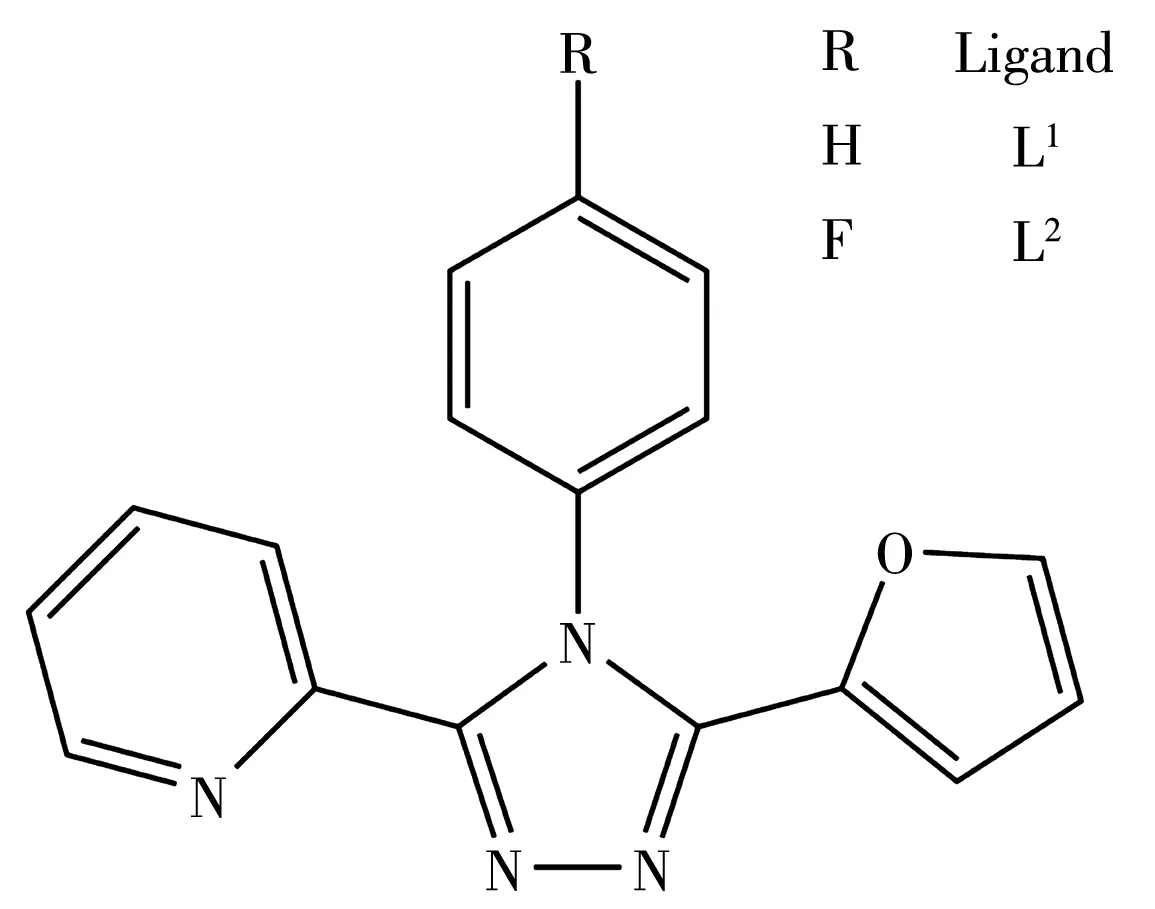
Scheme 1 Structures of ligands L1and L2
1 Experimental
1.1 Materials and measurements
All chemicals used were of analytical grade.Solvents were purified by conventional methods.The ligands L1and L2were synthesized via a similar litera‑ture method[21].Elemental analyses(C,H,N)were car‑ried out with a Thermo Finnigan Flash 1112A elemen‑tal analyzer.IR spectra were recorded on a Nicolet Avatar 380 FT‑IR instrument with KBr pellets in a range of 4 000~400 cm-1.Thermogravimetric analysis(TGA)was performed with a simultaneous NETZSCH STA 449C thermal analyzer under flowing nitrogen from 25 to 800℃ at a heating rate of 5℃·min-1.Powder X‑ray diffraction(PXRD)data were collected on a Bruker D8 ADVANCE diffractometer using Cu Kα radiation(λ=0.154 06 nm)at 40 kV and 40 mA in a range of 5°~50°.
1.2 Syntheses of complexes 1 and 2
trans‑[Cu(L1)2(NO3)2]0.5[Cu(L1)2(H2O)2]0.5(NO3)(1):A solution of Cu(NO3)2·3H2O(0.125 mmol)in water(1 mL)was added to a solution of L1(0.25 mmol)in MeOH(1 mL).The mixture was stirred for 4 h at room temperature.A resulting green product was filtered and washed with H2O,then dried under vacuum to give 0.116 mmol(92.8%)of complex 1.The well‑shaped single crystals of 1 suitable for X‑ray diffraction were obtained by slow evaporation from the MeOH solution of the complex.Elemental analysis Calcd.for C34H26CuN10O9(%):C,52.21;H,3.35;N,17.91.Found(%):C,52.46;H,3.17;N,17.76.FT‑IR(KBr,cm-1):3 479(w),3 058(m),3 011(w),1 615(m),1 504(s),1 384(vs),1 308(s),1 021(m),751(m),695(m).
trans‑[Cu(L2)2(NO3)2]0.5[Cu(L2)2(H2O)2]0.5(NO3)·2CH3OH(2):The prepared procedure was the same as that for 1 except using L2(0.25 mmol)to replace L1.Yield: 87.9%. Elemental analyses Calcd. for C36H32CuF2N10O11(%):C,49.01;H,3.66;N,15.88.Found(%):C,49.33;H,3.45;N,16.09.FT‑IR(KBr,cm-1):3 351(w),2 923(w),1 613(m),1 515(vs),1 384(s),1 149(m),836(m),743(m).
1.3 Crystal structure determination
The well‑shaped single crystals of 1 and 2 were selected for X‑ray diffraction study.The unit cell parameters and intensity data were collected at 296(2)K on a Bruker SMART APEXⅡCCD diffractometer using a graphite‑monochromated Mo Kα (λ=0.071 073 nm)radiation.The structures were solved by directmethods and refined onF2by full‑matrix least squares procedures using SHELXTL software[22].Allnon‑hydrogen atoms were refined anisotropically.All hydro‑gen atoms were fixed in calculated positions and refined isotropically.The coordinated NO3-ions in 2 were disordered over two positions with an occupancy of 0.698(13)for N9,O3,O4 and O5 and 0.302(13)for N9A,O3A,O4A and O5A.The crystallographic data of 1 and 2 are listed in Table 1 and the selected bond lengths and angles are provided in Table 2.
CCDC:2053558,1;2053559,2.
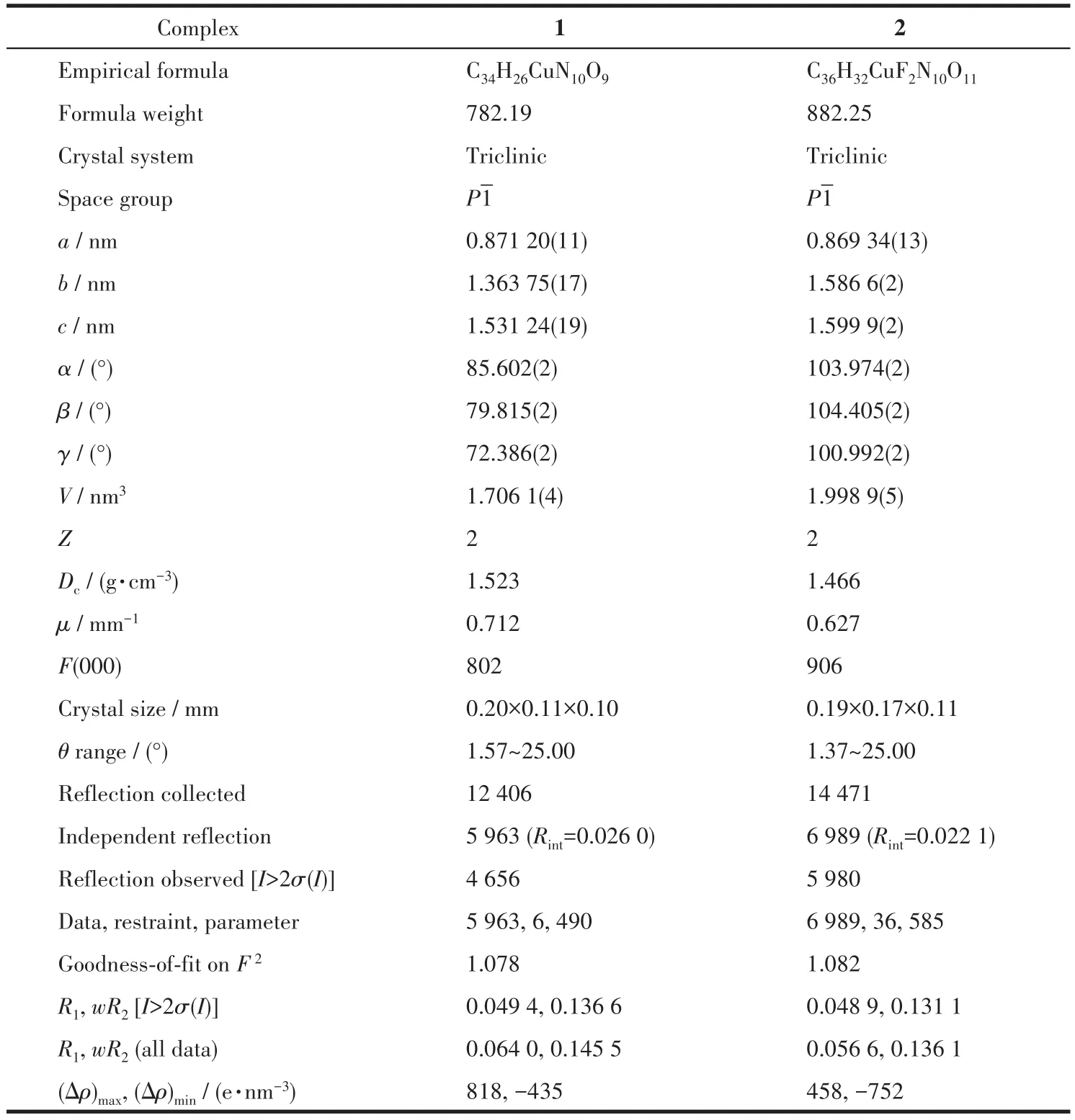
Table 1 Crystal data and structure refinements for 1 and 2
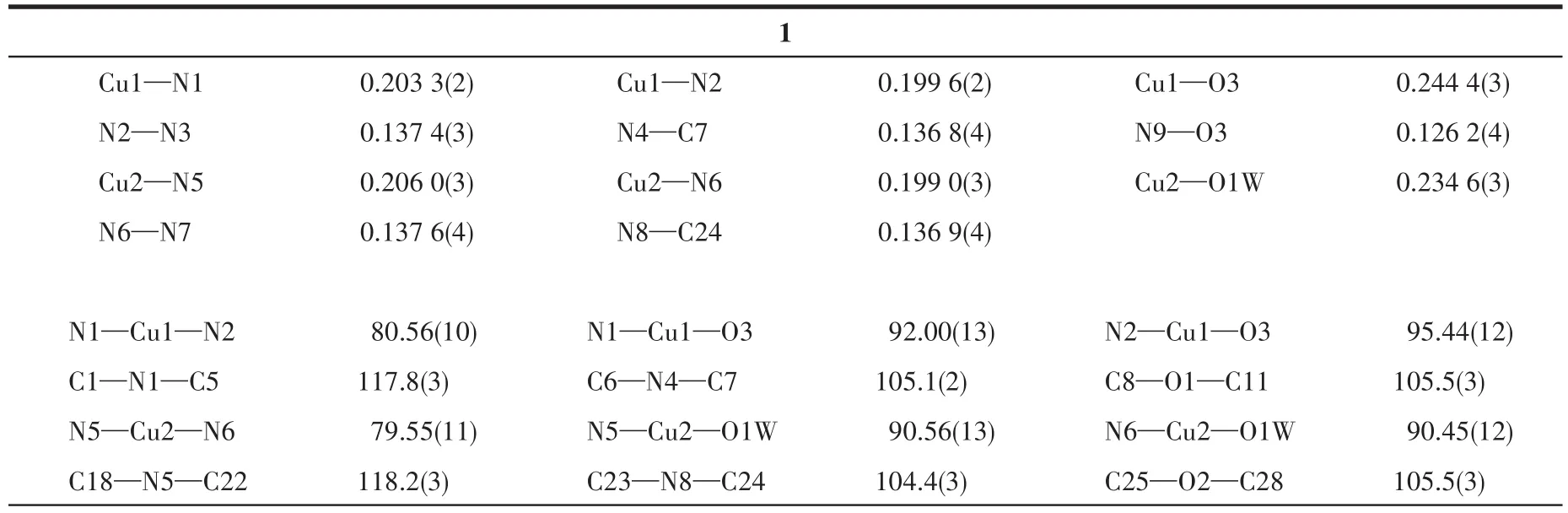
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2

Continued Table 2
2 Results and discussion
2.1 Synthesis
Asymmetrically 2‑furanyl substituted 3,4,5‑triaryl‑1,2,4 ‑triazoles(L1and L2)reacting with Cu(NO3)2·3H2O in molar ratio of 2∶1 produced two mononuclear complexes,trans‑[Cu(L1)2(NO3)2]0.5[Cu(L1)2(H2O)2]0.5(NO3)(1)andtrans‑[Cu(L2)2(NO3)2]0.5[Cu(L2)2(H2O)2]0.5(NO3)·2CH3OH(2),which were stable in air.Yields for 1 and 2 were 92.8% and 87.9%,respectively.The elemental analyses were satisfactory and reveal that both 1 and 2 contain one Cu(Ⅱ)ion,two triazole ligands(L1for 1 and L2for 2),two NO3-ions and one water molecule,except that 2 still has two methanol molecules.
2.2 Crystal structures of 1 and 2
The projection of structures of 1 and 2 is shown in Fig.1 together with the atomic labeling system.Both 1 and 2 crystallize in the triclinic space groupP1 and an inversion center is found in the Cu(Ⅱ)ion.Because 1 and 2 have a similar structure,herein,only the struc‑ture of 1 is described detailedly.The asymmetric unit of 1 contains two crystallographically different Cu(Ⅱ)ions(the occupancy factors of both Cu1 and Cu2 are 0.5),two L1ligands,two NO3-ions and one H2O mole‑cule,which is in consistent with the elemental analysis result.Both Cu1 and Cu2 ions lie in a distorted octahe‑dral[CuN4O2]environment with four N atoms from two L1ligands in the equatorial plane.However,in the axi‑al position the Cu1 ion is coordinated by two nitrate ions,while the Cu2 ion is coordinated by two water molecules.Each L1ligand coordinates to Cu1(or Cu2)via N1(or N5)atom of the pyridyl and N2(or N6)atom of the triazole,while the 2‑furanyl group does not par‑ticipate in coordination.It is worthwhile to note that two distinct Cu(Ⅱ) ions are observed to coexist in pres‑ent mononuclear Cu(Ⅱ)complexes for the first time,which is quite different from the mononuclear Cu(Ⅱ)complexes with the quinolyl or thienyl substituted triar‑yltriazoles[15‑16].The distance of Cu1—O3(0.244 4 nm)is longer than the Cu2—O1W one(0.234 6 nm)(Table 2).The Cu—N bond lengths are within the normal ranges found for the octahedral Cu (Ⅱ) complexes[10‑16].The ligand L1in 1 is non‑planar.The triazole coordinat‑ing to Cu1 makes dihedral angles of 47.5(2)°,8.4(2)°and 82.1(2)°with the 2‑furanyl ring,the pyridyl ring and phenyl ring,respectively,while the triazole coordi‑nating to Cu2 makes dihedral angles of 5.5(2)°,5.3(2)°and 88.9(2)°with the 2‑furanyl ring,the pyridyl ring and phenyl ring,respectively(Table 3).The corre‑sponding dihedral angles in L2of 2 are also given in Table 3.
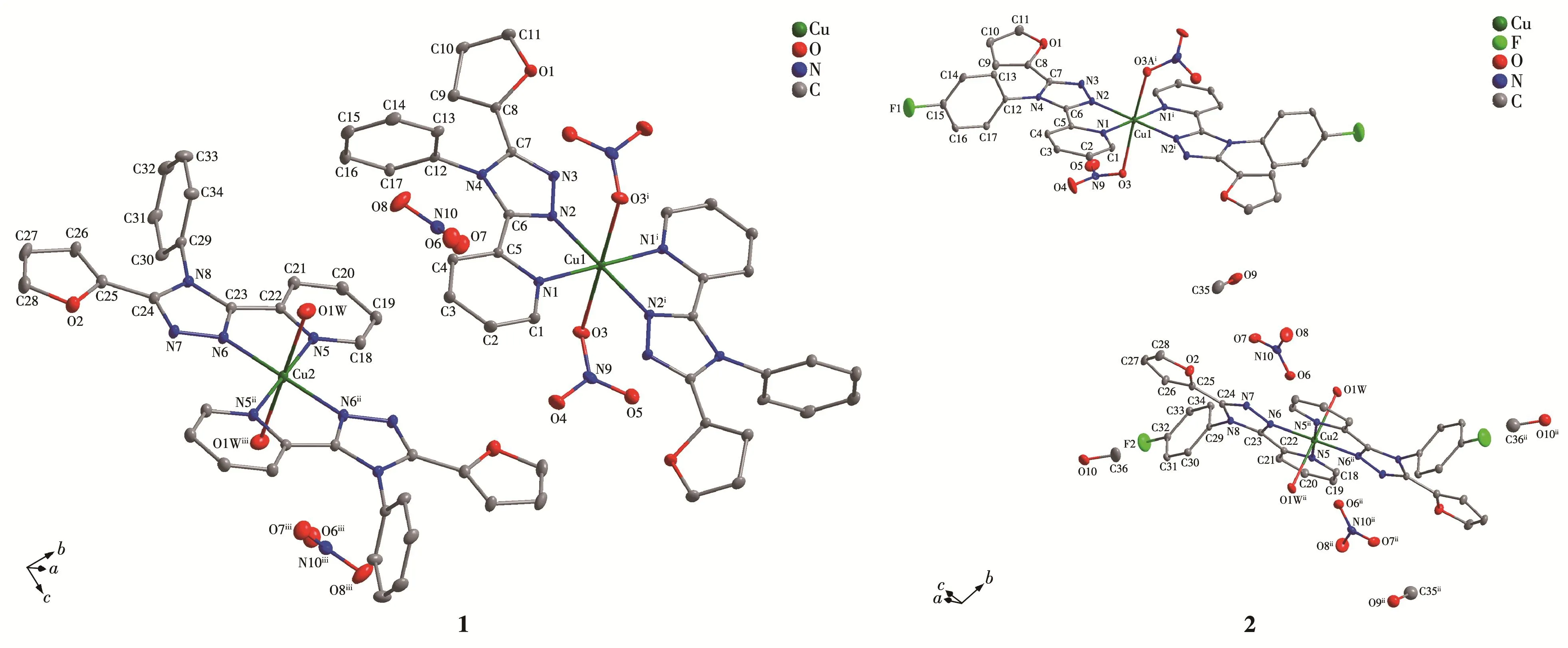
Fig.1 Projection of structures of 1 and 2 with 10% thermal ellipsoids probability
There are rich intermolecular hydrogen bonds and C—H…πinteractions in 1(Fig.S1,Table S1 in Sup‑porting information),associated with the closer crystal packing.These hydrogen‑bond interactions include:(1)between pyridyl and triazole rings(C1—H1A…N3iand C18—H18A…N7iii);(2)between pyridyl rings andNO3-anions(C2—H2A…O5ii,C3—H3A…O3iiand C20—H20A…O8);(3)between furan group and NO-3anion(C9—H9A…O8);(4)between phenyl and NO-3anions(C13—H13A…O5i,C17—H17A…O6,C30—H30A…O4ivand C34—H34A…O7ii);(5)between wa‑ter molecule and NO3-anions(O1W—H1WA…O7iiand O1W—H1WB…O3ii).In addition,there are an in‑termolecular edge‑to‑face C—H…πinteraction(C11—H11A…π(Ph)v)and three intramolecular edge‑to‑face C—H…πinteractions(C4—H4A…π(Ph),C21—H21A…π(Ph)and C26—H26A…π(Ph)).These extensive hydrogen‑bond and C—H…πinteractions connect the mononuclear units,NO3-anions and lattice water mole‑cules into a 2D layer(Fig.2).The corresponding hydro‑gen‑bonding and C—H…πinteractions of 2 are also shown in Fig.S1 and Table S1.
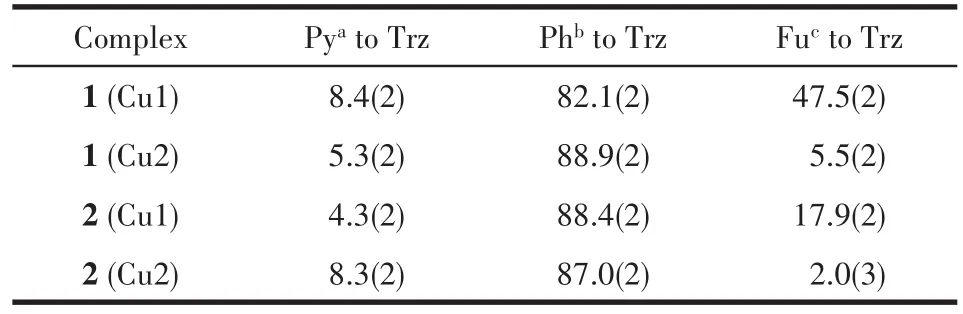
Table 3 Dihedral angles(°)for 1 and 2
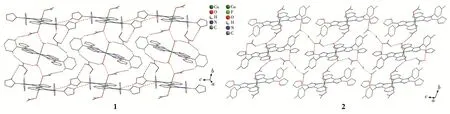
Fig.2 Two‑dimensional networks of 1 and 2
2.3 IR spectra
In the IR spectra of 1 and 2,a weak broad peak at 3 479 cm-1(1)or 3 370 cm-1(2)can be attributed to the O—H stretching vibrations of water molecules.A medi‑um band at 1 615,1 504 cm-1(1)or 1 613,1 515 cm-1(2)can be assigned to the coordinated pyridyl ring vi‑bration[11].A strong band at 1 384 cm-1is assigned to characteristic N=O stretching vibrations of NO3-in 1 and 2[15].In addition,the stretching vibration of F—C(Ph)in 2 can be observed at 1 149 cm-1[23].These fea‑tures are in consistent with the results of the X‑ray analyses.
2.4 Thermal stability and PXRD
As shown in Fig.3,the first weight loss of 2.3% between 25 and 178℃for 1 was observed due to the loss of one coordinated water molecule(Calcd.2.3%).Then 1 started to decompose.The remaining weight of 10.0% after heating to 500 ℃ is owing to the final resi‑due of CuO(Calcd.10.2%).
The first weight loss of 9.0% between 25 and268 ℃ in 2 was found because of the loss of one coordi‑nated water molecule and two lattice methanol mole‑cules(Calcd.9.3%).Then 2 began to collapse.The remaining weight of 10.8% after heating to 500℃is due to the final residue of CuO,in agreement with the calculated value of 9.2%.
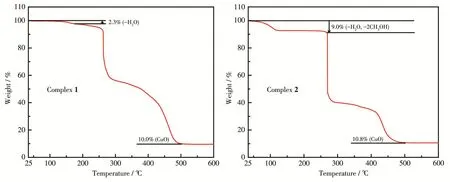
Fig.3 TGA curves of 1 and 2
The PXRD patterns and simulated ones of 1 and 2 are shown in Fig.S2.The experimental patterns were in well consistent with the simulated ones from the single X‑ray crystal data,indicating the high phase purity of the bulk products of 1 and 2.
3 Conclusions
Two new mononuclear Cu(Ⅱ)complexes based on the 2‑furanyl substituted triaryltriazoles have been syn‑thesized and structurally characterized by FT‑IR and X‑ray crystallography.It is first observed that two dis‑tinct Cu(Ⅱ)ions coexist in these mononuclear Cu(Ⅱ)complexes with furanyl substituted triaryltriazoles.
Supporting information is available at http://www.wjhxxb.cn
- 无机化学学报的其它文章
- Effect of Zn on Photocatalytic Activity of Block⁃Shaped Monoclinic WO3
- Synthesis,Structure,Luminescence,Photocatalytic and Magnetic Properties of a Neodymium Complex Constructed from Biphenyl⁃3,4′,5⁃tricarboxylic Acid
- Preparation and Nonlinear Absorption Properties of SiO2@CdTe@Au Composite Nanoparticles
- First⁃Principles Calculations on Electronic Structures and Optical Properties of g⁃C3N4Nanoribbons
- 二茂铁基-双酮锌配合物的合成、电化学活性及多光子吸收
- 基于(E)⁃N,N⁃二甲基⁃4⁃(2⁃(吡啶⁃4⁃基)乙烯基)苯胺的锌/镉有机-无机杂化金属卤化物的结构和发光性质

