Synthesis,Structure,Luminescence,Photocatalytic and Magnetic Properties of a Neodymium Complex Constructed from Biphenyl⁃3,4′,5⁃tricarboxylic Acid
ZHENG Huan JIAO Yuan FENG Si‑Si
(Institute of Molecular Science,Key Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education,Shanxi University,Taiyuan 030006,China)
Abstract:A Nd3+MOF,namely{[Nd(bpt)(DMF)(H2O)]·2H2O}n(1)(H3bpt=biphenyl‑3,4′,5‑tricarboxylic acid,DMF=N,N‑dimethylformamide),has been obtained by the self‑assemble reaction of NdCl3·6H2O and H3bpt ligand under solvothermal conditions(DMF/H2O).Complex 1 was characterized by means of elemental analysis,infrared spectros‑copy,powder X‑ray diffraction and single‑crystal X‑ray diffraction analyses.Single‑crystal X‑ray structural analysis reveals that complex 1 displays three‑dimensional frameworks featuring a(5,5)‑connected topological network with the point symbol of(44·63·83)(4862).In addition,the thermal stability,luminescent properties,photocatalytic behav‑iour,and magnetic property of complex 1 were also investigated in detail in the solid state.CCDC:2074972.
Keywords:Nd3+metal‑organic framework;biphenyl‑3,4′,5‑tricarboxylic acid;crystal structure;luminescence;photocatalysis;magnetism
0 Introduction
Metal‑organic frameworks(MOFs)constructed by coordination bonds between metal ions and organic ligands have attracted much attention not only because of their diverse striking structural topologies,but also for their wide range of potential applications,such as gas adsorption and separation,catalysis,luminescence,magnetism,chemical sensing,drug delivery[1‑5].In recent years,novel photocatalytic materials based on MOFs have been extensively studied due to their adjustable porosity,high crystallinity and semiconduc‑tor properties,which are largely driven by the need for green degradation of organic pollutants[6‑7].These appli‑cations make MOFs important functional materials.More significantly,these applications can be combined and integrated into individual framework to form multi‑functional MOFs[8‑10].
Lanthanide metal‑organic frameworks(Ln‑MOFs)are well known for their combination of both photolumi‑nescent centers and magnetic properties because of the characteristic of central cations,making them attrac‑tive candidates for the development of novel multifunc‑tional materials[11‑14].Moreover,proper organic bridging linkers are also significant for assembling the required MOFs materials.As is well‑known,multicarboxylate ligands are commonly used in the architectures of Ln‑MOFs due to their powerful topological and struc‑tural diversity capabilities[15‑17].Moreover,multicarbox‑ylate ligands are good candidates for H‑bonded accep‑tors and donors which serve as an effective building block for the formation of supramolecular skeleton[18].
In recent years,biphenyl‑3,4′,5‑tricarboxylic acid(H3bpt),a planar rigid ligand with three carboxylate groups,has attracted extensive attention[19‑24].The tricarboxyl oxygen‑donor linker with a variety of bridg‑ing/chelating functions could have diverse coordination modes to produce fascinating structures,while the coexistence of two benzene rings can make H3bpt a highly conjugated organic linker,resulting in the formed MOFs with potential optical properties[25‑29].We focused on the design and synthesis of MOFs contain‑ing different aromatic multicarboxylate ligands,and reported some novel MOFs with luminescent,magnetic and photocatalytic functions in our previous work[30‑33].In this study,a Nd‑MOF(1)with the chemical formula{[Nd(bpt)(DMF)(H2O)]·2H2O}n(DMF=N,N‑dimethylfor‑mamide)was synthesized from the Y‑shaped aromatic tricarboxylic ligand H3bpt under solvothermal condi‑tion(Scheme 1).The complex was characterized by ele‑mental analysis(EA),infrared(IR)spectroscopy,ther‑mogravimetric(TG)and X‑ray diffraction analyses.Crystal structure analysis shows that complex 1 con‑sists of one‑dimensional(1D)binuclear secondary building unit alongaaxiswhichisconnectedintoathree‑dimensional(3D)framework by bpt3-ligands.In addi‑tion,the fluorescence,magnetic properties and photo‑catalytic degradation of dyes of 1 have also been explored.

Scheme 1 Synthesis route of complex 1
1 Experimental
1.1 Materials and measurements
H3bpt ligand was bought from Jinan Henghua Sci.&Technol.Co.,Ltd.,and used directly without further purification.All solvents and reagents were of standard commercial grade and used directly without further purification.The sample for EA was dried under vacu‑um and performed with a CHN‑O‑Rapid instrument.IR spectra were obtained on KBr pellet by a BRUKER TENSOR27 spectrometer.Powder X‑ray diffractions(PXRD)patterns were collected on a Bruker D8 Advance X‑ray diffractometer employing Cu Kα radiation(λ=0.154 18 nm)with a 2θ range of 5°~50°.The operating voltage and current were 40 kV and 25 mA,respectively.TG analysis was performed on a Dupont thermal analyz‑er under a nitrogen atmosphere with a heating rate of 10 ℃ ·min-1.Luminescence analyses were performed on a Fluoromax‑4 spectrofluorometer with a xenon arc lamp as the light source.UV‑visible spectra were obtained with a JASCO V‑570 spectrophotometer.Mag‑netic susceptibility measurements data were obtained with a SQUID magnetometer(QuantumMPMS)in a temperature range of 2.0~300 K by using an applied field of 1 000 Oe.
1.2 Preparation of complex 1
A mixture of H3bpt(21.5 mg,0.075 mmol)andNdCl3·6H2O(71.7 mg,0.2 mmol)in H2O/DMF(4 mL,1∶1,V/V)was heated at 393 K for 72 h under autoge‑nous pressure in a sealed 23 mL Teflon‑lined stainless‑steel vessel.Purple block‑shaped crystals of 1 were obtained after cooling to room temperature.The crystal‑line samples were collected by filtration,washed with H2O and dried under vacuum overnight(Yield:40%,based on H3bpt).Anal.Calcd.for C18H20NO10Nd(%):C 38.97,H 3.61,N 2.53;Found(%):C 39.01,H 3.69,N 2.56.IR(KBr,cm-1):3 410w,2 932w,1 666s,1 624s,1 583s,1 533s,1 405s,1 252m,1 107m,1 062m,1 017w,871m,777s,720s,676s,469w.
1.3.1 X‑ray crystallography
Single‑crystal X‑ray diffraction data for complex 1 were collected at 100(2)K in a Beijing Synchrotron Radiation Facility(BSRF)beam‑line 3W1A,which was equipped with a MARCCD‑165 detector(λ=0.072 00 nm)with the storage ring working at 2.5×109eV.The data were collected by a MARCCD diffractometer and processed by using HKL 2000[34].The structures were solved by the direct method and refined by the full‑matrix leastsquares method onF2using the SHELXTL[35].All the non‑H atoms were refined aniso‑tropically.Hydrogen atoms attached to C and N atoms were placed geometrically and refined by using a rid‑ing model.Hydrogen atoms in hydroxyl and water mole‑cules were located from difference Fourier maps and refined using their globalUisovalue with the length of O—H being 0.082 nm.In the refinement of 1,the SQUEEZE routine of PLATON[36]was used to remove the contributions of disordered solvent molecules in the structure factors.EA and TGA results matched with the formula C18H20NO10Nd,corresponding to[Nd(bpt)(DMF)(H2O)]·2H2O.A summary of the crystallographic data as well as the data collection and refinement pa‑rameters for complex 1 is provided in Table 1.Selected bond lengths and angles for 1 are provided in Table 2.
CCDC:2074972.

Table 1 Crystal data and structure refinement for complex 1
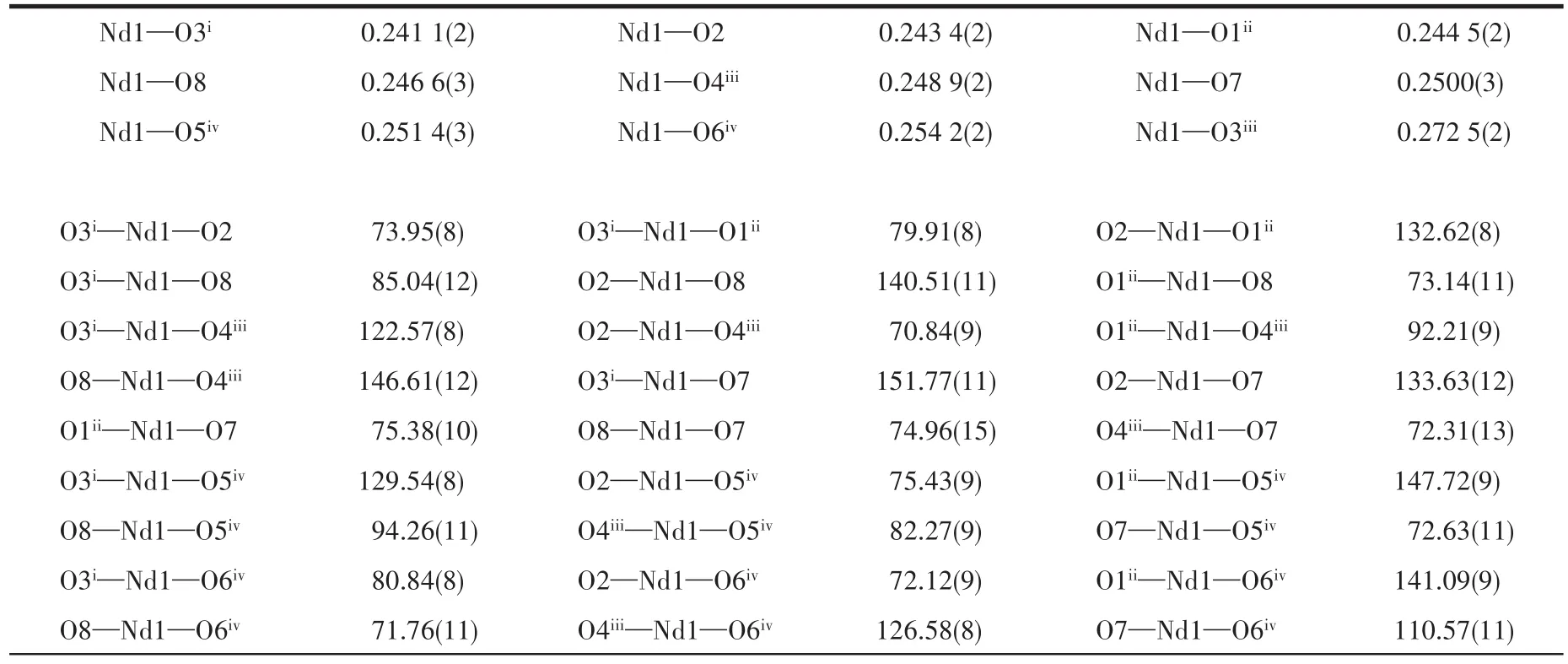
Table 2 Selected bond lengths(nm)and angles(°)for 1
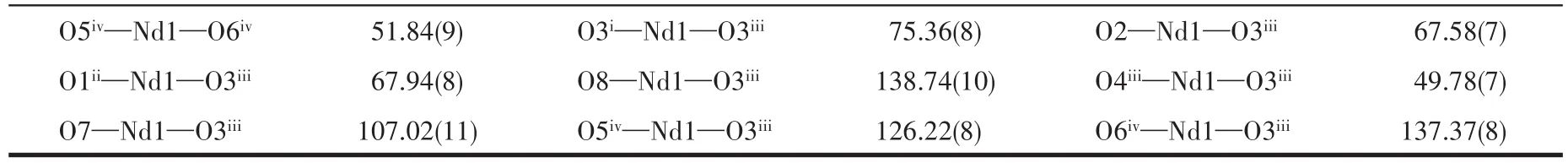
Continued Table 2
1.3.2 Photocatalytic activity study
The photocatalytic activity of the sample was eval‑uated by the degradation of three organic dyes,methyl orange(MO),rhodamine B(RhB)and methylene blue(MB),respectively,in aqueous solution.The experi‑mental operation was similar.Here we took MB as the representative to illustrate.An MB aqueous solution(17 μmol·L-1,15 mL)was mixed with complex 1(2.0 mg),and the mixture was stirred in the dark for 30 min to reach the adsorption‑desorption equilibrium,then it was exposed to the illumination.Then,the samples were periodically removed from the reactor and imme‑diately centrifuged to separate any suspended solids.The transparent solution was transferred to trace cuvette and analyzed by a UV‑Vis spectrometer.A 300 W medium pressure mercury lamp served as a source of ultraviolet light.The distance between the light and the solution was about 30 cm.
2 Results and discussion
2.1 IR spectrum
IR spectra of H3bpt and complex 1 were examined at room temperature(Fig.1).The main characteristic absorption peaks present the typical stretching vibra‑tions of COO-and O—H groups.The broad band in a region of 3 500~3 116 cm-1indicates O—H stretching of the coordinated water molecule[37].The strong bands at 1 107 and 1 666 cm-1are attributed to the ester C—O and acyl C=O stretching vibrations,respectively[38].The characteristic peaks of the asymmetric vibration and the symmetric stretching vibration of the carboxyl‑ate groups were at 1 624 and 1 405 cm-1,respectively.They were obviously shifted to lower wavenumbers rela‑tive to those of free H3bpt(1 720 and 1 410 cm-1),which suggests that the carboxylate groups in the complex are coordinated to the Nd3+ions[39].These structural features are in accord with the results of the X‑ray diffraction analysis.

Fig.1 IR spectra of H3bpt,complex 1 and the complex after the photocatalytic reaction
2.2 Crystal structure description
Complex 1 was well characterized by single crys‑tal X‑ray diffraction analysis.It crystallizes in the monoclinic systemC2/cspace group and displays a 3D structure.The asymmetric unit contains one Nd3+cat‑ion,one bpt3-anionic ligand,one coordinated DMF,one coordinated water molecule,and two free water molecules.As shown in Fig.2a,The Nd3+cation adopts a nine‑coordinated mode,coordinated by nine oxygen atoms from three monodentate(O2,O1iand O3ii)and two chelating(O3iii,O4iii,O5ivand O6iv)carboxyl groups from five different bpt3-ligands,one terminal water molecule(O7)and one terminal DMF molecule(O8)(Symmetry codes:i0.5-x,0.5-y,-z;iix,1-y,-0.5+z;iii0.5-x,-0.5+y,0.5-z;iv-x,y,0.5-z).The coordina‑tion environment of Nd3+cation is a slightly distorted tricapped trigonal prismatic.The Nd—O bond lengths vary from 0.241 1(2)to 0.272 5(2)nm,which corre‑spond to those reported for other lanthanide‑bpt3-com‑plexes[40‑42].The bpt3-ligands adopt three different coor‑dination modes:a bidentate bridging mode(modeⅠ),asymmetric chelating bridging mode(modeⅡ)and a chelating bidentate mode(modeⅢ)[20](Fig.2b).
Two adjacent Nd3+cations are connected by two chelating/bridging and two bis(monodentate)bridging carboxyl groups,forming binuclear[Nd2(COO)6(H2O)2(DMF)2]building units with the Nd1…Nd1iiiseparation of 0.407 0(1)nm,which is further extended into a 1D chain via the bpt3-ligand along a axis(Fig.3a).These binuclear building units are further cross‑linked by bpt3-ligands to form a 3D network with intersected channels[43](Fig.3b).The solvent accessible volume is 1.307 9 nm3per 4.713 0 nm3unit cell volume(27.8% of the total crystal volume)after the removal of the uncoordinated solvents calculated with PLATON.
Topologically,if each Nd3+cation and bpt3-ligand are considered as five‑connected nodes,respectively,the structure can be considered as a 5,5‑connected net with the point symbol of the topology as(44·63·83)(4862)(Fig.4).
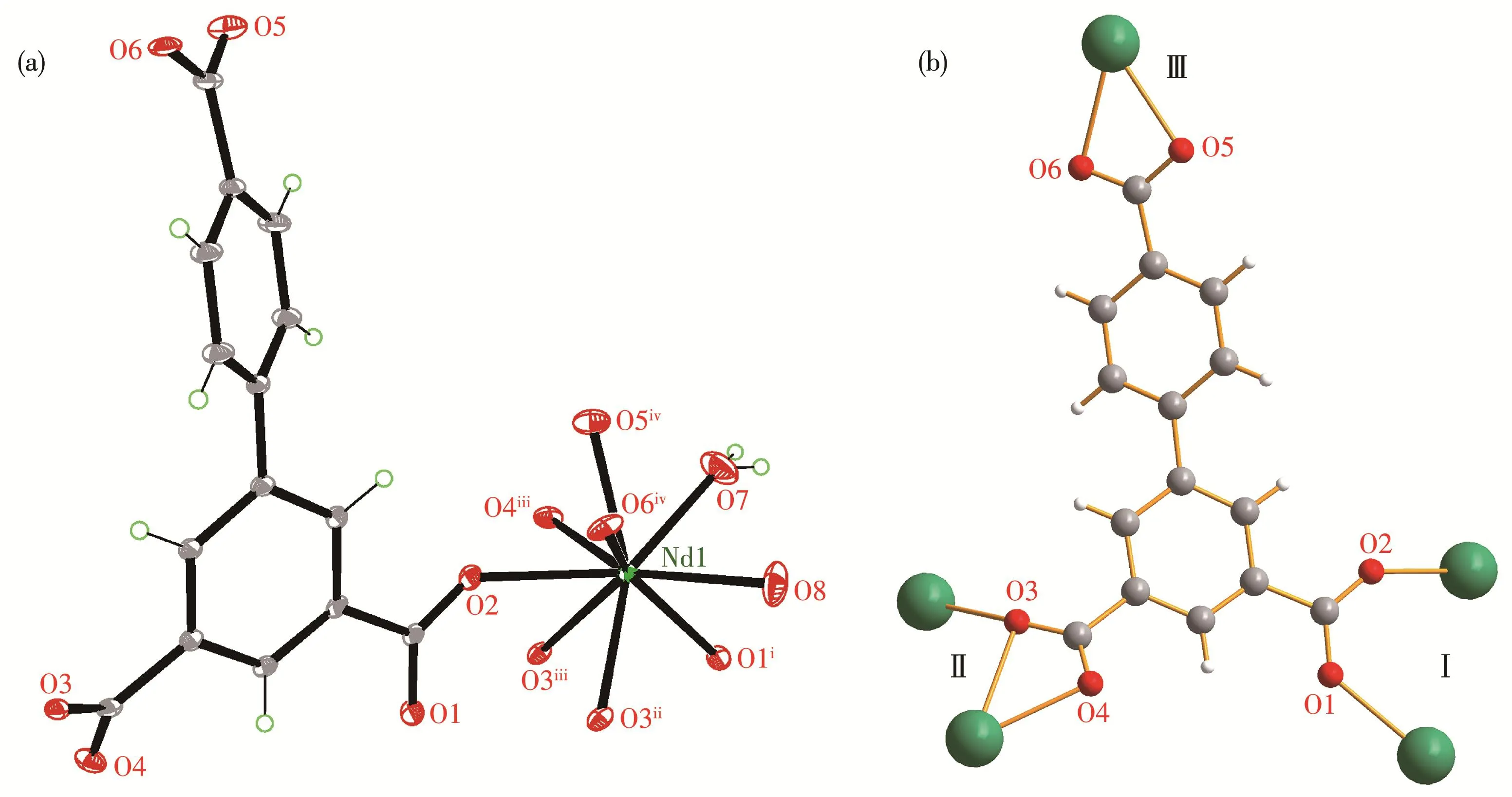
Fig.2 (a)Coordination environment of central Nd3+cation in 1;(b)Coordination modes for bpt3-in 1

Fig.3 (a)One‑dimensional chain and(b)3D network structure of complex 1
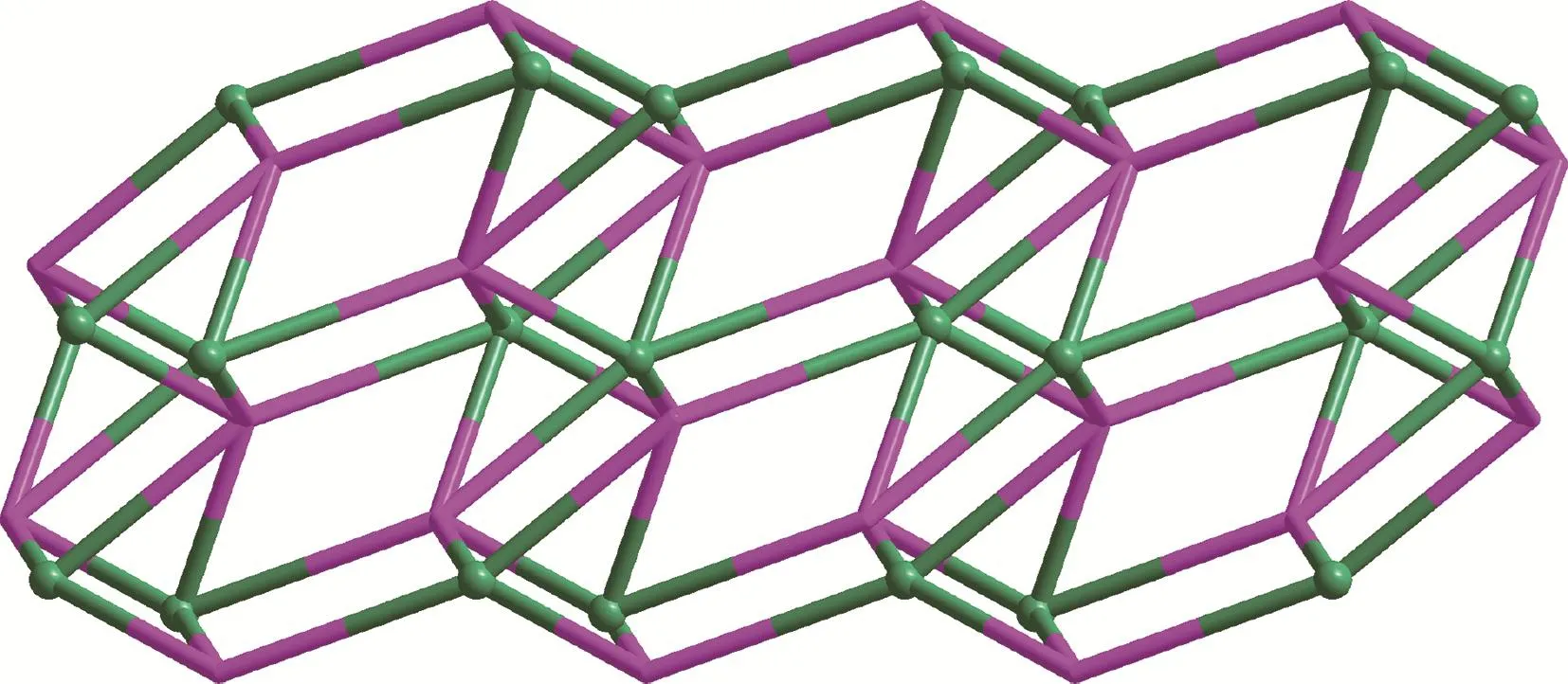
Fig.4 Topological structure of complex 1 with Nd3+cation and bpt3-ligand as 5‑coonected nodes,respectively
2.3 PXRD pattern and TG curve
To verify the phase purity of the complex,PXRD was performed.The experimental PXRD pattern was in agreement with the calculated ones based on the X‑ray single‑crystal data,indicating the high phase purity of complex 1(Fig.5a).In order to estimate the thermal stabilities,TG analysis for 1 was performed on bulk samples in a range of 25~800℃(Fig.5b).As shown in Fig.5b,the weight loss of 23.4%(Calcd.23.3%)occur‑ring between 25 and 220℃corresponds to the removal of two free H2O molecules and coordinated H2O and DMF molecules.After taking off the solvent molecules,with the temperature further heating the skeleton of 1 decomposed gradually without displaying any distinct plateau.
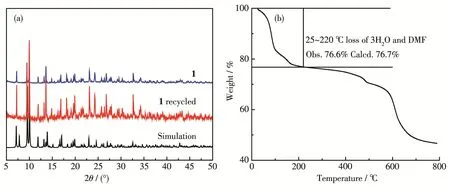
Fig.5 (a)PXRD patterns of complex 1 at room temperature and(b)TG curve of complex 1
2.4 Luminescence property
The solid‑state photoluminescent properties of H3bpt ligand and complex 1 were investigated at room temperature.It was found that complex 1 showed signif‑icant fluorescence enhancement and the strong emis‑sion band was observed at 386 nm(λex=280 nm)as shown in Fig.6.This band may be due to the emission of H3bpt ligand with a slight blue‑shift of 12 nm since the free H3bpt ligand exhibited emission at 398 nm attributed to the π ‑π*transitions(λex=280 nm)[40,44].The observed much stronger emission intensity of 1 indicates that the formation of MOF enhances the rigid‑ity of the aromatic backbone of the ligand and maximiz‑es the intramolecular/intermolecular interactions among the organic ligands,which are conducive to energy transfer[26].In addition,there was no obvious character‑istic Nd‑based emission in the region of 800~1 400 nm[45],indicating an inefficient energy transfer from ligand p‑excited states to neodymium f‑excited states.
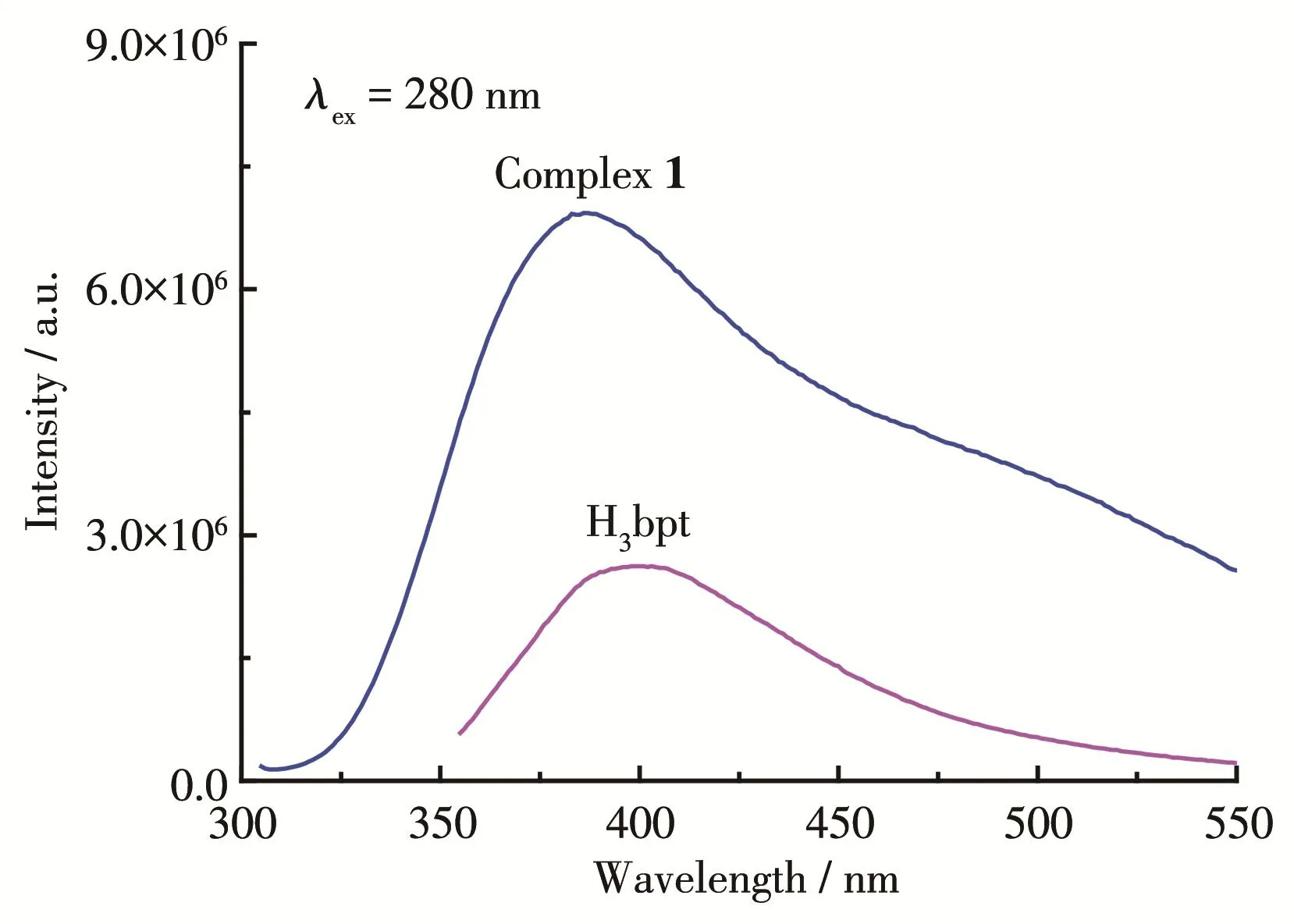
Fig.6 Luminescence spectra of H3bpt ligand and complex 1 at 298 K in the solid‑state
2.5 Photocatalytic property
Studies have shown that lanthanide complexes may have good photocatalytic activity due to the diverse and stable valence states of lanthanide cat‑ions[6].Although a variety of Ln‑MOFs based on H3bpt have been reported[20,22,29,40‑43],their photocatalytic prop‑erties have hardly been studied.So,in this research,three organic dyes,MO,RhB and MB,were used as the model pollutant in aqueous media to evaluate the photocatalytic activity of 1.The results showed that complex 1 displayed good specific effect to degradation of MB but little effect to MO and RhB under ultraviolet light irradiation.As shown in Fig.7a,the variation of UV‑visible absorption spectra of MB dye solution in the presence of 1 was measured at each 10 min inter‑val.The characteristic absorption(ca.665 nm)of MB was selected to monitor the adsorption and photocata‑lytic degradation process.The photocatalytic activity of 1 was gradually enhanced with time increasing from 0 to 100 min,and nearly 77% of MB was degraded(Fig.7a).As shown in Fig.7b,the control experiments as MB without catalyst,and MB with NdCl3,H3bpt and 1,respectively,were carried out under the same condi‑tions to ensure the results obtained from the photocata‑lytic experiments were consistent(where c0is the ini‑tial concentration of MB solution at the beginning of photocatalytic degradation,and c is the concentration of MB solution at each min interval).Under the ultravi‑olet light,the degradation of MB in the absence of cata‑lysts was negligible,implying that MB was relatively stable underillumination conditions.Furthermore,H3bpt ligand showed a certain catalytic degradation rate of MB(about 25%),which indicated that the ligand had certain optical activity.Complex 1 exhibit‑ed the best degradation performance for MB.The IR(Fig.1)and PXRD patterns(Fig.5a)of complex 1 after the photocatalytic experiment were almost the same as that of the as‑prepared complex,which indicates that MB is degraded rather than adsorbed and as a photocat‑alyst,complex 1 has good stability during the heteroge‑neous catalytic reaction in the solution.

Fig.7 (a)Variation in UV‑Vis absorption spectra of MB solution in the presence of 1 irradiated by visible light;(b)Photocatalytic degradation rate of MB under ultraviolet light in the absence and presence of NdCl3,H3bpt and 1,respectively
2.6 Magnetic property
The magnetic behaviour of complex 1 was studied at a temperature range of 2.0~300 K under a 1 000 Oe direct current magnetic field.The magnetic susceptibil‑ities and products χmT are presented as functions of the temperature in Fig.8.When the temperature decreased,complex 1 exhibited a regular increase of χmand a usual decrease of χmT from 1.51 cm3·mol-1·K at 300 K to 0.63 cm3·mol-1·K at 2.0 K.The Curie constant derived from χm-1vs T plots was 1.74 cm3·K·mol-1.Such behav‑iour is a typical isolated Nd3+complex[46]that matches the structure of complex 1.Although there are binuclear building units in complex 1,the Nd…Nd separation is long.For Nd3+cations,the spin‑orbit coupling is very large;the free‑ion ground state is4I9/2and the Zeeman factor gJis equal to 8/11 which leads to χmT value of 1.64 cm3·mol-1·K[47].At 300 K,the experimental χmT value of 1(1.51 cm3·mol-1·K)was slightly smaller than the expected one for the free Nd3+cation,which may be caused by the cumulative effects of crystal field varia‑tion,diamagnetic corrections or slight weighing errors.In a range of 50~300 K,the magnetic data obeyed theCurie‑Weiss law(Inset in Fig.8).The Weiss constant of-45.5 K agreed with previously reported values[46‑47].The susceptibility below 50 K did not conform to the Curie‑Weiss law,which may be attributed to the effect of crystal field splitting of4I9/2ground state into five Kramers doublets[46].
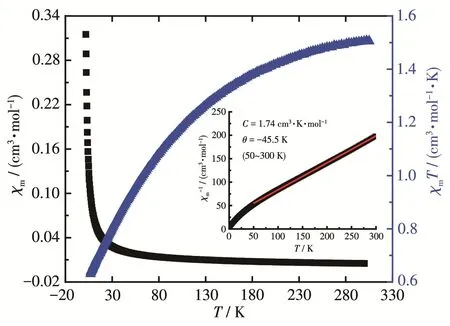
Fig.8 Plots of χm,χmT and χm-1(Inset)as functions of T for 1
3 Conclusions
In summary,one 3D Nd3+‑MOF based on a Y‑shaped tricarboxylic ligand (biphenyl‑3,4′,5‑tricarboxylic acid)was synthesized under solvothermal condition.The crystal structure shows that the frame‑work possesses(5,5)‑connected topological network.The MOF exhibited strong emission in the solid‑state at room temperature based on the ligand.The magnetic susceptibility displayed a typical isolated Nd3+com‑plex.In addition,the MOF showed high photocatalytic efficiency for the degradation of MB in aqueous solu‑tion.This study provides further insights into the ratio‑nal design of MOF‑based multifunctional materials.
- 无机化学学报的其它文章
- Coexistence of Two Unique Cu(Ⅱ) Ions in Mononuclear Cu(Ⅱ)Complexes with Furanyl Substituted Triaryltriazoles
- Effect of Zn on Photocatalytic Activity of Block⁃Shaped Monoclinic WO3
- Preparation and Nonlinear Absorption Properties of SiO2@CdTe@Au Composite Nanoparticles
- First⁃Principles Calculations on Electronic Structures and Optical Properties of g⁃C3N4Nanoribbons
- 二茂铁基-双酮锌配合物的合成、电化学活性及多光子吸收
- 基于(E)⁃N,N⁃二甲基⁃4⁃(2⁃(吡啶⁃4⁃基)乙烯基)苯胺的锌/镉有机-无机杂化金属卤化物的结构和发光性质

