Effect of Zn on Photocatalytic Activity of Block⁃Shaped Monoclinic WO3
XIAO Zhong‑LianWU Xuan‑Yi TAN He‑YunPaolo Aprea HAO Shi‑You*,
(1Xingzhi College,College of Chemistry and Life Sciences,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
(2Department of Chemical,Materials and Production Engineering,University FedericoⅡ,Naples 802125,Italy)
Abstract:Zn‑doped block‑shaped monoclinic WO3composite(Zn‑doped WO3)was synthesized via a facile method and the photocatalytic activity of rhodamine B(RhB)over Zn‑doped WO3was evaluated.The prepared samples were characterized by X‑ray diffraction,Raman spectrum,scanning electron micrograph,UV‑Vis diffuse reflection spec‑trum,Fourier transform infrared spectrum,and X‑ray photoelectron spectrum and other techniques,and the results showed that the block‑shaped monoclinic WO3did not be changed by appropriate amount of Zn doping.The photo‑catalytic results illustrated that mass ratio of 5% Zn doped WO3performed the best photocatalytic efficiency due to the formation of more oxygen vacancy and the increase of hydroxyl groups number.
Keywords:Zn;WO3;synthesis;photocatalysis;oxygen vacancy
It is well known that the pollution resulting from dye wastewater has become one of the most serious environmental problems due to the wide usage of dyes in textiles,leather,papermaking,food additives,cos‑metics,etc[1].These dye wastewater may cause direct se‑vere damage to the liver system,digestive system,and human beings because toxic by‑products can be pro‑duced from the discharged dyes via oxidation,hydroly‑sis,and other chemical reactions[2‑3].Therefore,the wastewater containing dyes must be eliminated before being discharged into the environment.At present,many methods such as physical adsorption[4],chemical precipitation[5],and photocatalytic degradation[6],have been used to remove dyes from wastewater.Amongthese approaches,semiconductor‑based photocatalysis is considered as a highly effective technology for the removal of organic dyes because organic pollutants can be degraded into H2O and CO2over the semiconductor photocatalyst.As is reported that WO3play an impor‑tant role in the field of photocatalysis due to its narrow band gap of about 2.8 eV[7]and hence potentially effi‑cient visible light absorbance.Generally,WO3possess‑es monoclinic,triclinic,orthogonal or hexagonal crystal structure at different temperatures[8].It can be conclud‑ed that monoclinic WO3has efficiently phothocatalytic performance because of its lowest band gap(about 2.65 eV at room temperature[9]).Recently,we found that the photocatalytic efficiency of monoclinic WO3was great‑ly affected by its morphology,and a block‑shaped mor‑phology was beneficial for the improvement of its photo‑catalysis[10].However,the photocatalytic activity of pure WO3is not satisfactory because of its inherent defects such as relatively low conduction‑band level[11].In order to improve the photocatalytic efficiency of WO3,doping with metal and nonmetal elements is often used to form WO3based composite structure such as WO3/TiO2[12],WO3/CuO[13],and WO3/C3N4[14].As a promising alternative semiconductor,ZnO has attracted wide attention in the field of photocatalysis[15‑17]because of potentially photocatalytic activity,low ‑cost and envi‑ronmentally friendly feature.Because of the similar ion‑ic radius of Zn2+to that of W6+,it can be concluded that Zn2+may penetrate into the WO3crystal lattice or sub‑stitute the W6+position in the crystal,resulting in easy generation of lattice defects and hence improvement of WO3photocatalysis.Recently,Zn doped WO3with dif‑ferent morphologys such as spherical,rod shaped or nanoporous morphology were synthesized and the pho‑tocatalytic activity of the resulted samples was also investigated[18‑19].However,to the best of our knowl‑edge,there have no study investigating the photocata‑lytic property of Zn doped monoclinic WO3with a block‑shaped morphology.
Herein,Zn doped block‑shaped monoclinic WO3was prepared via a facile method and the photocatalyt‑ic degradation of rhodamine(RhB)was carried out.The photocatalytic results show that appropriate amount of Zn doping can improve the photocatalytic activity of block‑shaped monoclinic WO3due to the formation of oxygen vacancy and the increase of hydroxyl groups number.
1 Experimental
1.1 Materials synthesis
Na2WO4·2H2O,Zn(NO3)2·6H2O,polyvinylpyrrol‑idone (PVP),absolute ethanol,sodium hydroxide(NaOH),37% fuming hydrochloric acid(concentrated HCl),RhB,terephthalic acid(TPA),1,4‑benzoquinone(BQ)and KI were purchased from Sinopharm Chemical Reagent Co.All the chemical reagents were used with‑out further purification.Deionized water,with a resis‑tivity larger than 18.2 MΩ,was obtained from Milli‑pore Milli‑Q®ultrapure water purification systems and used to prepare 0.1 mol·L-1HCl and 0.1 mol·L-1NaOH solutions(diluting the fuming hydrochloric acid and dissolving solid NaOH,respectively).
Typically,solution A was prepared by dissolving 4 g of PVP in 10 mL H2O at room temperature under stirring for 10 min,by adding 10 mL of concentrated HCl,and then by aging the solution for 60 min.Similar‑ly,solution B was prepared by dissolving 3.3 g of Na2WO4·2H2O in 10 mL H2O at room temperature.Afterwards,solution B was slowly added to solution A under stirring for 30 min to form a yellow precipitate(H2WO4).The mixed solution was stirred for another 30 min,transferred into a Teflon autoclave,and the syn‑thesis was carried out without agitation in oven at 180℃for 12 h.The product was filtered and the solid was washed three times with deionized water,followed by washing for another three times with absolute etha‑nol.The washed solid was then dried at 60℃overnight and a pale‑yellow pre‑product(a mixture of H2WO4and WO3)was obtained.
A typical synthesis of Zn(OH)2was performed as follows:at room temperature,1 g of Zn(NO3)2·6H2O was added to 60 mL H2O under stirring for 30 min,and then 2 mol·L-1NaOH was added dropwise until no for‑mation of white precipitant.Afterwards,Zn(OH)2was obtained by filtration,washed for 3 times with water and ethanol,respectively,and then dried at 60℃.
Zn‑doped WO3was synthesized by the following procedure.0.5 g of the resulted mixture of H2WO4and WO3and x g(x=0.015,0.025,0.035)of Zn(OH)2placed in the agate mortar were grinded for 30 min,and then calcined at 550℃for 2 h.Finally,different amounts of Zn doped WO3samples were obtained,and the samples were denoted as 3% Zn‑WO3,5% Zn‑WO3,and 7% Zn‑WO3,respectively.For comparison purpos‑es,WO3was synthesized under the same experimental conditions,except that no Zn(OH)2was added.
1.2 Characterization
The X‑ray diffraction(XRD)patterns were collect‑ed on a Philips PW3040/60 powder diffractometer using Cu Kα radiation(λ=0.154 nm).The X‑ray tube was operated at 40 kV and 40 mA,and scanning inter‑val ranged from 10°to 80°.Raman scattering analysis was performed on a Renishaw RM1000 Raman spec‑trometer with a 514 nm excitation laser light.Scanning electron microscope(SEM)images were obtained using a Hitachi S‑4800 instrument under an accelerating voltage of 20~40 kV,0.2~5 kV in 100 V steps,and 5~40 kV in 1 kV steps.The UV‑Vis diffuse reflectance(DRS)spectra of the samples over a range of 200~1 000 nm were recorded by a Nicolet Evolution 500 Scan UV‑Vis system with a scanning rate of 60 nm·min-1.The FT‑IR spectra were recorded by a Nicole Nexus 670 spectrometer with a resolution of 4 cm-1using KBr pellet method.The photoluminescence(PL)spectra of the samples were obtained at room tempera‑ture by a spectrofluorometer (NanoLOG‑TCSPC,Horiba Jobin Yvon,USA)with an excitation wave‑length of 325 nm.X‑ray photoelectron spectroscopy(XPS)measurement was carried out on a RBO upgrad‑ed PHI‑5000 C ESCA system(Perkin Elmer)using monochromated Al Kα X‑rays(E=1 486.6 eV)as a radi‑ation at 250 W operating at an accelerating voltage of 15 kV.All binding energies were calibrated using car‑bon(C1s,284.6 eV)as a reference.
1.3 Photocatalytic tests
The photocatalytic activities of WO3,3% Zn‑WO3,5% Zn‑WO3,and 7% Zn‑WO3were evaluated by the photodegradation of RhB under visible light irradia‑tion.In a typical experiment,50 mg of photocatalyst(WO3,3% Zn‑WO3,5% Zn‑WO3or 7% Zn‑WO3)was dis‑persed into 50 mL of RhB solution(5 mg·L-1)under magnetic stirring for 15 min.The pH of all the solu‑tions containing RhB used for the photocatalytic experi‑ments was adjusted to the desired value using 0.1 mol·L-1HCl and/or NaOH solutions.Afterwards,the sus‑pensions were stirred in the dark for 30 min to reach the equilibrium.At given time intervals,a small amount of suspension was withdrawn and centrifuged to remove the photocatalyst.The residual RhB levels in the filtrates were then analyzed by recording the varia‑tions of the absorbance at 552 nm with a UV‑Vis spec‑trophotometer(Evolution 500LC).The removal efficien‑cy of RhB was evaluated as η:

Where A0is the initial absorbance of RhB and A is the absorbance of RhB in the filtrates.
2 Results and discussion
The crystalline structure of WO3and the samples prepared with different Zn amounts were characterized by XRD technique,which is presented in Fig.1a.It can be seen from Fig.1a that the XRD patterns of all the samples can be identified as monoclinic WO3(PDF No.46‑1096),whose characteristic peaks are located at 23.1°,23.6°,24.4°,33.3°,34.2°which corresponding to(002),(020),(200),(120),(202)[20].It is obvious from Fig.1a that the characteristic peak located at about 30.68°(marked with five pointed star)can be detected for the Zn‑doped samples,which is the(100)reflection of ZnO.It also can be found from Fig.1a that the inten‑sity of(100)reflection increased with the increasing of Zn doping amount,implying that Zn can effectively entry into WO3lattice,in good agreement with our above inference.The Raman spectra of as‑prepared Zn‑doped WO3were also recorded and compared with that of WO3in the range of 200~1 000 cm-1(Fig.1b).The peaks at around 270.4,715.8 and 805.8 cm-1are typi‑cal features of the monoclinic structure of WO3[21],which is consistent with the XRD results.The lack of the peak at approximately 950 cm-1attributed to the stretching mode of W6+=O[22],confirms the crystallini‑ty of the catalysts.After Zn doping,the two mostintense peaks at 715.8 and 805.8 cm-1,corresponding to O—W—O vibration mode,became wider.Further‑more,the Raman band at about 325 cm-1assigned to 2E2(M)vibration mode of hexagonal wurtzite ZnO[23]was observed in Zn doped WO3samples,confirming the presence of Zinc in the catalyst.The result(Fig.S1)further prove the presence of Zinc in the synthesized samples.It can be seen from Fig.1c and 1d that WO3and 5% Zn‑WO3have a block‑shaped morphology.Oth‑er Zn doped samples also have similar structures to that of WO3,indicating that Zn doping amount arrang‑ing from 3% to 7% can not change the block‑shaped morphology of initial WO3.
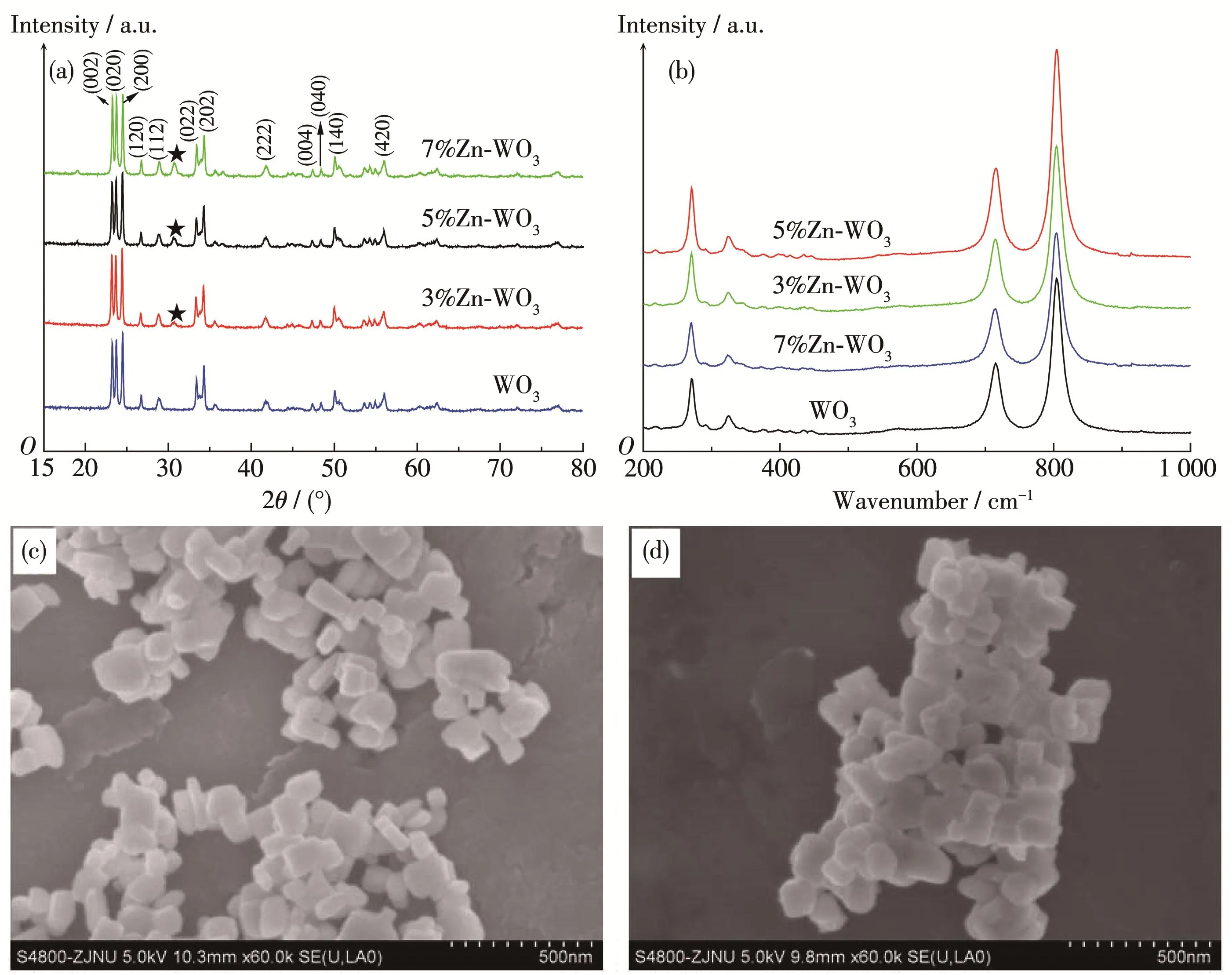
Fig.1 XRD patterns(a)and Raman spectra(b)of WO3,3% Zn‑WO3,5% Zn‑WO3,and 7% Zn‑WO3;SEM images of WO3(c)and 5% Zn‑WO3(d)
The photocatalytic activities of WO3,3% Zn‑WO3,5% Zn‑WO3,and 7% Zn‑WO3are showed in Fig.2.It is clear that the photocatalytic efficiency of WO3increased when Zn doping amount increased from 3% to 5%,but decreased when the doping amount exceeded 5%.Fig.3 can explain the above experimental results.The photocatalytic results show that an appropriate amount of Zn doping is good for the improvement of WO3photo‑catalysis performance.It can be concluded from Fig.S2 that RhB was actually degraded over 5% Zn‑WO3.
In order to explain the above photocatalytic re‑sults,UV‑Vis DRS and PL spectra of WO3,3% Zn‑WO3,5% Zn‑WO3,and 7% Zn‑WO3were recorded and the results are shown in Fig.3.It can be seen from Fig.3a that the light(especially visible light)absorption efficiency of 5% Zn‑WO3was higher than that of WO3,resulting in efficient generation of photogenerated elec‑trons and holes over 5% Zn‑WO3under the irradiation of visible light.The PL spectra of pure WO3and Zndoped WO3are shown in Fig.3b.It is obvious that the position and pattern of the emission peaks of all sam‑ples were almost similar,but the PL intensities of the samples were noticeably different.Generally,the lower the PL intensity,the higher the separation efficiency for photogenerated electron‑hole pairs[24].From Fig.3b,it is easy to find that the PL intensity of 5% Zn‑WO3was the lowest,indicating that the charge separation efficient in 5% Zn‑WO3was better than that in WO3.This may be due to the fact that the photogenerated electrons and holes are separated by the charge trans‑fer at the heterojunction interfaces of 5% Zn‑WO3.Con‑sequently,the photocatalytic activity of 5% Zn‑WO3was higher than that of WO3.It can be seen that the light absorption efficiency of 7% Zn‑WO3was lower than those of other samples,and the PL intensity of it was the highest one,resulting in the lowest photocata‑lytic activity.
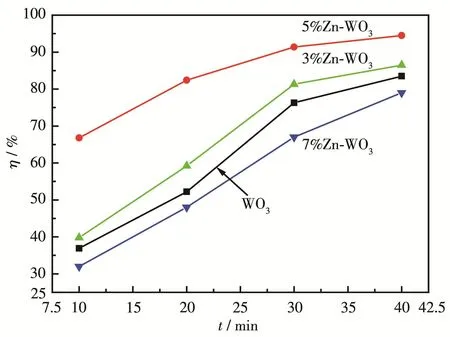
Fig.2 Photodegradation of 5 mg·L‑1RhB in the presence of different photocatalysts under visible light irradiation at pH of 6(VRhB=50 mL,mphotocatalyst=0.05 g)
In order to study the reason why 5% Zn‑WO3had higher separation efficiency of photogenerated electron‑hole pairs,W4f XPS spectra for 5% Zn‑WO3and WO3were carried out(Fig.4).It is clear from Fig.4 that the W4f7/2and W4f5/2peaks centered at 35.4 and 37.6 eV are typical binding energies corresponding to W6+oxi‑dation state[25].Moreover,the peak at about 36.2 eV cor‑responding to orbital spin of W5+4f5/2[26]was detected in 5% Zn‑WO3and WO3,but the peak intensity of the for‑mer was higher than that of the latter,implying that Zn doping is beneficial for the formation of W5+.The possi‑ble reason is that Zn2+is beneficial to the interaction between WO3precursor(H2WO4)and PVP.Therefore,the W6+is easier to be reduced by PVP in 5% Zn‑WO3precursor than in the WO3precursor,resulting in a larger number of oxygen vacancies arising from the replacement of W6+by W5+in 5% Zn‑WO3,as expressed by the following equation:
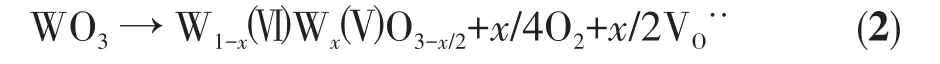
Where VO··represents an oxygen vacancy.From our previous report[27],it can be concluded that the photo‑generated electrons can be easily captured by oxygen vacancy,which can cause efficient separation efficien‑cy for photogenerated electron‑hole pairs.Therefore,the photocatalytic efficiency of 5% Zn‑WO3was higher than that of WO3.
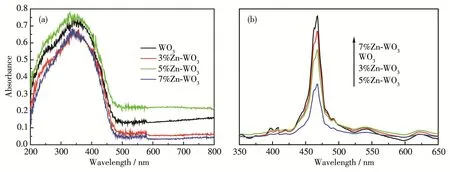
Fig.3 UV‑Vis DRS(a)and PL(b)spectra of WO3,3% Zn‑WO3,5% Zn‑WO3,and 7% Zn‑WO3
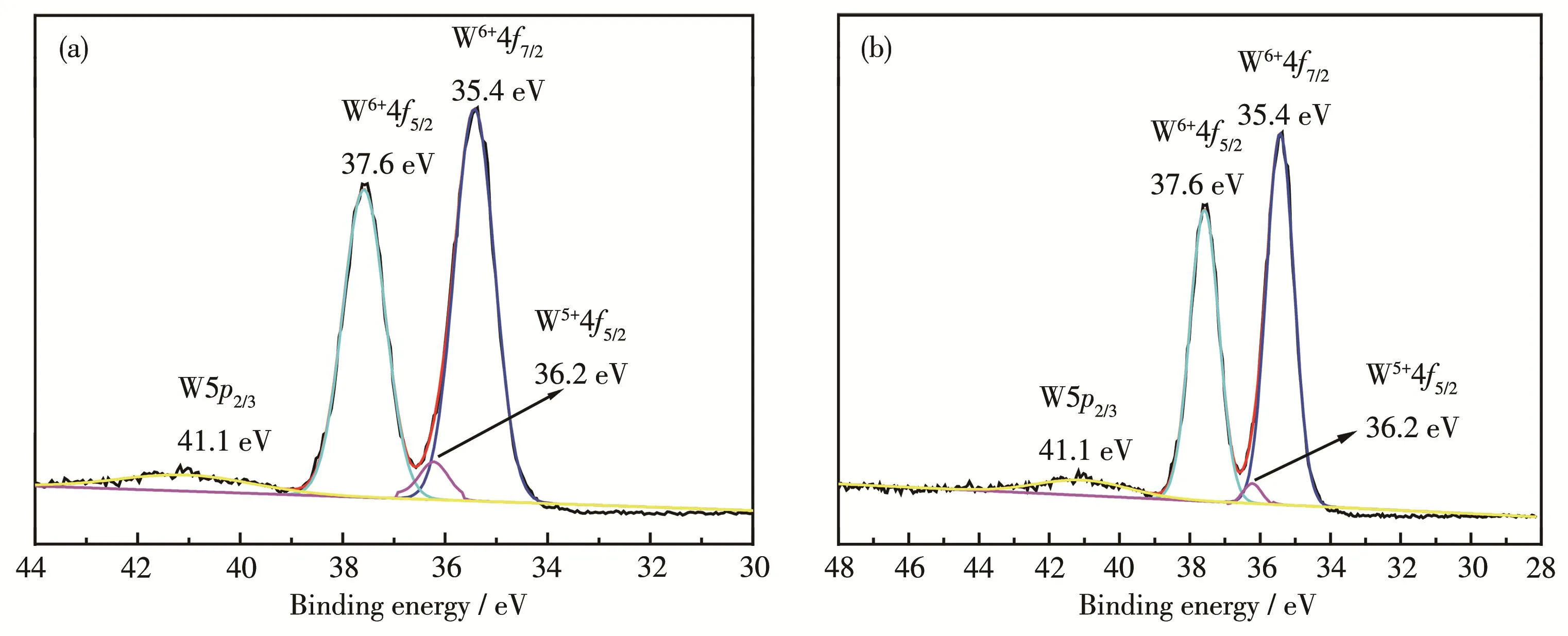
Fig.4 W4f XPS spectra for 5% Zn‑WO3(a)and WO3(b)
Besides the above factor affecting the photocata‑lytic activity,the adsorption ability of dyes on the sur‑face of photocatalyst also play an important role.It is reported that the content of hydroxyl groups on the sur‑face of photocatalyst can greatly influence the adsorp‑tion ability of RhB and hence the photocatalytic effi‑ciency[28].Generally,the content of hydroxyl groups can be reflected by the O1s XPS spectra[29].In order to in‑vest the effect of Zn doping on the content of hydroxyl groups on the surface of WO3,the O1s XPS spectra of 5% Zn‑WO3and WO3were obtained(Fig.5).According to Han et al.,the peak at about 530.5 eV is related to oxygen in the lattice(O2-,OⅡ),and another peak,located at about 531.5 eV,corresponds to adsorbed oxygen(OⅠ)in the form of O—H on the surface[30].Generally,the content of hydroxyl groups can be reflected by the ratio of SOⅠ(the peak area of adsorbed oxygen in the form of O—H)to SOⅡ(the peak area of oxygen in the lattice).The higher the value of SOⅠ/SOⅡ,the richer the content of hydroxyl groups in the pre‑pared sample.It can be found from Fig.5 that the value of SOⅠ/SOⅡfor 5% Zn ‑WO3was higher than that for WO3,implying that the content of hydroxyl groups in 5% Zn‑WO3was higher than that in WO3.The reason may be that Zn2+is easy to combined with OH-to form[Zn(OH)4]2-coordination ion,which is good for the improvement of hydroxyl content in the precursor of 5% Zn‑WO3.Consequently,the adsorbed amount of RhB on 5% Zn‑WO3was higher than that on WO3,re‑sulting in a higher photocatalytic activity.The results of Fig.S3 show that·O2-and h+are the main active species to degrade RhB.

Fig.5 O1s XPS spectra for 5% Zn‑WO3(a)and WO3(b)
3 Conclusions
In summary,Zn‑doped WO3was synthesized by a facile method.The photocatalytic results show that the photocatalytic activity of WO3is enhanced after doping of Zn because the photoelectrons and holes can be effi‑ciently separated due to the formation of oxygen vacan‑cies.Furthermore,Zn doping can improve the content of hydroxyl groups,which is beneficial for the improve‑ment of RhB adsorption ability and hence the photocat‑alytic efficiency.
Acknowledgement:This work was supported by the National Natural Science Foundation of China (Grant No.21876158).
Supporting information is available at http://www.wjhxxb.cn
- 无机化学学报的其它文章
- Coexistence of Two Unique Cu(Ⅱ) Ions in Mononuclear Cu(Ⅱ)Complexes with Furanyl Substituted Triaryltriazoles
- Synthesis,Structure,Luminescence,Photocatalytic and Magnetic Properties of a Neodymium Complex Constructed from Biphenyl⁃3,4′,5⁃tricarboxylic Acid
- Preparation and Nonlinear Absorption Properties of SiO2@CdTe@Au Composite Nanoparticles
- First⁃Principles Calculations on Electronic Structures and Optical Properties of g⁃C3N4Nanoribbons
- 二茂铁基-双酮锌配合物的合成、电化学活性及多光子吸收
- 基于(E)⁃N,N⁃二甲基⁃4⁃(2⁃(吡啶⁃4⁃基)乙烯基)苯胺的锌/镉有机-无机杂化金属卤化物的结构和发光性质

