Preparation and Nonlinear Absorption Properties of SiO2@CdTe@Au Composite Nanoparticles
CHANG QingGUAN JingMENG Tian‑Ming
(1College of Media Engineering,Communication University of Zhejiang,Hangzhou 310018,China)
(2College of Electronic Engineering,Heilongjiang University,Harbin 150080,China)
Abstract:SiO2@CdTe@Au nanocomposites were prepared by using SiO2nanoparticles,CdTe quantum dots and Au nanoparticles as raw materials by layer‑by‑layer adsorption.The samples were tested and characterized to show the successful preparation of nanocomposites.The Z‑scan technique was used to measure the nonlinear absorption opti‑cal properties of SiO2@CdTe and SiO2@CdTe@Au nanocomposite samples under nanosecond laser pulses.The anal‑ysis of the experimental results showed that SiO2@CdTe and SiO2@CdTe@Au nanocomposites all exhibited saturat‑ed absorption properties.SiO2@CdTe@Au nanocomposite presented enhanced nonlinear optical characteristics com‑pared to SiO2@CdTe nanocomposite,and the mechanism was analyzed.
Keywords:SiO2@CdTe@Au;composite nanoparticles;Z‑scan;nonlinear absorption
0 Introduction
Currently,the performances of nanomaterials com‑prising only one substance are no longer sufficient to meet the needs of an increasingly developed society.A variety of nanocomposites with excellent properties have become prevalent in the field of materials[1‑2].
Nanosilica materials have become crucial carrier materials on account of their large specific surface ar‑ea,good chemical stability,nontoxicity,hydrophilicity and easy functionalization[3‑4].For these reasons,silica is the optimal material for nanocomposite matrices,andthe morphology and dispersion of its materials are inno‑vations of researchers.
Quantum dots(QDs)are ideal fluorophores for bio‑imaging and broadly serve the major field of biomedi‑cal sciences with their fluorescent labeling ability and chemical properties[5‑6].They can emit or absorb light at specific frequencies.Researchers can precisely control these frequencies by changing the size,shape and type of QDs to select the specific wavelengths required.Fur‑thermore,Au nanoparticles(NPs)have inestimable potential in terms of light,electricity and magnetism due to their surface effects and quantum size effects[7].In addition to their special electronic and optical prop‑erties,Au NPs also exhibit interesting nonlinear optics and strongsurfaceplasmon resonanceproperties,which are exactly what we need to develop[8].Effective‑ly regulating and utilizing the surface plasmon reso‑nance properties of Au NPs can promote related research.Moreover,the surface plasmon resonance properties of particles can promote related research,and it is beneficial to develop the practical application of composite nanomaterials.
Many researchers have reported the preparation and application of SiO2and CdTe QDs or SiO2and Au NPs combinations.Ge et al.synthesized SiO2@CdTe NPs by hydrothermal method on mercapto capped sili‑ca NPs(SH‑SiO2).SiO2@CdTe NPs have good fluores‑cence preservation properties and are used for the de‑tection of H2O2in chemistry and biology[9].Pan and Jie et al.used EDC/NHS cross‑linking to connect CdTe QDs on the surface of amino SiO2to form CdTe func‑tionalized SiO2,which has a great application prospect in clinical diagnosis[10‑11].Wang et al.reported a method of sound chemical auxiliary seed growth on the surface of SiO2self‑assembly cationic polyethyleneimine(PEI).The cationic thin layer with lots of primary amine groups formed,which is easy to absorb density of Au seed,and the preparation of SiO2@Au NPs used highly uniform size and surface‑enhanced Raman scattering(SERS)active,which are ideal SERS tags for SERS‑based immunoassay[12].Yang et al.prepared gold‑embedded silica by in⁃situ deposition.Au(OH)3was formed on the surface of amines coated silica NPs,which was used as the nuclear site to form gold clus‑ters,and SiO2@Au core‑shell NPs were obtained,which could control heat generation at the nanometer level[13].
In the field of materials,it is common that silica combines with CdTe QDs or Au NPs.Because of the complex preparation process and other issues,it rarely occurs in nanocomposites comprised of three materials.In order to get ternary composite NPs,Liu and Jiang et al.coated gold particles with silica by amino function‑alization,and then the composite was covalently bond‑ed with CdTe QDs through EDC/NHS crosslinking to form core‑shell NPs for biosensors and biomarkers[14‑15].Zhou et al.synthesized gold nanorods@SiO2@CdTe QDs hybrid nanostructures,and photoluminescence(PL)spectra showed that the quenching effect of gold nanorods is reduced through the isolation of silica lay‑er[16].Wang et al.put CdTe encapsulated into SiO2,and synthesized bovine serum albumin(BSA)modified gold cluster(Au@BSA).Then the composite was covalently connected by amino and formed a new type of satellite core CdTe/Silica/Au replication hybrid NPs which were used as dual emission ratio fluorescent probes for Cu2+[17].Although these nanocomposites have been pre‑pared,the preparation processes are complex and expensive.
Therefore,we have developed nanocomposites to overcome these shortcomings and improve material per‑formance.Three basic nanomaterials were combined into one material to complement each other.This new material not only retained the advantages of the three original materials but also improved the nonlinear opti‑cal properties of the final product.Adding a charged polymer solution to the surface of each material can promote the equilibrium of positive and negative phase adsorption so that the silica,QDs and Au NPs firmly adsorbed layer‑by‑layer.More unexpectedly,the layer‑by‑layer method allowed the polymer to be more com‑pact without affecting the other layers or destroying its own properties.The nondispersibility and noninfluence of this method bring great benefits of the preparation of nanocomposites.
The paperdescribesthe tightlayer‑by‑layeradsorption of QDs and Au NPs on silica spheres.Au NPs were supported on the matrix material,effectively utilizing the advantages of both,improving the optical properties and stability of the composite.This phenom‑enon was accompanied by the unique fluorescent prop‑erties of the QDs,making the material a refined nano‑composite.In addition,the prepared SiO2@CdTe and SiO2@CdTe@Au nonlinear absorption coefficients were measured by a Z‑scan device to investigate the benefi‑cial nonlinear optical properties.
1 Experimental
1.1 Synthetic procedures
The formation process of SiO2@CdTe@Au NPs is shown in Fig.1.First,we used an improved Stöber method to prepare SiO2NPs[18].Phase A(150 mL ammonia and 880 mL ethanol)was added to a 2 L three‑necked flask and heated in a water bath to 30 ℃ with rapid mechanical stirring at 280 r·min-1.At the same time,another 1 L three‑necked flask was prepared,and phase B(36 mL tetraethylorthosilicate and 880 mL eth‑anol)was likewise heated in a water bath to 30℃.When the temperature of both samples reached 30℃,phase B was quickly added to phase A,and the reac‑tion was stirred for 12 h.SiO2NPs of different particle sizes were prepared by varying the reaction time and temperature.HAuCl4,sodium citrate,and deionized wa‑ter were used to prepare Au NPs according to the litera‑ture[19],and aqueous CdTe QD solutions were prepared according to a previous report[20].Subsequently,we ad‑opted the properties of the positive and negative charge phases of the electrolyte solution to synthesize the final composite sample.We added 0.4 mL of the positively charged polymer solution P+(100 μL 3.5%poly(dial‑lyldimethylammonium chloride)(PDADMAC)solution with 1 mol·L-1NaCl solution as base solution)while stirring.The adsorption time was at least 30 min,and the mixture was centrifuged three times.CdTe QDs were added while stirring continuously.After reacting for 30 min,0.4 mL of the negatively charged polymer solution P-(10 mg·mL-1P-solution:poly(sodium 4‑styrenesulfonate)(PSS)with 1 mol·L-1NaCl solution as base solution)was added.After centrifugation,0.4 mL of solution P+was added.Then,after sufficient reac‑tion for 30 min,sodium citrate‑modified Au NPs were added by centrifugation.The nanocomposite particle solution obtained after sufficient reaction time was cen‑trifuged and stored.The above method for coating CdTe QDs and Au NPs layer by layer on SiO2spheres is referred to as the layer‑by‑layer(LBL)method[21].
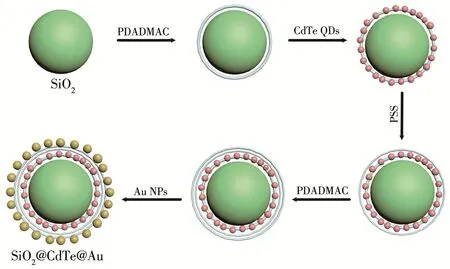
Fig.1 Process of forming SiO2@CdTe@Au composite NPs with LBL method
1.2 Characterization
The morphology required for each prepared sam‑ple was characterized by scanning electron microscopy(SEM,S ‑4800SEM,HITACHI,Japan)and transmis‑sion electron microscopy(TEM,FEI Tecnai F20,FEI,American).The accelerating voltage of SEM was 5 kV,and the working distance was 8.9 mm.The accelerating voltage of TEM was 220 kV.The elemental mapping of the product was gained by using a Quanta 200 FEG scanning electron microscope(FEI,American)to cap‑ture energy dispersive X‑ray spectroscopy(EDS).The corresponding crystals were studied by X‑ray diffrac‑tion(XRD,D8 advance,Bruker,German)with Cu Kα(λ=0.154 nm).The tube current was 100 mA and the tube voltage was 50 kV,and the scanning range(2θ)was 5°~80°,with a scanning rate of 5(°)·min-1.The UV‑Vis spectra of Au NPs,CdTe QDs and SiO2@CdTe@Au composite NPs were measured by a UV‑Vis spectrophotometer(TU‑1901,Puxi Instruments,China).A continuous wave laser at 405 nm(MLL‑Ⅲ,CNI)served as the excitation source for steady‑state laser excitation.Steady‑state fluorescence spectra were col‑lected by a spectrometer(IHR550,HORIBA,Japan)with a CCD(Synapse,HORIBA Jobin Yvon,Japan).
1.3 Z⁃scan technique
The third‑order nonlinear absorption properties were detected and measured by open‑aperture Z‑scan according to a previous report[22].
The laser source used in this experiment was gen‑erated by a Q‑switched Nd∶YAG laser(Surelite Ⅱ,Continuum)with a pulse width of 4 ns(full width at half maxima,FWHM)at 532 nm and 4.8 μJ single‑pulse energy.A low repetition rate was used under all conditions(10 Hz in nanosecond pulses)to eliminate spurious cumulative effects originating from thermally induced nonlinearities.A water solution with a linear transmittance of 56%measured in a 2 mm quartz cell was used as the sample.A nonlinear absorptive back‑ground from the solvent was removed from the data.The nonlinear absorption properties of both SiO2@CdTe and SiO2@CdTe@Au were tested with an open aperture Z‑scan device.
2 Results and discussion
The method we have adopted to combine SiO2with CdTe and Au is obviously different from the existing methods.In order to get the ternary‑composite NPs,the gold particles were coated with silica and then cova‑lently bonded with CdTe QDs or CdTe QDs was encap‑sulated into SiO2,then covalently bonded with gold[14‑17].The main purpose of these preparation meth‑ods is to avoid contact which leads to gold quenching.Although these nanocomposites have been prepared,the preparation processes are complex.The LBL meth‑od we adopted is relatively simple and it can protect Au NPs from quenching.
As shown in Fig.2,SiO2nanoparticle samples with a particle size of approximately 120 nm were obtained by a modified Stöber method.SiO2NPs exhibited a uni‑form spherical shape in the SEM image,which was con‑sistent with the TEM image(Fig.3).The adsorption pro‑cess of Au NPs from less to more is also shown in the related figures.
Consequently,according to the TEM image,we successfully prepared the product.Obviously,CdTe QDs and Au NPs were adsorbed on the SiO2spheres.In addition,Au NPs were uniform in size and dispersed,and the diameters of these particles were 5~20 nm.
Fig.4 shows an EDS mappings,and it is confirmed again that(a)Si,(b)Cd,(c)Te and(d)Au nanoparticles are present in SiO2@CdTe@Au.The XRD patterns of various samples prepared in the experiment are shown in Fig.5.The pattern of CdTe QDs in the observation showed three distinct diffraction peaks at 25.61°,42.61°and 50.31°.In contrast to the standard card(PDF No.15‑0700),these nanocrystals have cubic sphalerite structures.The diffraction peaks of synthe‑sized SiO2and SiO2@CdTe were relatively dissimilar;three diffraction peaks that were obvious in the XRD pattern of CdTe QDs were not present,but a new peak of 21.09°(2θ)appeared.Although the peak positions of some samples were not obvious,the TEM image andfluorescence spectrum can totally prove the presence of CdTe QDs,despite of the low diffraction peak of CdTe QDs.For the composite nanomaterial,we measured the XRD diffraction peak of the sample with a small num‑ber of Au NPs,and then we measured that of the sam‑ple containing a large number of Au NPs.Both of their diffraction peaks were unclear compared to the images of Au NPs themselves.
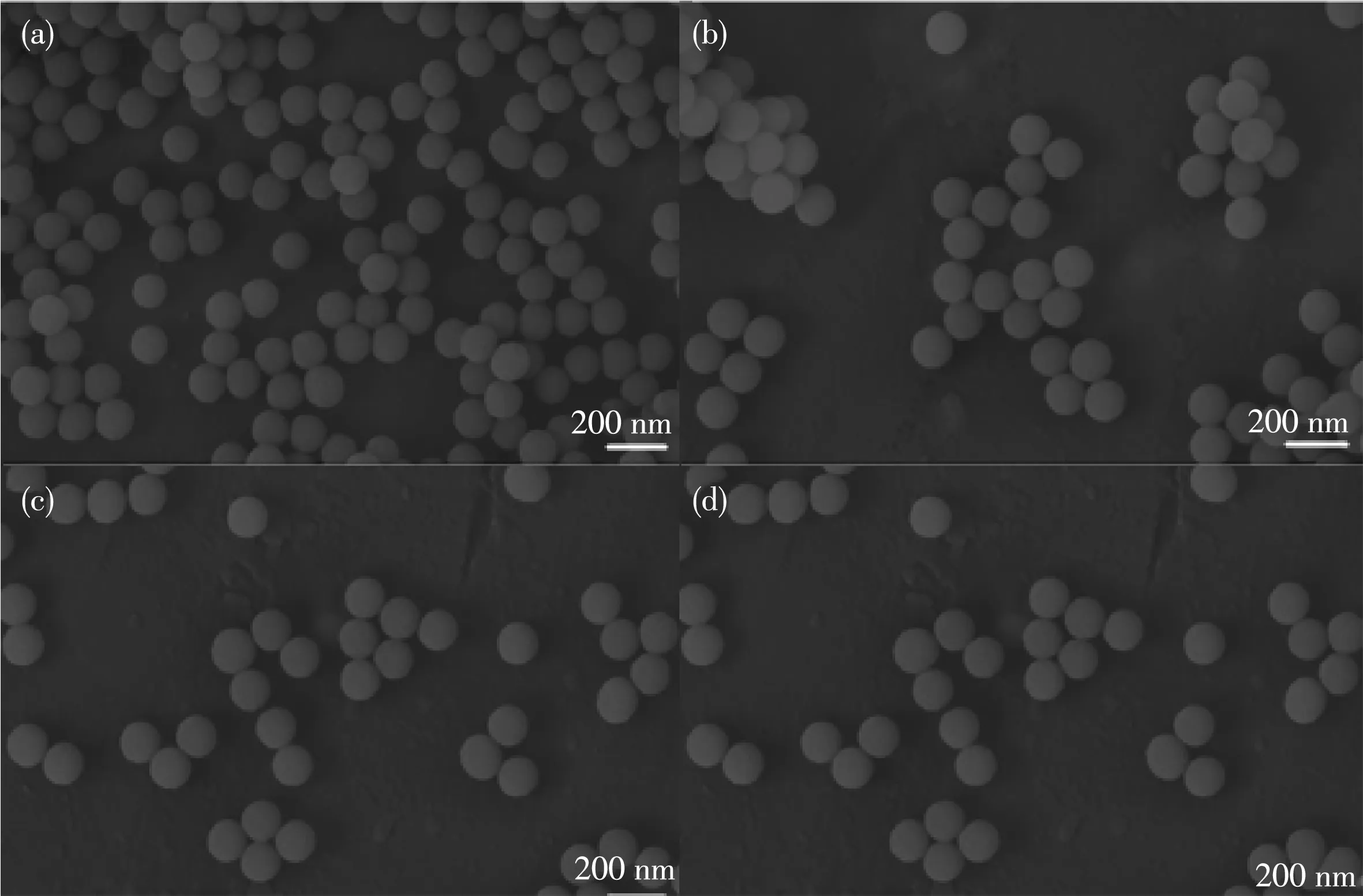
Fig.2 SEM images of(a)SiO2NPs,(b)SiO2@CdTe,(c)SiO2@CdTe@Au(less)and(d)SiO2@CdTe@Au(more)

Fig.3 TEM images of(a)SiO2NPs,(b)SiO2@CdTe,(c)SiO2@CdTe@Au(less)and(d)SiO2@CdTe@Au(more)
Fig.6 shows that the maximum absorption peak for CdTe was at 516 nm.Yu′s team[23]summarized the rela‑tionship between the size of QDs and the first absorp‑tion peak.The size of CdTe was approximately 2.2 nm.Au NPs had a characteristic surface plasmons peak at 520 nm.SiO2@CdTe,SiO2@CdTe@Au(less)and SiO2@CdTe@Au(more)nanocompositesshowed the phenomenon of red shift,which is due to the increase of the sample size resulting from the adsorption of QDs and gold NPs.The absorption spectra of these nano‑composite materials were not as strong as those of the corresponding monomer CdTe QDs.This is because SiO2NPs also absorb light,which partially covers the absorption spectrum of QDs.In the fluorescence spec‑tra(Fig.7),as the layers of the polymer were added,the sample gradually redshifted.Due to the size effects of QDs,their sizes have a great relationship with their own absorption and emission.
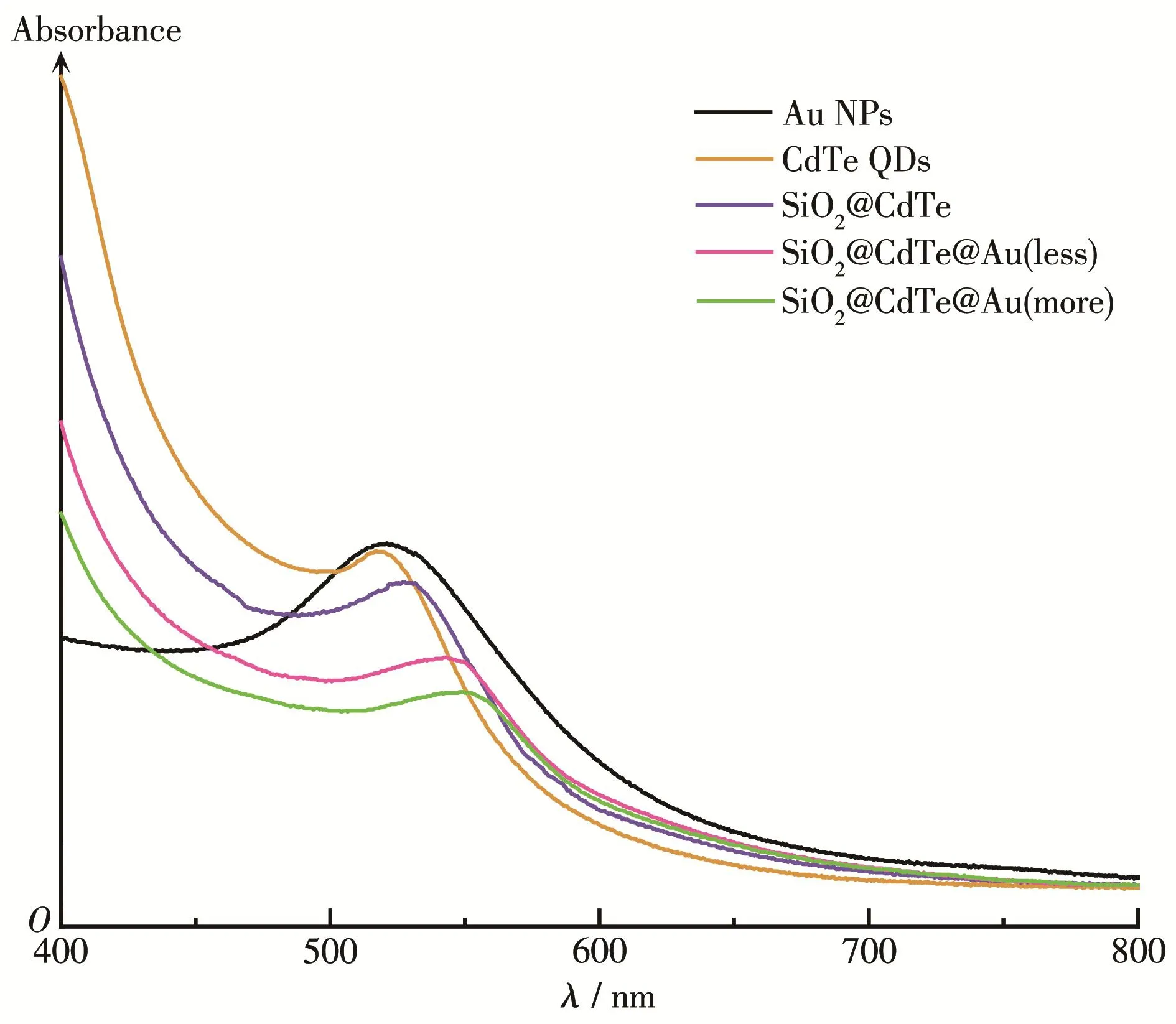
Fig.6 UV‑Vis absorption spectra of as‑prepared samples
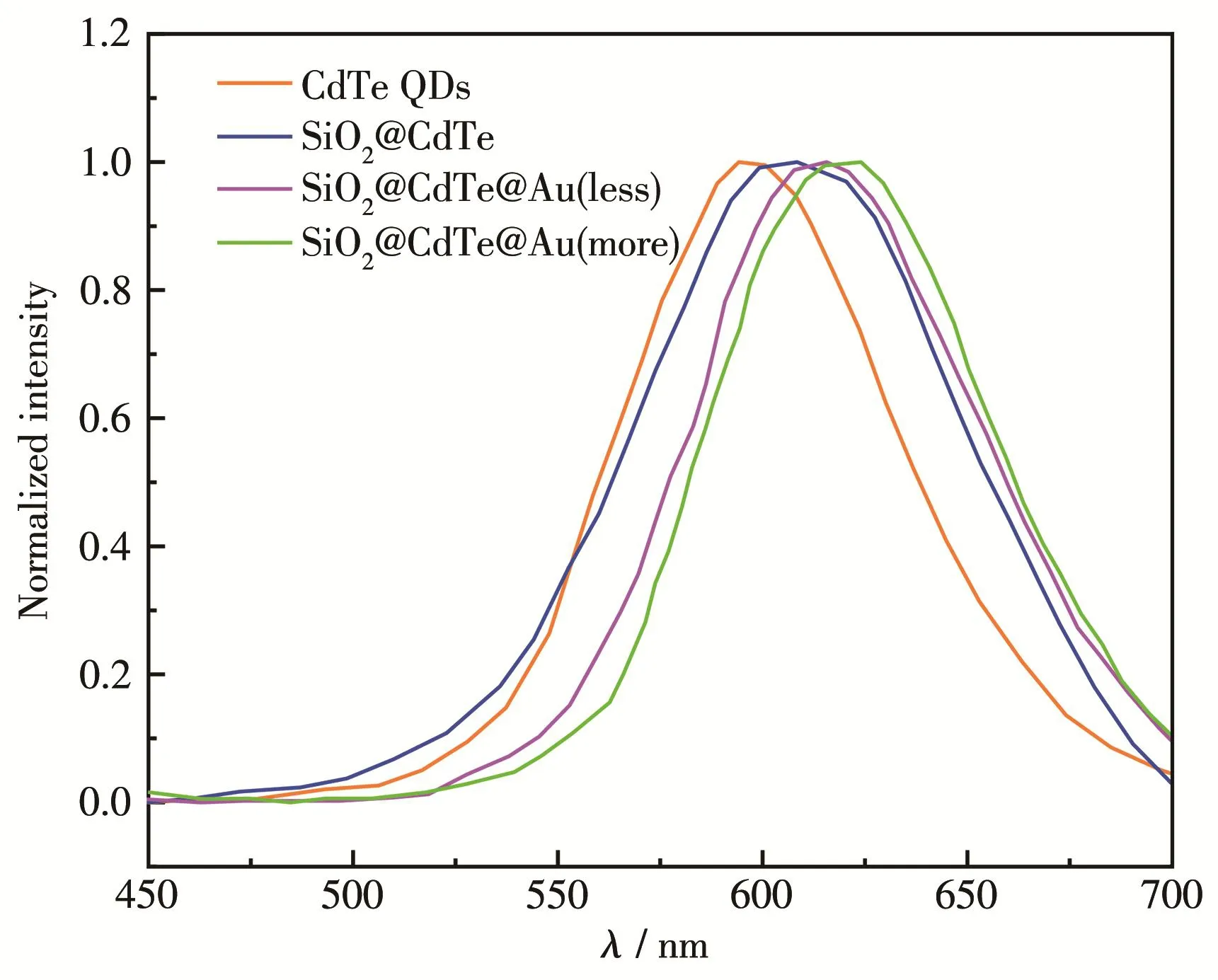
Fig.7 Fluorescence spectra of as‑prepared samples
As the particle size of the nanocomposite material with adsorbed QDs and gold increased,the correspond‑ing fluorescence spectrum also shifted to the long wave‑length direction,showing a clear redshift phenomenon.In the images and characterization experiments of all SiO2@CdTe@Aucompositenanoparticlesdescribed above,it is apparent that CdTe QDs and Au NPs were adsorbed on SiO2spheres to form nanocomposite parti‑cles.
The nonlinear absorption characteristics(Fig.8)exhibited by SiO2@CdTe are derived from CdTe QDs.The QD material is a three‑dimensionally constrained quantum system.When its size is close to the Bohr radius,its energy level structure changes with the size,which in turn leads to a change in nonlinear absorption characteristics.The wavelength of the incident light of the laser was 532 nm,and the single photon absorption of CdTe QDs was responsible for the saturated absorp‑tion of the sample.In addition,Au NPs are attracting attention due to their surface plasmon resonance;they exhibit a distinct nonlinear optical response whileexcited near surface plasmon resonance.As a result,the local field resonance in the NPs is strengthened;this phenomenon is called the local field effect.At the near‑plasma resonance excitation frequency, an enhancement phenomenon occurs[24].
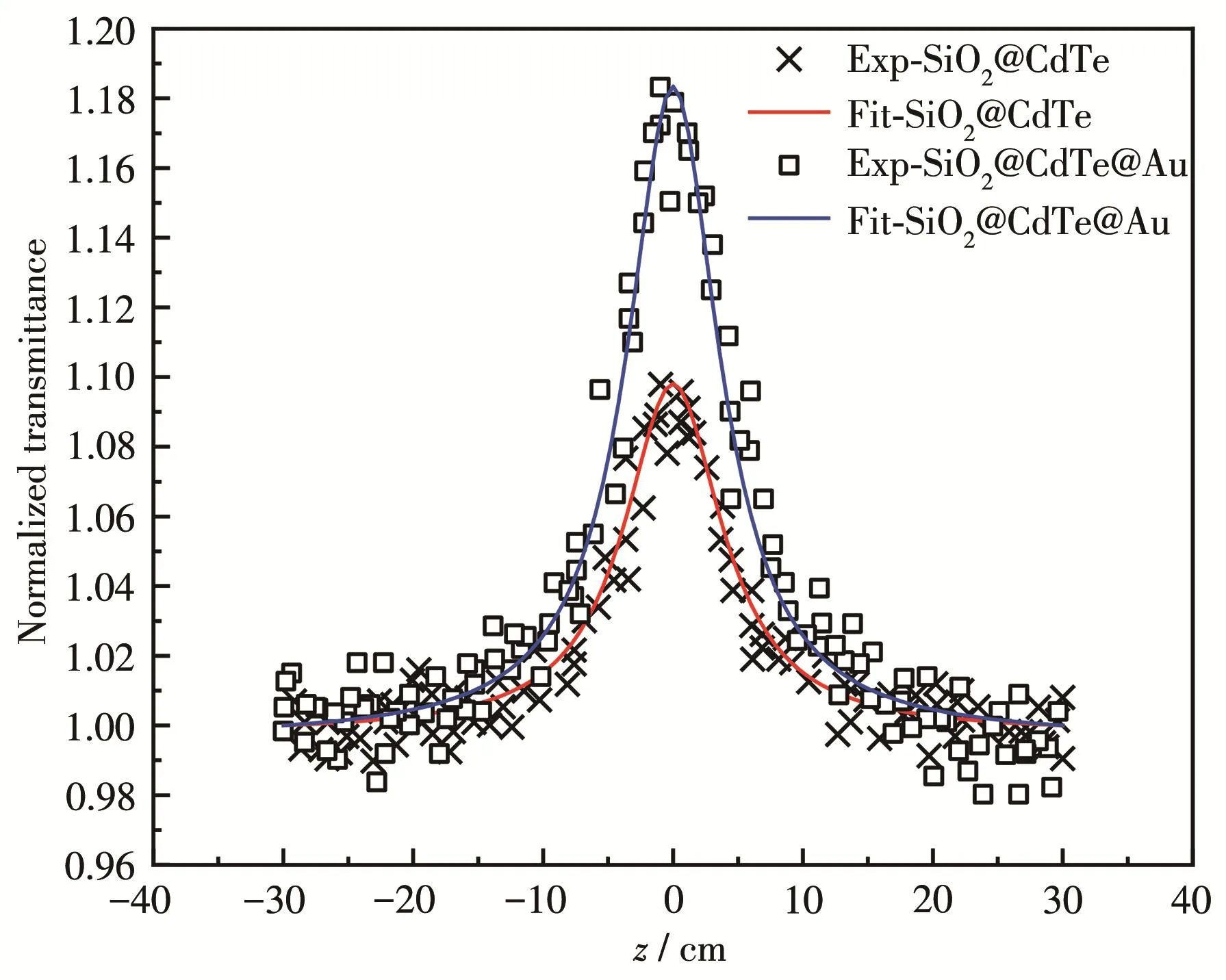
Fig.8 Open aperture Z‑scan normalized transmittance curves of SiO2@CdTe and SiO2@CdTe@Au NPs at 4 ns
Desirable results have been further obtained by fitting the open‑apertureZ‑scan experimental data to a theoretical formula[22].The nonlinear absorption trans‑mittance of theZ‑scan experimental medium can be expressed by Eq.1,and the nonlinear absorption coeffi‑cientβcan be calculated[22]:
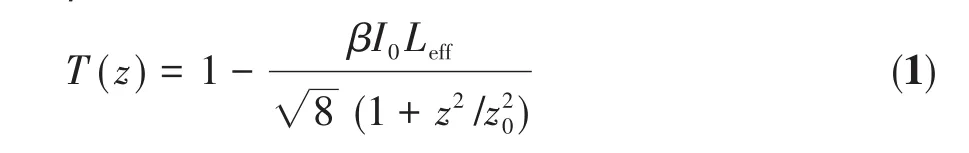
whereI0is the maximum light intensity at the laser focus;Leffis the effective length of the sample,andLeff=(1-T0)l/(-lnT0);T0is the linear transmittance of the solution;lis the sample pool thickness;z0is the diffrac‑tion length of Rayleigh diffraction,andz0=πω02/λ;λis the laser incident wavelength;ω0is the radius of the laser beam waist at the focal point.By the calculation of Eq.1,we can obtain the nonlinear absorption coeffi‑cients of SiO2@CdTe and SiO2@CdTe@Au,as shown in Table 1.
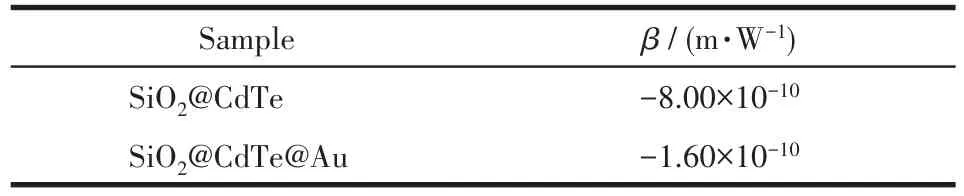
Table 1 Nonlinear absorption coefficients of SiO2@CdTe and SiO2@CdTe@Au
Fig.8 indicated that the nonlinear optical absorp‑tion properties increased with increasing coatings and thatthe materialsexhibited saturated absorption,which means that the transmittance of the laser became larger as the light intensity increased.Various experi‑mental results have proven that the nonlinear absorp‑tion characteristics of SiO2@CdTe@Au are stronger than those of SiO2@CdTe.We speculate that this is all due to the unique surface plasmon resonance phenome‑non of Au NPs.On this basis,we are confident that the nanocomposite material has promising nonlinear opti‑cal absorption properties.
3 Conclusions
Herein,nanocomposites were prepared by taking advantage of the positive and negative charge proper‑ties of the electrolyte solution to adsorb CdTe quantum dots and Au nanoparticles firmly onto SiO2nano‑spheres by layer‑by‑layer adsorption to prepare SiO2@CdTe and SiO2@CdTe@Au nanocomposite mate‑rials.Through a variety of characterization techniques and from different perspectives,the morphologies of the materials were obtained,and the synthesis of the nanocomposites was verified.Finally,the nonlinear optical absorption characteristics of SiO2@CdTe and SiO2@CdTe@Au nanocomposites were studied compar‑atively under the action of nanosecond and picosecond laser pulses usingZ‑scan technology.The experimen‑tal results show that nanocomposite SiO2@CdTe@Au experiences a nonlinear optical property enhancement compared to SiO2@CdTe.The main reason for this enhancementis the surface plasmon resonance enhancement effect of Au nanoparticles.
- 无机化学学报的其它文章
- Coexistence of Two Unique Cu(Ⅱ) Ions in Mononuclear Cu(Ⅱ)Complexes with Furanyl Substituted Triaryltriazoles
- Effect of Zn on Photocatalytic Activity of Block⁃Shaped Monoclinic WO3
- Synthesis,Structure,Luminescence,Photocatalytic and Magnetic Properties of a Neodymium Complex Constructed from Biphenyl⁃3,4′,5⁃tricarboxylic Acid
- First⁃Principles Calculations on Electronic Structures and Optical Properties of g⁃C3N4Nanoribbons
- 二茂铁基-双酮锌配合物的合成、电化学活性及多光子吸收
- 基于(E)⁃N,N⁃二甲基⁃4⁃(2⁃(吡啶⁃4⁃基)乙烯基)苯胺的锌/镉有机-无机杂化金属卤化物的结构和发光性质

