Oxymatrine inhibits cell migration and invasion in non-small-cell lung cancer by down-regulating Toll-like receptor 4*
HUYa-e,YANGPing,MAOJia-hui
(Department of Pathophysiology,Medical School of Nantong University,Nantong 226001,China.E-mail:huli0120@163.com)
[ABSTRACT]AIM:To investigate the therapeutic effect of oxymatrine on non-small-cell lung cancer(NSCLC)A549 cells and a xenograft mouse model,and to explore the underlying molecular mechanisms.METHODS:The effect of oxymatrine on the A549 cell viability was assessed by CCK-8 assay.After the A549 cells were treated with Toll-like re⁃ceptor 4(TLR4)stimulator lipopolysaccharide(LPS)and oxymatrine(5,10 and 15 mmol/L),the mRNA and protein ex⁃pression levels of TLR4 and myeloid differentiation factor 88(MyD88)were analyzed by RT-qPCR and Western blot,re⁃spectively.The migration and invasion abilities of the cells were measured by Transwell assay,and the mRNA and protein expression levels of matrix metalloproteinases-2(MMP-2),MMP-9 and vascular endothelial growth factor(VEGF)were also determined.A xenograft model in nude mice was utilized to evaluate the effect of oxymatrine on tumor growth.RE⁃SULTS:Oxymatrine inhibited the viability of A549 cells,decreased LPS-induced expression of TLR4,MyD88,MMP-2,MMP-9 and VEGF in A549 cells,and suppressed LPS-increased migration and invasion abilities of A549 cells.In the xe⁃nograft model,oxymatrine both reduced tumor growth and inhibited TLR4 expression in the tumor.CONCLUSION:Oxy⁃matrine exerts anti-tumor properties in NSCLC in vitro and in vivo by down-regulating the TLR4/MyD88 signaling pathway,suggesting that oxymatrine can be a potential therapeutic agent for NSCLC.
[KEY WORDS] Oxymatrine;Lipopolysaccaride;Non-small-cell lung cancer;Cell migration;Cell invasion;Toll-like receptor 4
Non-small-cell lung cancer(NSCLC)is account⁃ing for 85%of all lung malignancies and its incidence has a gradual upward trend over the past few de⁃cades[1].Current treatment for the patients with ad⁃vanced NSCLCremains surgery,chemotherapy and tar⁃get-therapy[2].Unfortunately,the 5-year survival rate of the patients with lung cancer is less than 15%,main⁃ly due to its malignant biological behaviors,including tumor migration,invasion and metastasis[3].In addi⁃tion,chemotherapy is often associated with multidrug resistance and toxic side effect.Therefore,further in⁃vestigation into the mechanism of invasion and metasta⁃sis of lung cancer as well as searching for novel treat⁃ment strategies have become increasingly important.
Studies have showed that many chronic inflamma⁃tory pulmonary diseases have a close relationship with lung cancer[4].Lipopolysaccharide(LPS),the major component of the outer membrane of Gram-negative bacteria,triggers Toll-like receptor 4(TLR4)signaling in immune cells and tumor cells.The TLR4/myeloid differentiation factor 88(MyD88)signaling pathway is a classic signaling pathway associated with inflamma⁃tion.LPScan promote the malignant proliferation,in⁃vasion and migration of human NSCLC cells by activa⁃tion of TLR4[5].Recent experiments show that TLR4 over-expresses in different metastatic tumor cells and positively correlated with drug resistance,tumor cell survival and metastasis[6].TLR4 is a viable therapeutic target for NSCLC metastasis augmented by gram-nega⁃tive pneumonia[7].
Oxymatrine(OMT;molecular formula:C15H24N2O2)is an alkaloid extracted from the dried root ofSophora flavescensAiton.Several studies have found multiple pharmacological effects of OMT,including anti-inflam⁃matory,anti-virus,anti-fibrosis and anti-arrhythmia ac⁃tivities[8-11].Furthermore,OMT has been reported to have anti-tumor properties,including the inhibition of cancer cell proliferation,cell cycle and angiogenesis,and the promotion of apoptosis in gastric,bladder and breast cancers[12-14].Recently,studies showed that OMT induced apoptosis in human lung cancer A549 cells[15].In our previous study,we found that OMT inhibited LPS-induced inflammation by down-regulating TLR4 signaling in macrophages[10].However,the anti-tumor effects of OMT on the malignant biological behaviors of human NSCLC,including tumor migration,invasion and metastasis are largely unknown.
Therefore,the present study aimed to evaluate the therapeutic effect of OMT on NSCLC A549 cells and a xenograft mouse model,to explore the underlying mo⁃lecular mechanisms.
MATERIALSAND METHODS
1 Cell culture and drug treatment
A549 cells,a human NSCLC cell line(American Type Culture Collection),were cultured in RPMI-1640 medium(HyClone)with 10%fetal bovine serum,1×105U/L penicillin and 100 mg/L streptomycin in the in⁃cubator with a humidified atmosphere(5% CO2,37℃).At about 80%confluence,the cells were di⁃gested with a solution containing 0.25%trypsin and 0.002%EDTA(Gibco)and collected for subsequent experiments.
A549 cells were seeded into 6-well culture plate(5×105cells/well)and incubated overnight.The cells were randomly divided into 6 groups:untreated group,the cells were cultured with RPMI-1640 medium;LPS group,the cells were treated with LPS(10 mg/L;Sig⁃ma);LPS+OMT groups,the cells were pretreated with various concentrations of OMT(5,10 and 15 mmol/L,respectively;Baoji F.S.Biological Development Com⁃pany)1 h prior to the treatment of LPS(10 mg/L)for another 24 h;LPS+TAK-242 group,the cells were pre⁃treated with TAK-242(100 nmol/L;MedChemEx⁃press)for 1 h,and then were treated with LPS(10 mg/L)for 24 h.
2 Cell Counting Kit-8(CCK-8)assay
A549 cells were seeded in 96-well plates(2×103/well)and cultured overnight.Subsequently,various concentrations of OMT(0,3.75,7.5,15 and 30 mmol/L)were added to cells for 24 h,48 h and 72 h.Then the supernatant was replaced by 100µL of RPMI-1640 medium containing 10µL of CCK-8(Beyotime Biotechnology)and the cells were incubated for 2 h at 37℃.The absorbance(A)was measured at a wave⁃length of 450 nm by an ELx800 microplate assay reader(BioTek).The inhibition rate of the viability of A549 cells was calculated by(1−A450in treatment group/A450in untreated group).
3 RT-qPCR
Total RNA was extracted with Trizol™(Fermen⁃tas),and then 2µg of total RNA was reversely tran⁃scribed into cDNA according to the reverse transcrip⁃tion protocol(Fermentas).The primers used were showed in Table 1.The PCR was run in a 10µL reac⁃tion system,which was composed of 5µL SYBR Green Master Mix(Life),0.5µL template cDNA,0.2µL each of forward and reverse primers,and 4.1µL of nu⁃clease-free water.The amplifying conditions were as follows:95℃for 10 min,followed by 40 cycles of 95℃for 15 s,60℃for 30 s,and 72℃for 30 s.Each sample was subjected to PCR in triplicate,and three in⁃dependent experiments were done.For quantification,the mRNA of interest was normalized with GAPDH that served as an internal control.The specificity of the am⁃plified PCR products was valued by the relative cycle threshold(Ct)method,which was calculated by the 2−ΔΔCt.
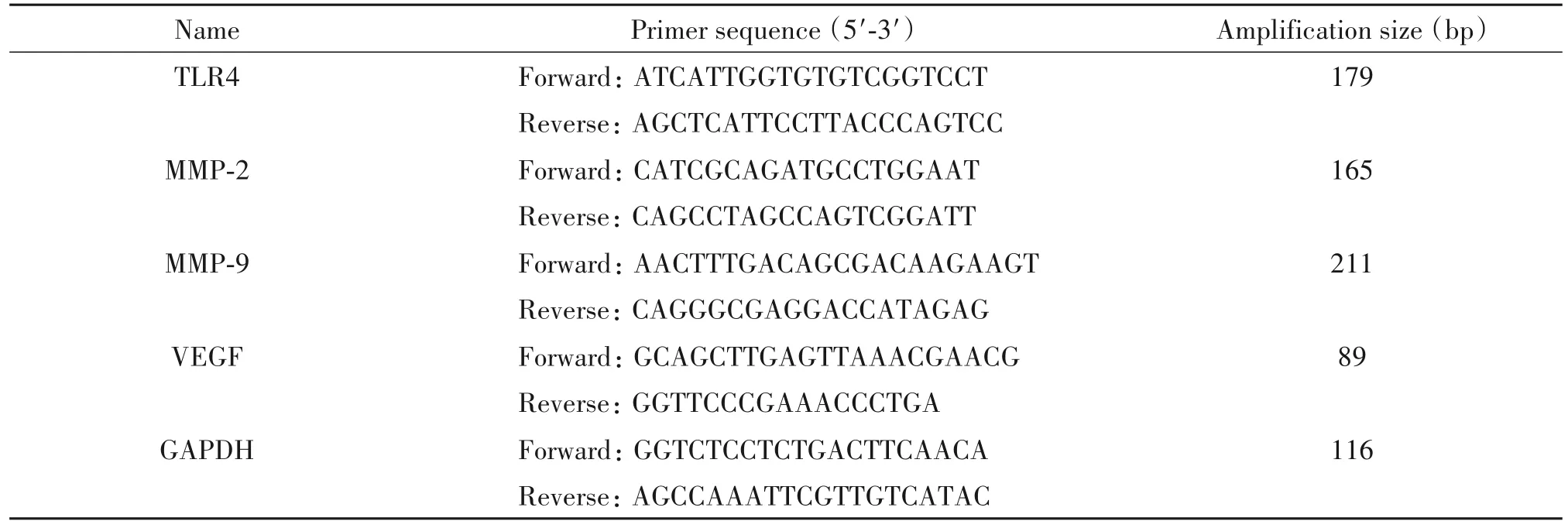
Table 1.The sequences of the primers for RT-qPCR
4 Western blot analysis
A549 cells or tumor tissue were washed and lysed in buffer(50 mmol/L Tris,pH 7.4,150 mmol/L so⁃dium chloride,1%Triton X-100,1%sodium deoxy⁃cholate,0.1%sodium dodecyl sulfate,supplemented with protease inhibitor cocktail)on ice for 30 min.Then the lysates were centrifuged at 12 000 r/min for 10 min at 4℃and the supernatant was stored at−80℃.The protein was separated on a 10%SDS-PAGE and electroblotted onto a polyvinylidene difluoride mem⁃brane by Mini-Protean transmembrane system under constant current(200 mA)for 120 min.Membraneswere blocked in 5%non-fat dried milk for 1 h at room temperature and then incubated with primary antibodies that recognized TLR4(1∶200,Abcam),MyD88(1∶1 000,CST),matrix metalloproteinase-2(MMP-2;1∶1 000,Abcam),MMP-9(1∶1 000,Abcam),vascular endothelial growth factor(VEGF;1∶1 000,Abcam)and GAPDH(1∶2 000,CST)overnight at 4℃.Then,the membranes were incubated in the horseradish peroxidase(HRP)-conjugated goat anti-rabbit IgG(1∶5 000,Santa Cruz)at room temperature for 2 h.En⁃hanced chemiluminescence was used for detection.Im⁃munoreactive bands were scanned and quantified by ImageJ software,and the amount of target protein was normalized with GAPDH in the same lane.
5 Transwell migration and invasion assays
Polycarbonate membrane culture inserts with 8-µm pore size(Corning)were put into the wells of 24-well culture plates to separate upper and lower cham⁃bers.In migration assay,cells(1.5×104)were seeded into upper chambers in serum-free medium,and 500µL complete medium(RPMI-1640 medium with 10%fetal bovine serum)was added to the lower chambers.In invasion assay,Matrigel(BD)was diluted(1∶5)in RPMI-1640 medium,and the mixture was added to each chamber at low temperature(on ice)and main⁃tained at 37℃for 30 min.After Matrigel was poly⁃merized sufficiently,600µL complete medium was added to the bottom of the chambers and neutralized for 1 h.The cells(3×104)in serum-free RPMI 1640 me⁃dium were added into the upper chambers,and 300µL of complete medium was added to the lower chamber.Seeded cells were incubated at 37℃overnight and then treated with or without LPS,OMT or TAK-242 de⁃scribed in drug treatment.The numbers of migratory or invasive cells on the back side of the polycarbonate membrane were quantified by counting 5 independent visual fields under the microscope(Olympus 600 Auto⁃biochemical Analyzer),and the morphological changes of the cells were observed by staining with Wright-Giemsa Stain Kit(Nanjing Jiancheng Bioengineering Institute).
6 Animal model
A xenograft model was conducted in athymic BALB/c(nu/nu)mice(Slake).All experimental proto⁃cols used in this study were approved by the Animal Ethics Committee of Nantong University.Six-week-old mice were housed in a specific-pathogen-free facility with 12 h/12 h light-dark cycles and free access to food and water.After treated with LPS(10 mg/L)for 24 h,A549 cells(1×107cells suspended in 200µL PBS)were subcutaneously injected into the lower right flank of the mice under sterile conditions.When average tu⁃mor volume reached about 50 mm3,the mice were ran⁃domly divided into 2 groups(n=5):vehicle control group and OMT-treated group.The mice in vehicle con⁃trol group were intraperitoneally injected with PBSonce daily,while the mice in OMT-treated group were intra⁃peritoneally injected with OMT(60 mg/kg)once daily.Tumors were measured using vernier calipers every 5 d.Tumor volumes were calculated according to the fol⁃lowing formula:volume=length×width2/2.On the 30th day,all mice were anethetized,and were sacri⁃ficed via cervical dislocation.The tumors were dissect⁃ed,weighed,and frozen at−80℃for further work.
7 Statistical analysis
All data were expressed as mean±standard devia⁃tion(SD),and analyzed using SPSS 17.0.Graphs were plotted with GraphPad Prism 8.0 software.The data were analyzed using non-linear regression,t-test,and one-way analysis of variance(ANOVA)with Schef⁃fe post-hoc test or repeated measures two-way ANOVA with Greenhouse-Geisser post-hoc test.P<0.05 was considered as statistically significant.
RESULTS
1 Oxymatrine inhibited the viabilityof A549 cells
The results of CCK-8 assay showed that OMT in⁃hibited the viability of A549 cells in a dose-and timedependent manner(Figure 1).The half-maximal in⁃hibitory concentration(IC50)of OMT for A549 cells at 24,48 and 72 h were approximately(15.85±1.11),(10.98±1.13)and(6.87±1.12)mmol/L,respective⁃ly.Based on the curves,we chose 0,5,10 and 15 mmol/L as the concentration range in the following ex⁃periments.

Figure 1.Oxymatrine(OMT)time-and dose-dependently inhibited the viability of A549 cells.The A549 cells were treated with OMT at 0,3.75,7.5,15 and 30 mmol/L for 24,48,or 72 h,and the inhibi⁃tion rate was evaluated by CCK-8 assay.Non-linear regression was used for analysis of the data.Mean±SD.n=6.
2 Oxymatrine inhibited LPS-induced expression of TLR4 and MyD88 in A549 cells
RT-qPCR results revealed that LPS(10 mg/L)ele⁃vated the mRNA levels of TLR4 and MyD88 in A549 cells,which were inhibited by OMT(10 and 15 mmol/L)and almost completely blocked by TLR4 an⁃tagonist TAK-242(P<0.01,Figure 2A).The protein levels of TLR4 and MyD88 stimulated by LPSwere also reduced by 10 and 15 mmol/L OMT and by TAK-242(Figure 2B).

Figure 2.Oxymatrine(OMT)inhibited LPS-induced expression of TLR4 and MyD88 in A549 cells.A:relative mRNA levels of TLR4 and MyD88 were assessed by RT-qPCR;B:Western blot was used to detect the changes of TLR4 and MyD88 protein levels.Mean±SD.n=3.**P<0.01 vs untreated control group;#P<0.05,##P<0.01 vs LPSgroup.
3 Oxymatrine inhibited LPS-induced migration and invasion of A549 cells
Transwell assays were used to evaluate the effect of OMT on the migration and invasion of A549 cells.The results showed that LPS enhanced the migration and invasion ablilties of A549 cells compared with the non-stimulation control,whereas OMT at concentra⁃tions of 10 and 15 mmol/L significantly decreased LPS-induced migration and invasion abilities of A549 cells,exhibiting a effect similar to TAK-242(Figure 3),indi⁃cating that OMT inhibited LPS-enhanced migration and invasion of 549 cells.

Figure 3.Oxymatrine(OMT)inhibited LPS-induced migration and invasion of A549 cells.Both migration(A)and invasion(B)were evaluated with Transwell assays except that in the latter case the culture insert was pretreated with Matrigel.The migratory/invasive cells were stained with Wright-Giemsa Stain Kit and visualized under microscope(×100).Mean±SD.n=3.**P<0.01 vs untreated control group;##P<0.01 vs LPSgroup.
4 Oxymatrine down-regulated LPS-induced ex⁃pression of MMP-2/9 and VEGF in A549 cells
The genes related to invasion and angiogenesis were detected in A549 cells.After the exposure to 10 mg/L LPS for 24 h,both mRNA and protein levels of MMP-2,MMP-9 and VEGF in A549 cells were signifi⁃cantly increased compared with the non-stimulation control(P<0.01).However,when treated with OMT(10 and 15 mmol/L)or TAK-242,the LPS-induced in⁃creases in the expression of MMP-2,MMP-9 and VEGF were significantly reduced(P<0.01,Figure 4).
5 Oxymatrine inhibited tumor growth in vivo
We next tested whether OMT suppresses tumor growthin vivoin a xenograft model.We performed timelapse measurements of the tumor dimensionsin vivoand found that the tumors in vehicle control group showed an exponential growth from 10 d after implantation on⁃wards,while the tumor growth was markedly inhibited by OMT treatment(Figure 5A).At 30 d after initiation of drug administration,the weight and size of tumors in OMT-treated group were markedly reduced as com⁃pared with vehicle control group(P<0.01,Figure 5B and C).In addition,the protein expression level of TLR4 in the tumor was significantly inhibited by OMT(P<0.01,Figure 5D).
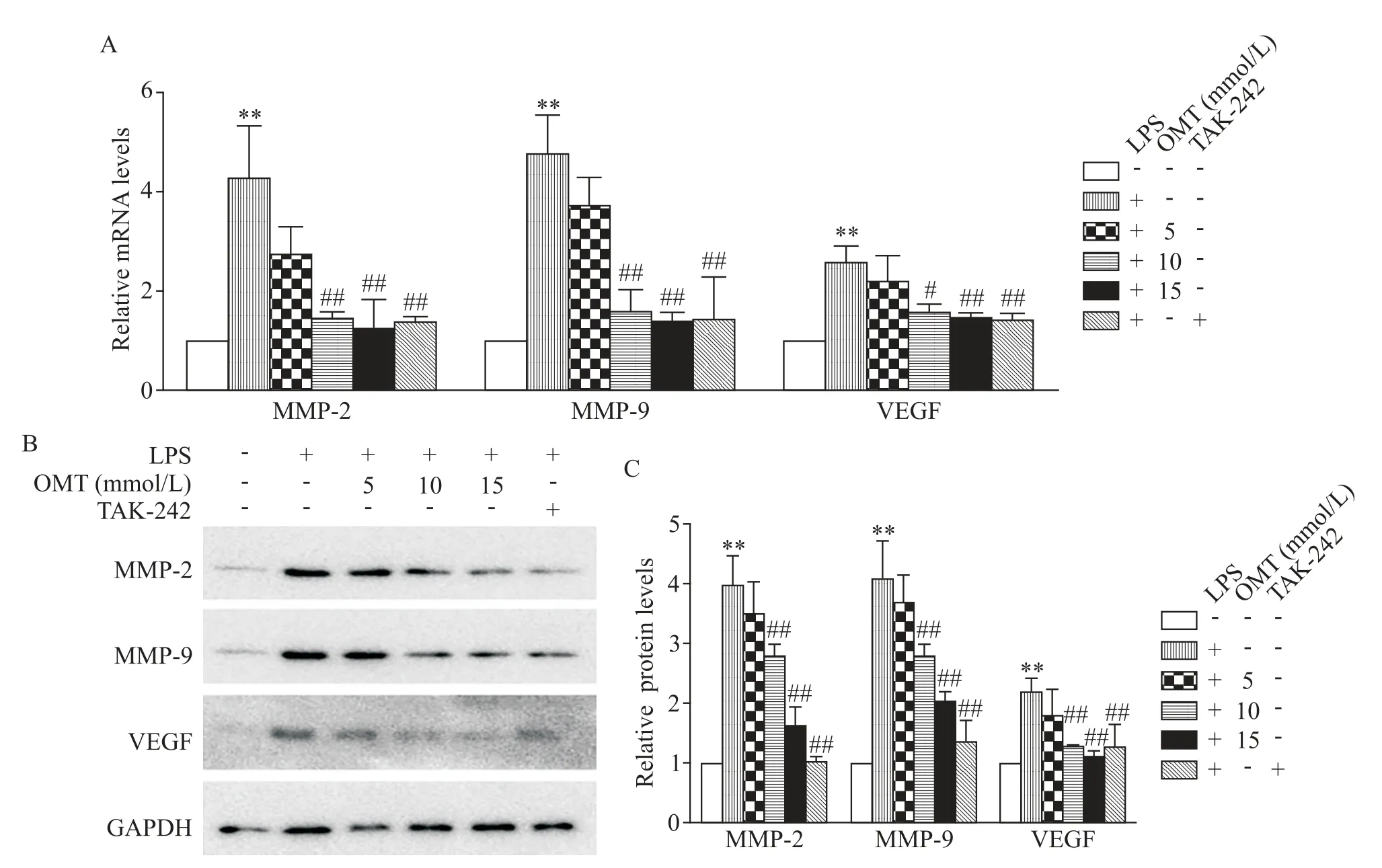
Figure 4.Oxymatrine(OMT)reduced LPS-induced expression of MMP-2,MMP-9 and VEGF in A549 cells.A:relative mRNA levels of MMP-2,MMP-9 and VEGF were assessed by RT-qPCR;B:Western blots was used to detect the protein levels of MMP-2,MMP-9 and VEGF.Mean±SD.n=3.**P<0.01 vs untreated control group;#P<0.05,##P<0.01 vs LPS group.
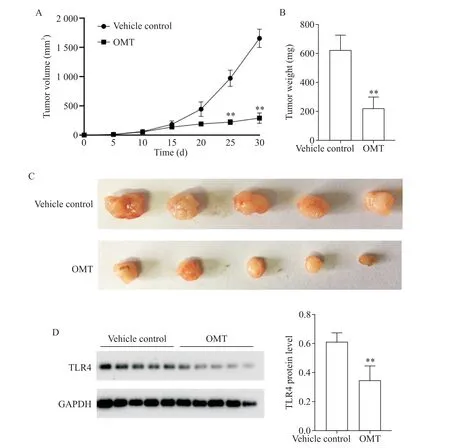
Figure 5.Oxymatrine(OMT)inhibited tumor growth and the expression of TLR4 in the tumor in a human lung cancer xe⁃nograft mouse model.LPS-treated A549 cells were subcutaneously injected into the lower right flank of athymic BALB/c(nu/nu)mice,which were treated with daily intraperitoneal injection of OMT(60 mg/kg)or PBSas a vehicle control,starting on the day when average tumor volume reached almost 50 mm3.A:time-lapse measurements of tumor growth in vi⁃vo;B:weight of the tumor 30 d after inoculation;C:photographs showing the size of tumors dissected 30 d after inocula⁃tion;D:Western blot was used to detect the protein level of TLR4.Mean±SD.n=5.**P<0.01 vs vehicle control at the same time point.
DISCUSSION
Recently,the TLR4 signaling pathway has at⁃tracted lots of attention in the f ield of anti-cancer drug development.This pathway is mainly responsible for in f lammation and is known as a major contributor to me⁃tastasis[16].TLR4 ligation with LPS induces a range ofeffects in human tumor cells,depending on the cell type,culture conditions and ligand concentrations[17].In addition,studies found that Gram-negative bacteria facilitated outgrowth and metastasis of human lung can⁃cer cells through TLR4 signaling[18].TLR4 is also me⁃diates MyD88 dependent pathways by interaction with a series of cytokines to promote tumorigenesis.MyD88 is a key mediator in the process of MyD88-dependent sig⁃nal transduction[19].Evidences show that TLR4 is overexpressed in various tumor cell lines and TLR4 sig⁃naling has been implicated in invasion,metastasis,and survival of various cancers[20-21].It has been re⁃ported that OMT exhibits significant anti-cancer effects on a variety of tumor cells by modulation of multiple sig⁃naling pathways[22-23].In this study,our results showed that TLR4 and MyD88 were expressed in human NSCLCA549 cells,and the expression level of these 2 proteins was increased notably in response to LPS stimulation and significantly reduced by OMT and TLR4 antagonist TAK-242.
In the case of NSCLC,the high metastatic poten⁃tial remains the major therapeutic challenge.Previous study has reported that the TLR4/MyD88 signaling path⁃way is involved in cancer metastasis[24].Cancer metas⁃tasis,the main causes of morbidity and mortality in mil⁃lions of patients with cancer,depends on the invasion and migration of cancer cells.Transwell chamber assay was used to detect the anti-metastatic effect of OMT.Our findings of transwell migration and invasion assay indicated that activation of TLR4 played an effective role in the migrasional and invasive potential of A549 cells and provided evidence of the antimetastatic effect of OMT.Therefore,it was likely that TLR4/MyD88 sig⁃naling contributed to cell migrational and invasive ac⁃tivity and OMT may affect cell migration and invasion in human NSCLC cells through a synergistic effect on the TLR4/MyD88.
The MMPs,especially MMP-2 and MMP-9,play a major role in promoting tumor metastasis involved in the degradation of extracellular matrix(ECM)[25].Loss of ECM of blood or lymph vessels facilitates cancer cells to invade into the blood or lymphatic system and spread to other tissues and organs.Angiogenesis is the physiological process formed by vascular endothelial ortumor cells.An anti-angiogenic approach has been widely accepted as a most promising strategy to control tumor growth and metastasis[26].The VEGF is a critical driver of sprouting angiogenesis that functions by regu⁃lating vascular formation,remodeling and permeabili⁃ty.Previous reports indicate that silencing TLR4 de⁃creases the expressions of MMP-2 and MMP-9 and thereby inhibits the proliferation,migration and inva⁃sion of prostate cancer cells[27].Meanwhile,the inhibi⁃tion of TLR4/MyD88 pathway restrains angiogenesis and pulmonary metastasis by suppressing VEGFexpres⁃sion in hepatocellular carcinoma[28].The present study indicates that after LPS stimulation,human NSCLC A549 cells expressed higher level of MMP-2,MMP-9 and VEGF,while OMT inhibited LPS-induced expres⁃sion of MMP-2,MMP-9 and VEGF.Therefore,OMT and TLR4 antagonist TAK-242 exert antimetastatic ef⁃fect in human NSCLC A549 cells by reducing MMP-2,MMP-9 and VEGFexpressions.
In a recent study,Liu et al[29]reported that OMT enhanced the inhibitory effect of 5-fluorouracil on hepa⁃tocellular carcinoma bothin vitroandin vivo.More⁃over,OMT prominently suppressed tumor growth in a lung cancer mouse model[30].After establishing the sig⁃nificant anti-tumor effects of OMT by inhibiting TLR4 in human NSCLC A549 cellsin vitro,we investigated whether OMT also inhibited tumorigenicity in a mouse tumor model in the present study.In vivo,the tumor volume and weight in OMT-treated group were signifi⁃cantly lower than those in vehicle control group.Our re⁃sults indicate that OMT suppressed the growth of A549 lung tumors in nude mice and decreased TLR4 expres⁃sion in the tumor.Therefore,OMT inhibited tumor growthin vivoby decreasing TLR4 in cancer cells.
In conclusion,OMT exhibited significant inhibito⁃ry effect on proliferation,migration and invasion of A549 cells and markedly inhibited NSCLC tumor growth in xenografted mice,and the inhibitory effect is likely to be attributable to inhibition of the expression of MMP-2,MMP-9 and VEGF and blockade of the ac⁃tivity of TLR4/MyD88 signaling pathway.These results point to OMTas a promising therapeutic agent to inhibit metastasis of NSCLC.

