双臂夹结构的高效抗菌剂及其治疗伤口感染研究
王晓菊,李颖,周思荣,冯丽恒*
(1. 山西大学 分子科学研究所,教育部化学生物学与分子工程实验室,山西 太原 030006;2. 山西大学 化学化工学院,山西 太原 030006)
0 Introduction
Bacterial infections have always threatened the health of people, especially those in developing coun‐tries[1-5]. It is reported that 70%-80% morbidities and mortalities is ascribed to wound infections[6], a most common acquired infection in hospital.Pseudo‐monas aeruginosa(P. aeruginosa) is generally found(about 15%) in hospital acquired wound infection[7].Except for acquired infection,sepsis caused byEsche‐richia coli(E.coli) leads to more than 8 500 deaths according to the health-care system of USA[8]. In ad‐dition,disease such as gastroenteritis triggered bySal‐monellaalso torments people around the world[9]. Al‐though the use of antibiotics has played a great role in killing pathogenic microorganisms,drug resistance re‐sulting from the misuse and overuse of antibiotics be‐come an another big medical issue[10-13]. Therefore, it still needs endeavors to develop more effective bacte‐ricides to eradicate pathogenic microorganisms[14-15].
Various materials were developed to be antibacte‐rial agents, including inorganic and organic materi‐als[16-26]. Organic small molecules have precise molec‐ular structures and superior properties, and without threaten of long-term biotoxicity of inorganic met‐al[27-30]. Among these organic materials, quaternary ammonium salts(QAS)are considered to be a class of broad-spectrum antimicrobial agent[31-37]. Ionic form makes them water-soluble and beneficial to interact with membrane of microbes. Positive charges facili‐tate QAS to absorb onto negative charged bacteria through electrostatic interactions, then hydrophobic part can penetrates the cell wall of bacteria causing the bacteria to die[38]. QAS not only kill bacteria and inhibit their growth, but also hardly generate resis‐tance by destructing cell membrane[39-40]. Therefore,numerous QAS-based organic materials were devel‐oped to kill bacteria and some of them were market available, such as benzalkonium chloride and cetyl‐pyridinium chloride[41]. It is necessary to develop more potent QAS-based biocides to address the trou‐ble in killing bacteria for the moment.
We synthesized a novel biocide, termed as BPBDN, based on pyridine QAS for wound infec‐tions treatment. The compound BPBDN was de‐signed to have two pyridine moieties by reacting with 2,2'-bis(4-(bromomethyl)benzyloxy) -1,1'- dinaphtha‐lene. Increased positive charges and electron donat‐ing action endow BPBDN with potent antibacterial ac‐tivity against three common pathogenic bacteria, such asE.coli(ampicillin-resistant),P. aeruginosaand
Salmonella typhimurium(S. typhimurium). The membrane-disrupted action of BPBDN was shown to be the killing mechanism of bacteria,and lipopolysac‐charide (LPS) is demonstrated to play a role in the killing process. Therefore, BPBDN can effectively kill bacteria,but have little cytotoxicity,which is ben‐eficial for further treatment of wound infection. Final‐ly, BPBDN was successfully applied in the treatment of wound infection of living mice.
1 Materials and Methods
1.1 Materials and measurements
All chemical reagents were obtained from com‐mercial companies and used without further treat‐ments. The ampicillin-resistantE. coliTOP 10 was purchased from Beijing Bio-Med Technology Devel‐opment Co., Ltd.P. aeruginosawas purchased from Beijing Solarbio Science & Technology Co., Ltd.,andS. typhimuriumwas purchased from China Cen‐ter of Industrial Culture Collection.
UV-vis absorption spectrum was recorded on a UH5300 spectrophotometer and fluorescence emis‐sion spectrum was measured on a Hitachi F-4600 fluo‐rescence spectrophotometer with a Xenon lamp as the excitation source. Zeta potentials were measured by using Malvern ZetaSizer Nano ZS90. The morpholo‐gy ofE.coliwas observed by using scanning elec‐tron microscopy (SEM, JEOL JSM-6510). Stained bacteria were determined by a confocal laser scanning microscopy(Zeiss LSM 880).
1.2 Synthesis of 2,2'-bis(4-(pyridine-methylene)-benzyloxy)-1,1'-di-naphthalene dibromoides(BPBDN)
2, 2'-Bis(4- (bromomethyl)benzyloxy) -1, 1'-di‐naphthalene was synthesized according to the previ‐ous literature[42]. The synthetic process of BPBDN was shown in Scheme 1. The specific process was de‐scribed as follows: 2, 2'-dihydroxy-l, l'-dinaphthyl(0.57 g, 2.00 mmol) in 15 mL acetone was heated at 65 °C and stirred vigorously to obtain homogeneous solution. Under nitrogen atmosphere, 1,4-bis(bromo‐methyl) benzene (2.11 g, 8.00 mmol) and K2CO3(0.30 g, 2.20 mmol) were added into the homoge‐neous solution. Then, the mixture was heated and re‐fluxed for 6 h. After filtering at high temperature, the solid was extracted with dichloromethane. The col‐lected organic layer was washed with water and dried over anhydrous Na2SO4. The solvent was removed under vacuum to obtain crude product. Silica gel chromatography was used to purify the crude product with dichloromethane/petroleum ether (V/V=1:3) as the eluent to obtain 2,2'-bis(4-(bromomethyl) benzy‐loxy)-1,1'-dinaphthalene(0.45 g,34.5%).
A mixture of 2,2'-bis(4-(bromomethyl)-benzy‐loxy)-1,1'-dinaphthalene (1.96 g, 3.00 mmol) and pyridine (0.48 mL, 6.00 mmol) in 20 mL DMF was stirred at 70 °C for 48 h under nitrogen atmosphere.After cooling to room temperature, 200 mL acetone was added into resulting solution under stirring. Then white precipitate was obtained and collected by filtra‐tion. After washed with DMF, acetone, and ether, re‐spectively, the white product BPBDN (0.92 g,37.8%) was obtained by drying under vacuum.1H NMR (DMSO-d6, 600 MHz)δ9.12 (d, 4H), 8.60 (t,2H), 8.14 (t, 4H), 8.04 (d, 2H), 7.95 (d, 2H), 7.61(d, 2H), 7.35 (t, 2H), 7.30 (d, 4H), 7.24-7.25 (m,2H), 7.02 (d, 4H), 6.97 (d, 2H), 5.75 (s, 4H), 5.15(m, 4H);13C NMR (DMSO-d6, 150 MHz)δ63.01,69.86, 115.23, 120.38, 123.41, 124.51, 127.46,128.35, 132.68, 133.86, 140.23, 145.72, 147.35,149.82, 153.49; HRMS-ESI for C46H38N2O22+(m/z):325.1461.
1.3 Antibacterial experiments
BPBDN (5, 10, 15, 20 µmol/L) was respectively incubated withE. coli(OD600= 0.2),P. aeruginosa(OD600= 0.2),S. typhimurium(OD600= 0.2) for 20 min in PBS. Then theE. coliandP. aeruginosasus‐pensions were serially diluted 3×104fold with PBS,respectively. TheS. typhimuriumsuspension was di‐luted 2.5×104fold with PBS. Then a 100 µL portion of the diluted bacteria solution was spread on the sol‐id medium agar plate. ForE. coli, the agar plate was supplemented with 50 µg/mL ampicillin. After incu‐bation at 37 °C for 16 h, the colonies formed and were counted. The bacteria without BPBDN treat‐ment was performed as blank group. The viability rate (VR) was calculated according to the following equation:

WhereCis the colony forming unite (cfu) of experi‐mental groups andC0is the cfu of blank group with‐out BPBDN treatment.
1.4 Cytotoxicity assay
The cytotoxicity of BPBDN against HeLa and HEEC cells was evaluated by a standard methyl thia‐zolyl tetrazolium (MTT) assay. HeLa and HEEC cells were seeded into 96-well plates at a density of 5×103cells/well, respectively. After incubation for 24 h,the cells were further incubated with fresh culture me‐dium containing different concentrations of BPBDN(5, 10, 20, 30 μmol/L) for 12 h. After removed the previous medium, 10 μL of MTT (5 mg/mL) solution was added to each well. After another incubation for 3 h, 100 μL DMSO per well was added to replace the supernatant for dissolve formazan produced by cells.Then the absorbance at 490 nm was measured using iMark microplate absorbance reader (Bio-Rad). All data were conducted in triplicate and presented as mean ± SD compared to the OD values of untreated cells.

Scheme 1 The Synthesis route of BPBDN
1.5 Confocal laser scanning microscopy (CLSM)characterization
E. coli(OD600= 0.2) were incubated with BPBDN (20 µmol/L) for 20 min. Untreated bacteria(without BPBDN) were incubated under exactly the same conditions, which performed as control group.After removal of unbounded BPBDN by centrifuga‐tion (10 000 r/min, 2.0 min), the obtained pellets were suspended in 100 μL PBS. A 30.0 μL portion of mixture of PI and SYTO9 was added to suspension to incubate for 20 min. Another centrifugation(10 000 r/min, 2.0 min) was performed to obtain pellets and then were suspended in 30 μL PBS. A 4.0 μL portion of the suspension was added to a clean glass slice to be observed by CLSM using a 488 nm laser for SY‐TO9 and PI.
1.6 Zeta potential measurements
E. coliin PBS (OD600=1.0) was incubated with BPBDN (20 μmol/L) for 20 min. After removal of unbounded BPBDN by centrifugation (10 000 r/min,3 min), the obtained pellets were suspended in 1.0 mL of sterile water for zeta potential measurements.As control,untreated bacteria and BPBDN alone were also measured under exactly the same conditions.
1.7 Scanning electron microscopy(SEM)character‐ization
After the antimicrobial experiments, a portion of bacteria samples were dropped onto clean silicon slic‐es and dried in clean bench. After dried,1.0%glutar‐aldehyde was used for fixation overnight. The speci‐mens were washed with sterile water thrice after re‐moval of glutaraldehyde. Ethanol was added in a graded series (40%, 70%, 90%, and 100% for 6 min,respectively) and the specimens were dried naturally in the air. The dried specimens were processed to be observed by SEM.
1.8 Animal experiments
All animal procedures were performed in accor‐dance with the relevant laws and guidelines approved by the Animal Care and Use Committee of Shanxi University. Female BALB/c mice (six weeks old)were obtained from Vital River Company in this ex‐periment. To build an infected wound, a surgical pro‐cedure was performed on the back of mouse andE.coli(1.0×108CFU/mL, 10 μL) was dropped on the wound (5.0 mm in diameter). All mice were divided into 2 groups (3/groups): PBS and BPBDN. After in‐fection for 24 h, 20 μL BPBDN solution (60 μmol/L)was dropped on the infected wound in BPBDN group,and 20 μL PBS was added in the same way in PBS group. The wounds of the mice were observed and imaged every two days following the treatment. After 12-day treatment, all mice were executed and major organs (such as heart, liver, lung, kidney and spleen)were dissected from the mice, processed by freezing microtome, and stained with Hematoxylin and Eosin(H&E). The pathologies photographs were obtained by using an optical microscope.
2 Results and Discussion
2.1 Synthesis and characterization of BPBDN
We choose pyridine salts to construct antibacteri‐al QAS. 2,2'-dihydroxy-l,l'-dinaphthyl reacted with 1,4-bis(bromomethyl)benzene under the previously re‐ported method, and the binaphthyl derivative with two reaction sites was added into pyridine in DMF un‐der a nitrogen atmosphere. After stirring at 70 ℃for 48 h, the target compound BPBDN was obtained(37.8% yield). The structure of BPBDN was con‐firmed by1H-NMR,13C-NMR, and MS-ESI, respec‐tively.
After verified the structure of BPBDN, the solu‐bility and photophysical properties of BPBDN were then evaluated. For absorption spectrum of BPBDN,there are two absorption peaks located at 260 nm and 337 nm,respectively. Excited at 346 nm,BPBDN dis‐plays a maximum fluorescence peak at 422 nm (Fig.1a). As shown in Fig. 1b, BPBDN has good disper‐sion in water with a diameter of 12 nm. In addition,BPBDN was measured to possess a positive potential of (26.3 ± 3.3) mV due to the presence of two pyri‐dine units (Fig. 1c). Good water solubility and posi‐tive charges promote BPBDN to interact with nega‐tively charged bacteria in biological system and fur‐ther disrupt the membrane stability. Moreover, densi‐ty functional theory (DFT) was used to perform theo‐retical calculation of BPBDN,the optimize configura‐tion and electron density distributions of the HOMO and LUMO energy levels of BPBDN were shown as Fig. 1d. The HOMO electron cloud of BPBDN was mainly distributed on binaphthyl moiety, and the LU‐MO electron density was mainly distributed on pyri‐dine moiety. Such electron cloud may affect the anti‐bacterial activity of pyridine QAS.

Fig. 1 a)Normalized absorption and emission spectra of BPBDN in DMSO;b)Hydrodynamic size distribution of BPBDN(20 μmol/L)in water;c)Zeta potentials of BPBDN in water(20 μmol/L);d)HOMO and LUMO of BPBDN
2.2 Cytotoxicity and antibacterial activity of BPBDN
Pyridine,as an active antibacterial site,was intro‐duced to the construction of many QAS-based anti‐bacterials[43-44]. It was reported that electron donating group in the structure of pyridine QAS could increase the antibacterial activity[45]. Hence, we chose modi‐fied 2,2'-dihydroxy-l,l'-dinaphthyl to link two pyri‐dine molecules, resulting in increased positive charge density. To demonstrate the antibacterial activity of synthesized BPBDN,three common pathogenic bacte‐riaE.coli(ampicillin-resistant),P. aeruginosa,S. ty‐phimuriumwere used to perform the antibacterial ex‐periments. As shown in Fig. 2a, BPBDN showed an excellent antibacterial activity toward three kinds of bacteria at a low concentration (20 μmol/L) of BPBDN. The bacterial colonies growth ofE.coli,P.aeruginosaandS. typhimuriumafter treatment of BPBDN were shown in Fig. 2b. These results sug‐gested that BPBDN had a concentration-dependent ef‐fect on the bacterial growth, and could kill bacteria at a treated concentration above 20 μmol/L. For further application of infection treatment, the cytotoxicity of BPBDN was evaluated by using standard MTT assay(Fig. 2c and 2d). Within the evaluated concentration range, BPBDN had a little cytotoxicity toward HeLa and HEEC cells. It provided the possibility for anti‐bacterial experiment of BPBDNin vivo.
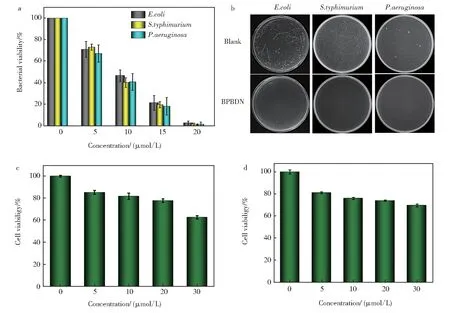
Fig. 2 a)Antibacterial activity of BPBDN toward E. coli,S. typhimurium and P. aeruginosa;b)Representative photo‐graphs of E. coli,S. typhimurium and P. aeruginosa on agar plate without and with treatment of BPBDN(20 μmol/L);c)Cytotoxicity of BPBDN against mammalian cells of HeLa;d)Cytotoxicity of BPBDN against Human esophageal epithelium cells of HEEC
2.3 Antibacterial mechanism of BPBDN
In order to investigate the antibacterial mecha‐nism of BPBDN,E.coliwas used as model bacteria to conduct further experiment. The good interaction between biocide and bacteria is the precondition of killing bacteria. To demonstrate the interaction be‐tween BPBDN andE.coli, zeta potential measure‐ment was performed. As shown in Fig. 3a,E.colishowed negative potential of(-50.1±0.2)mV. Af‐ter treatment of BPBDN, the zeta potential ofE.colibecame more positive as (-39.5 ± 0.5) mV. It indi‐cated that positive charged BPBDN could interact with bacteria resulting in potential changes. After ver‐ification of the interaction between BPBDN and bac‐teria, we employed BacLight Live/Dead viability kit to evaluate the antibacterial effect of BPBDN under CLSM. As shown in Fig. 3b, after treatment of BPBDN, the fluorescence color fromE.colibecame green to red, indicating the bacteria were killed by
LPS was used to pretreat BPBDN and then inves‐tigated the changes of antibacterial activity. As shown in Fig. 4a, BPBDN alone could almost kill allE.coli, while after treatment with LPS, BPBDN had nearly no antibacterial activity. The corresponding photographs of bacterial colonies on agar plates were BPBDN and then stained by PI. Moreover, scanning electron microscopy was used to observe the morphol‐ogy of bacteria with treatment of BPBDN (Fig. 3c).Obviously,the surface ofE.colitreated with BPBDN became collapsed and broken compared with that of blank group. It illustrated that BPBDN interacted with bacterial membrane and further damaged it re‐sulting in the death of bacteria. As Gram-negative bacteria,E.colihas an outer membrane covered with negative charge LPS. We suspected that LPS might play a role in the process of killing bacteria by BPBDN.shown as Fig. 4b. It indicated that BPBDN might damage the bacterial membrane by interacting with LPS.
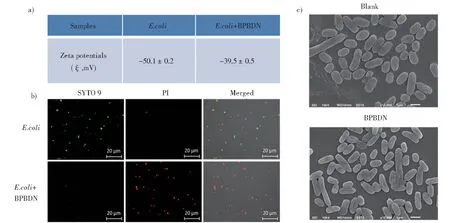
Fig. 3 a)Zeta potentials of E. coli without and with treatment of BPBDN(20 μmol/L);b)Confocal images of E. coli stained by SYTO 9 and PI without and with treatment of BPBDN;c)Representative SEM images of E. coli without and with treatment of BPBDN(20 μmol/L)

Fig. 4 a)Antibacterial activity of BPBDN(20 μmol/L)before and after incubation with LPS(100 μg/mL);b)Representative agar plates photographs of E. coli after different treatment

Fig. 5 a)Representative photographs of E. coli-infected wound after treatment of PBS and BPBDN;b)Histological images of mice organs after treatment of PBS and BPBDN
2.4 Bactericidal activity of BPBDN in vivo
Considering that BPBDN has effective antibacte‐rial capability and a little cytotoxicity, we further in‐vestigate the treatment of wound bacterial infection.E. coli-infected wound model was constructed on BALB/c mouse, and divided into the experimental group and the control group. The experimental group was treated with BPBND, while the blank group was treated with the same amount of PBS. After 12-day treatment, the changes of infected wounds were shown as Fig. 5a. Notably, the wounds treated with BPBDN had faster healing speed than those treated with PBS. On day 10, wounds in BPBDN group al‐most healed. The results indicated that BPBDN had antibacterial activityin vivoand facilitated the wound healing. Furthermore,the damage of major organs af‐ter treatment of BPBDN was evaluated by using he‐matoxylin and eosin(H&E). As shown in Fig. 5b,or‐gans including heart, liver, spleen, lung, and kidney had no noticeable damage because no disturbed struc‐tures were found. It demonstrated that BPBDN had good biosafetyin vivo. All above results illustrate BPBDN have promise potential to treat wound bacte‐rial infection.
3 Conclusions
In summary, a novel pyridine quaternary ammo‐nium salt BPBDN was designed and synthesized as an effective antibacterial agent for treatment of wound infection. BPBDN is designed to have two pyridine molecules that bridged by a binaphthyl deriv‐ative. The increased positive charge density and elec‐tron donating action make BPBDN have high antibac‐terial activity. For three common pathogenic bacteriaE.coli,P. aeruginosaandS. typhimurium, BPBDN can kill them at a low concentration of 20 μmol/L even theE.coliis ampicillin-resistant. And it is dem‐onstrated that lipopolysaccharide, covered on the out‐er membrane of Gram-negative bacteria, plays an im‐portant role in the membrane-disrupted process. Fur‐thermore, BPBDN can also be used to kill bacteriain vivoand treat the bacteria-infected wound of mice with good biosafety. It not only provides a potential antibacterial agent for treatment of wound infection,but also expended the design of pyridine-based quater‐nary ammonium salt with potent antibacterial activity.

