中链脂肪酸抗菌和诱导防御肽表达的功能及其在仔猪饲料中的应用
喻正旺,周忠新
中链脂肪酸抗菌和诱导防御肽表达的功能及其在仔猪饲料中的应用
喻正旺,周忠新
农业动物遗传育种与繁殖教育部重点实验室/生猪健康养殖协同创新中心/华中农业大学动物营养与饲料科学系,武汉 430070
近年,越来越多的研究表明,中链脂肪酸(MCFAs)抵抗病原菌是哺乳动物先天防御系统的重要组成部分,而且MCFAs也能够诱导人、猪和鸡内源性防御肽的表达,MCFAs的这些新功能尚未引起重视。MCFAs还和饲用有机酸或饲用植物精油具有协同抗菌增效作用,可以减少这些活性物质的使用量。相比于长链脂肪酸,日粮中添加MCFAs能显著地提高动物机体内氧气消耗量和线粒体呼吸速率,但产生活性氧自由基少,特别适合幼龄动物肠代谢和肝代谢所需的快速能量供应特点。日粮添加低浓度MCFAs(0.1%—0.5%,质量比)能显著提高新生或断奶仔猪存活率、粗蛋白粗脂肪消化率、饲料转化率,调节肠道菌群和改善肠上皮结构,进而促进生长。基于MCFAs的上述功能,将MCFAs与饲用有机酸或植物精油复配制备成微囊颗粒,可能是其用于仔猪抗生素替代品的尚佳方式。
中链脂肪酸;先天防御系统;协同抗菌;防御肽;仔猪
0 引言
瑞典雀巢公司基于大量医学和兽医学文献分析后对抗生素促生长机理进行了概述,称“抗生素对人和动物的促生长效应主要是减少了病原菌感染及其伴随的炎症响应造成的热量负担(因为在良好卫生状况下不能观察到抗生素的促生长效应)”[1]。中链脂肪酸( medium-chain fatty acids,MCFAs)不仅具有良好的抑菌或杀菌、抗病毒和抗寄生虫作用[2-4],而且能够改善肠道健康[5-7]和提高饲料消化率[8-9]。近年新的研究发现,MCFAs抵抗病原菌是幼龄动物先天防御系统的重要组成部分,而且MCFAs能够诱导人和动物体内宿主防御肽(host defensin peptides,HDPs)的表达[10-12],被认为是一种良好的免疫增强剂。早前报道的综述主要针对MCFAs的杀菌或抑菌活性[8,13-14],以及MCFAs对仔猪生产性能的影响[3,8,15-19],均未涉及MCFAs诱导宿主防御素表达的内容。本文主要综述了MCFAs在哺乳动物先天防御中的重要作用、诱导宿主防御肽表达、协同抗菌增效、代谢优势机理几个方面的新进展及其在仔猪抗生素替代品中的应用前景。本文还介绍了微囊化技术在避免中链脂肪酸难闻气味、减少添加量和延长肠道作用时间中的优势。
1 中链脂肪酸具有良好体外抑菌或杀菌作用,化学性质稳定
脂肪酸是重要的有机化工和精细化工原料,目前从天然动植物油脂经水解、精馏生产的脂肪酸占脂肪酸总量的4/5以上,是世界脂肪酸的主要来源[20]。脂肪酸根据碳链的长度分为短链、中链和长链脂肪酸3种,通常把含有6—12个碳原子的直链饱和脂肪酸称为中链脂肪酸。MCFAs资源来源广,自然界中天然存在的MCFAs都是偶数碳长,包括己酸(C6)、辛酸(C8)、癸酸(C10)和月桂酸(C12),主要存在于椰子油(含60%)[21-23]、棕榈油(含8%)、萼距花属植物油中[24]。MCFAs具有良好的抑菌或杀菌作用,很早就被用于饲料(尤其用于青贮饲料)和食品防腐中[4]。体外试验已经证明MCFA及其单甘油酯都能够抑制或杀死致病菌、病毒和寄生虫[3, 25]。总的来讲,中链脂肪酸(己酸、辛酸、癸酸和月桂酸)及其脂肪酸甘油一酯(辛酸甘油酯、癸酸甘油酯和月桂酸甘油酯)和长链不饱和脂肪酸(棕榈烯酸、棕榈油酸、油酸、顺式-8-十八碳烯酸和亚油酸)抑菌活性较强,脂肪醇(正辛醇、正葵醇和月桂醇)次之,长链饱和脂肪酸(肉豆蔻酸、软脂酸和硬脂酸)抑菌效果相对较弱[26-27]。MCFAs主要作用于细菌的细胞膜,其两亲性的化学结构可以破坏细菌细胞膜的结构,导致细胞内容物流出和细胞裂解,从而导致细菌死亡[28-30],这种机制也使得致病菌很难对其产生耐药性[31-32]。
值得注意的是,中链脂肪酸(辛酸、葵酸、月桂酸)与有机弱酸(乙酸、乳酸、苹果酸、柠檬酸)对O157:H7具有极强的协同杀菌作用,葵酸与柠檬酸的协同杀菌能力最强;例如,无杀菌效应的0.125mmol·L-1的葵酸(约0.002%)与无杀菌效应的0.125mmol·L-1的乳酸、0.125mmol·L-1苹果酸、0.125 mmol·L-1柠檬酸(约0.0015%)分别组合后能显示极强的协同杀菌效应,其杀菌活力的Log CFU值在协同前后的变化分别为2、3、6,如果提高组合的浓度,协同杀菌能力更强[33]。中链脂肪酸(辛酸,癸酸和月桂酸)与食用植物精油(香芹酚,丁香酚,β-间苯二酸,反式肉桂醛,百里酚和香兰素)复合,对O157:H7的杀菌作用同样大大增强[34]。采用辛酸和牛至精油的组合处理沙门氏菌、单核细胞增生性李斯特菌、大肠杆菌和金黄色链球菌时,发现辛酸和牛至精油表现出良好的协同效应[35]。比例为2:1:1:5:2:2的辛酸、香芹酚、癸酸、肉桂醛、丁香酚、己酸复合配方对大肠杆菌、沙门氏菌和金黄色葡萄球菌的抑菌性能高且在模拟胃肠液环境中同样具有良好的抑菌作用[36]。断奶是养猪生产中最具挑战性和关键性的阶段之一[37]。仔猪断奶后通常有一个生长延滞期,主要是因为胃内盐酸和胰酶分泌不足导致消化能力低,以及日粮和环境因素改变等应激导致的采食量下降和腹泻[38]。日粮中加入弱的有机酸降低胃肠道pH值能一定程度缓解这个问题,常用的有机弱酸主要是柠檬酸、甲酸、丙酸、乳酸、富马酸、苯甲酸等[39]。体外模拟盲肠系统证实辛酸钠可以显著减少大肠杆菌和沙门氏菌的数量[40],通过断奶仔猪口服鼠伤寒沙门氏菌或产肠毒素大肠杆菌构建的两个实验模型,证实了从椰子油中提取的中链脂肪酸盐混合物能够减小沙门氏菌或大肠杆菌等条件致病菌在肠道定植的活性[41]。因此MCFAs在预防和治疗仔猪腹泻方面将有较大的应用前景,且与有机酸或植物精油复合使用杀菌效果可能更好。
2 中链脂肪酸抵抗病原菌是哺乳动物先天防御系统的重要组成部分
脂质的抗菌作用在20世纪60年代前后被发现,但近年来人们才开始认识到脂肪酸是许多生物抵抗病原菌的先天防御系统的重要组成部分,尤其是在皮肤和黏膜表面的防御中,包括哺乳动物、两栖动物、软体动物、植物、藻类[26, 28, 42-43]。哺乳动物母乳[24, 42]、皮肤[26, 44-45]、粘膜[42]中都存在高含量的中链饱和脂肪酸和长链不饱和脂肪酸,它们是这些组织抵御病原微生物最活跃的抗菌剂,其作用比以前想象的更为重要[26, 42, 44-47]。例如人的皮肤中每平方厘米含有10—15μg的游离脂肪酸,其中月桂酸 (C12)、肉豆蔻酸 (C14)、棕榈酸 (C16)、sapienic acid (C16:1n-10)、 cis-8-octadecenoicacid (C18:1n-10)含量最丰富[28]。测试细菌对丙酮提取的前臂皮肤的敏感性试验发现,化脓性链球菌和金黄色葡萄球菌在脂质减少的情况下的存活时间明显长于未经处理的皮肤[48]。动物母乳中含有大量抗菌的MCFAs(己酸C6、辛酸C8、葵酸C10、月桂酸C12)和LCFAs(主要是油酸C18:1、亚油酸C18:2、亚麻酸C18:3),其中中链脂肪酸占乳脂的比例高,兔(58%)、大鼠(58%)、马(48.1%)、山羊(34.7%)、奶牛(20.7%)、人(11.7%)、猪(4.1%)[24, 49]。以低脂牛奶为唯一牛奶来源的儿童发生急性胃肠道疾病的可能性比服用全脂牛奶的儿童高5倍[50]。高脂肪牛奶饮食喂养可以减少李斯特菌在大鼠肠黏膜的定植[51]。由此可见,MCFAs在皮肤和黏膜屏障功能中发挥重要作用,是哺乳动物先天防御系统的重要组成部分。
3 中链脂肪酸能够诱导人、猪和鸡内源防御肽的表达
宿主防御肽(Host defensin peptides, HDPs)是小于50个氨基酸的短肽,一般带正电荷,具有两亲性,广泛存在于植物界和动物界[52-53]。HDPs是先天免疫系统的重要组成部分,对细菌、真菌、原生生物和多种病原体都具有直接的抗菌活性[54-56]。此外,HDPs还具有强大免疫调节活性,可以抑制炎症反应和促进伤口愈合[57-60]。例如含有猪宿主防御肽(PR-39/pBD-1)的重组质粒可调节仔猪肠道的先天性和适应性免疫反应,可以降低仔猪的腹泻率[61]。
近年的研究表明,MCFAs可以诱导人和动物体内HDPs的表达。国外ZENG 等报道认为,碳链长度为3—10的脂肪酸(包含奇数碳和偶数碳)能显著诱导体外培养的猪肠上皮细胞中至少3种防御素基因的表达,但其诱导机制和动物体内效应不得而知[62];这些脂肪酸也能诱导鸡巨噬细胞和单核细胞中防御素基因的表达[10]。JIANG 等也报道,短链、中链、长链脂肪酸都能不同程度地诱导人体内抗菌肽LL-37基因和蛋白的表达[63]。WANG等在探索辛酸(C8)和葵酸(C9)对肠上皮屏障功能的影响时,发现辛酸(C8)和葵酸(C9)可促使内源性防御肽pBD-1、pBD-2的表达显著升高,其作用机制为辛酸(C8)和葵酸(C9)减弱了经典组蛋白脱乙酰基酶途径的活性,促进了启动子pBD-1和pBD-2上组蛋白3赖氨酸9(H3K9)的乙酰化,从而增强了PBD-1和PBD-2基因表达[12]。基于HDPs在先天免疫和适应性免疫中的重要作用,近来一些外源性的HDPs正在被开发和利用,但体外合成的HDPs还存在稳定性较差、易在动物消化道及胃中发生降解的问题[64]。BECHINGER等[65]和CHEUNG等[66]报道指出使用外源HDPs成本较高且仍然存在耐药风险,并且还可能会影响内源性HDPs在先天性免疫中的保护功能。所以通过MCFAs调控内源性HDPs的合成与分泌已成为一种用于疾病控制和预防的有前途的抗生素替代方法[67]。
4 中链脂肪酸不同于长链脂肪酸的消化、吸收和代谢特点
MCFAs水解能力和吸收速度是长链脂肪酸(Long Chain Fatty Acids,LCFAs)的6倍,代谢速度是其10倍,能够快速为肠细胞和肝代谢提供能量[3, 68]。利用同位素标记技术比较仔猪对中链脂肪酸和长链脂肪酸的吸收效果和氧化效果,结果表明辛酸的吸收和氧化速率要显著高于油酸[69]。其主要原因是MCFAs比LCFAs分子量小、呈极性且水溶性较好,对胆盐和胰酶的依赖性低,更容易被水解和吸收;MCFAs吸收后不依赖肉毒碱的转运,主要以游离形式进入门静脉,能够自由通过线粒体的双层膜进入线粒体氧化,进而快速为机体供能,而LCFAs必须与肠细胞中的脂肪酸结合蛋白(FABP)结合,转运到滑面内质网,重新酯化形成甘油三酯,然后与载脂蛋白结合形成乳糜微粒,进入到淋巴系统,通过血液循环被运往机体肌肉、肝脏、脂肪组织细胞[68,70]。体外研究表明,中链脂肪酸可以显著提高肝细胞氧气的消耗量、NAD(P)H的水平[71]及氧化丙酮和乳酸的活性[70]。用葵酸(C10)或月桂酸(C12)处理的C2C12肌管产生的ROS要显著低于肉豆蔻酸或棕榈酸处理组,且耗氧量比肉豆蔻酸(C14)或棕榈酸(C16)处理组高[72]。喂食MCFAs的小鼠肌细胞比LCFAs组线粒体氧化能力显著增强,活性氧自由基的产生显著降低[73-74],这与体外研究结果一致。对其机理的解释方面,喂食MCFA的小鼠试验表明,MCFA会诱导ω氧化基因Cyp4a10和Cyp4a14的表达,增加二羧基脂肪酸的产量,激活过氧化物酶体增殖物激活受体(peroxisome proliferators- activated receptors,PPARs),进而激活脂肪酸在微体中的ω氧化途径、在线粒体和过氧化物酶体中的β氧化途径,从而加速MCFA的氧化供能水平[74]。由此可见,MCFAs在体内具有消化吸收快、代谢快、产生活性氧自由基少的特点,特别适合幼龄动物肠代谢和肝代谢所需的快速能量供应特点。
5 中链脂肪酸对仔猪存活率、生长性能、营养物质表观消化率和肠道微生物的影响
MCFAs在新生或断奶仔猪的研究报道较多[75],在母猪、蛋鸡、肉鸡、牛饲料中也有一些零星报道[76-79]。这些研究表明,MCFAs及其甘油酯可以调节畜禽营养代谢、提高畜产品品质,在动物生产中使用MCFAs是发展绿色畜产品生产的有效途径之一。前人关于中链脂肪酸的添加浓度和形式以及对仔猪存活率、生长性能、营养物质表观消化率和肠道微生物的影响的研究在表1中进行了总结。
HANCZAKOWSKA等基于252头新生仔猪的试验结果表明,日粮中添加0.1%(1g·kg-1)的辛酸(C8)或葵酸(C10)可使84日龄体重增加14%—22%,平均日增重增加15%—23%,料肉比降低20%—30%,粗蛋白或粗脂肪的表观消化率提高5%,粗纤维的表观消化率提高10%—13%,死亡率下降8.2%—11.3%,无氮浸出物的表观消化率变化不显著;同时,添加0.1%的辛酸(C8)或葵酸(C10)也都使肠绒毛高度、隐窝深度、及绒毛高/隐窝深度比值显著增加,有害菌产气荚膜梭菌的数量显著减少,葵酸的效应更显著[80]。在断奶仔猪饲料中添加0.1%的中链脂肪酸和植物精油复合物(桉树油+辛酸+葵酸),显著提高了仔猪日增重和采食量,并且与抗生素组、ZnO组没有差异,同时也提高了干物质、粗蛋白、钙、磷、能量和氨基酸消化率[81]。HANCZAKOWSKA等基于326头断奶仔猪试验,在酸化剂(0.5%的甲酸和丙酸)的基础上再添加0.2%的辛酸(C8)或葵酸(C10)可以进一步提高84日龄体重和平均日增重,进一步降低料肉比,粗纤维表观消化率进一步提高11%—14%(干物质、粗蛋白、粗脂肪消化率也有提高)[82]。0.3%的中链脂肪酸和有机酸混合物(MCFA +甲酸钙+乳酸钙+柠檬酸)可以替代仔猪日粮中的氧化锌,显著提高仔猪采食量、促进动物生长,同时还可以显著增加直肠和回肠中乳酸杆菌的数量并显著降低沙门氏菌和肠球菌数量[83]。而日粮中添加高浓度(8%)的中链脂肪酸(60%辛酸和40%葵酸)可以提高饲料转化效率和氨基酸的消化率,但差异不显著。
综上所述,高浓度(8%)的中链脂肪酸可以提高饲料的转化效率以及能量和氨基酸的消化率,但差异不显著。低浓度(0.1%—0.5%)的中链脂肪酸可显著提高新生或断奶仔猪存活率、营养物质消化率、饲料转化率、改善肠道健康,促进生长。中链脂肪酸对生长性能提升效果较好的为辛酸(C8)和葵酸(C10)。体外抗菌效果方面,0.002%的葵酸和0.0015%的柠檬酸组合对O157:H7的杀菌能力最强,0.004%葵酸和0.006%-间苯二酸组合对O157:H7的杀菌能力最强[33-34]。体内应用效果方面,0.1%的桉树油-辛酸-葵酸组成的复合物、0.3%的MCFA-甲酸钙-乳酸钙-柠檬酸组成的复合物和0.2%的中链脂肪酸(辛酸或葵酸)与0.5%的有机酸(甲酸和丙酸)组成的复合物均可以达到替代仔猪饲料中氧化锌的作用。不同的动物或相同动物的不同生长阶段,以及养殖场管理方式和环境等都是影响中链脂肪酸组合效果的重要因素,特别是不同的养殖场对组合效应的报道结果缺乏一致性,这可能是不同养殖场的不同管理和环境导致的。
6 微囊化技术是提高中链脂肪酸溶解度和生物利用度,避免难闻气味的有效方法
微囊化技术是将小的固体颗粒,液滴或气体包裹在涂层中的一种技术,已经被用于食品工业、化妆品及制药等多个领域,它可以保护活性成分免受外部环境的影响,掩盖难闻的气味,还可以将活性成分运送到动物体内特定的作用靶点[87]。MCFAs难溶于水、有难闻气味、刺激缩胆囊素释放而减少采食量[3]。MCFAs浓度随着胃肠道逐渐降低,在仔猪日粮中添加包被的中链脂肪酸,中链脂肪酸在胃肠道各部位的浓度显著高于对照组(添加相同浓度的未包被中链脂肪酸)[88],这表明利用包被技术减少中链脂肪酸的用量及延长中链脂肪酸在胃肠道的作用时间的可行性,将有利于降低仔猪的腹泻率。中链脂肪酸和有机酸或精油具有协同抗菌作用,在断奶仔猪饲料中添加中链脂肪酸和植物精油复合物制成的微囊可以达到替代日粮中氧化锌和抗生素的作用[82]。体外胃肠道模拟试验表明,月桂酸和百里香酚制成的微粒可以被运输到肠道后段以防止断奶仔猪腹泻[89]。日粮中添加有机酸和中链脂肪酸制成的脂质微囊,显著提高了生长猪的生产性能[90]。目前国内鲜有关于包被中链脂肪酸用作饲料添加剂替代抗生素的报道。
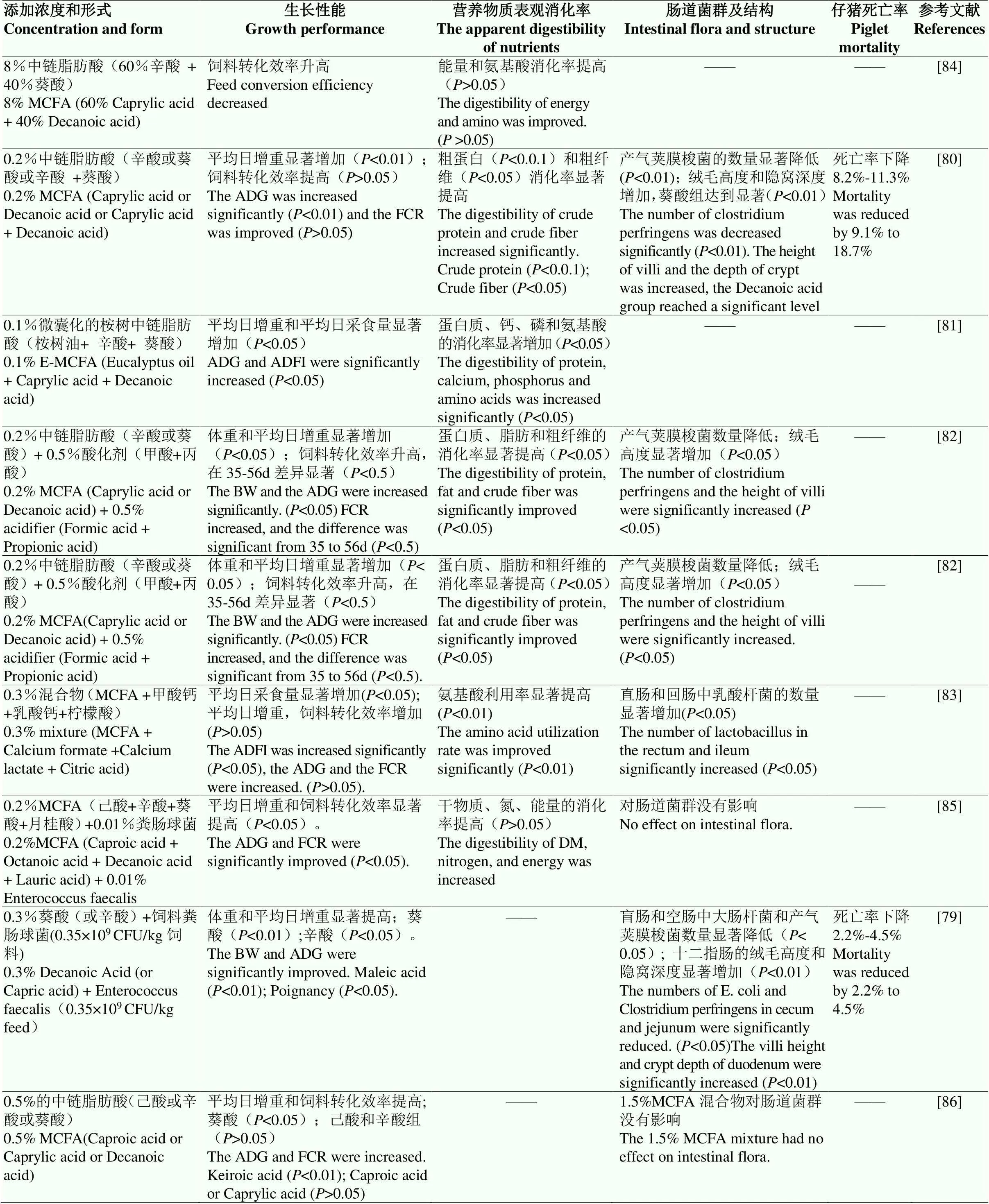
表1 基础仔猪日粮中添加一定浓度的中链脂肪酸对仔猪生产性能、营养物质表观消化率、肠道微生物及结构和仔猪死亡率的影响
BW:体重;FCR:饲料转化率;ADFI:平均日采食量;ADG:平均日增重;DM;干物质
BW: weight; FCR: feed conversion rate; ADFI: average daily food intake; ADG: average daily weight gain; DM; Dry matter
7 小结
中链脂肪酸是哺乳动物母乳、皮肤、粘膜先天防御系统的重要组成部分,中链脂肪酸诱导人、猪和鸡内源性防御肽表达现象的发现,是对已有中链脂肪酸体外抗菌功能认知的有益补充,这些新功能将促进中链脂肪酸在仔猪饲料抗生素替代品开发中的应用。日粮中添加低浓度(0.1%—0.5%)的中链脂肪酸可提高新生或断奶仔猪存活率、营养物质消化率、饲料转化率,改善肠道健康,促进仔猪生长。中链脂肪酸与植物精油或有机酸具有协同抗菌增效功能,将其混合制备成微囊颗粒,能很好地提高其溶解性,避免难闻气味,提高生物利用度。
[1] BRUSSOW H. Growth promotion and gut microbiota: insights from antibiotic use. Environmental Microbiology, 2015, 17(7): 2216-2227. DOI:10.1111/1462-2920.12786.
[2] SHILLING M, MATT L, RUBIN E, VISITACION M P, HALLER N A, GREY S F, WOOLVERTON C J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on clostridium difficile. Journal of Medicinal Food, 2013, 16(12): 1079-1085. DOI:10.1089/ jmf.2012.0303.
[3] ZENTEK J, BUCHHEIT-RENKO S, FERRARA F, VAHJEN W, VAN KESSEL A G, PIEPER R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Animal Health Research Reviews, 2011, 12(1): 83-93. DOI:10.1017/ s1466252311000089.
[4] WOOLFORD M K. Microbiological screening of straight chain fatty-acids (c1-c12)as potential silage additives. Journal of the Science of Food and Agriculture, 1975, 26(2): 219-228. DOI:10.1002/jsfa. 2740260213.
[5] LIU Y L. Fatty acids, inflammation and intestinal health in pigs. Journal of Animal Science and Biotechnology, 2016, 7(3): 321-329.
[6] 刘聪聪, 王树辉, 涂治骁, 陈少魁, 汪龙梅, 秦琴, 张琳, 王秀英, 刘玉兰, 朱惠玲. 中链脂肪酸对脂多糖诱导的断奶仔猪肠黏膜免疫屏障损伤的保护作用. 中国畜牧杂志, 2018, 54(10): 70-74.
LIU C C, WANG S H, TU Z X, CHEN S K, WANG L M, QING Q, ZHANG L, WANG X Y, LIU Y L, ZHU H L. Protective effect of medium-chain fatty acids on injury of intestinal mucosal immune barrier induced by Lipopolysaccharide in weaned piglets. China Animal Husbandry & Veterinary Medicine,2018, 54(10): 70-74. (in Chinese)
[7] 赵晓, 张永, 张新胜, 徐庆, 于晓明, 李惠子, 杨雪艳, 刘英华, 薛长勇. 不同脂肪酸组成的油脂对LPS诱导的小鼠肠道炎症的影响. 中国食物与营养, 2017, 23(1): 60-63.
ZHAO X, ZHANG Y, ZHANG X S, XV Q, YU X M, LI H Z, YANG X Y, LIU Y H, XUE C Y.Effects of oil composed of different fatty acids on intestinal inflammation induced by LPS in C57BL/6J mice. Food and Nutrition in China, 2017, 23(1): 60-63. (in Chinese)
[8] Hanczakowska E. The use of medium-chain fatty acids in piglet feeding – a review. Annals of Animal Science, 2017, 17(4): 967-977. DOI:10.1515/aoas-2016-0099.
[9] ZHANG J Y, BAEK D H, KIM I H. Effect of dietary supplemental medium chain fatty acids instead of antibiotics on the growth performance, digestibility and blood profiles in growing pigs. Journal of Animal Physiology and Animal Nutrition, 2019, 103(6): 1946-1951. DOI:10.1111/jpn.13175.
[10] SUNKARA L T, JIANG W, ZHANG G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS One, 2012, 7(11). DOI:10.1371/journal.pone.0049558.
[11] WU J, MA N, JOHNSTON L J, MA X. Dietary nutrients mediate intestinal host defense peptide expression. Advances in Nutrition, 2020, 11(1): 92-102. DOI:10.1093/advances/nmz057.
[12] WANG J, LU J, XIE X, XIONG J, HUANG N, WEI H, JIANG S, PENG J. Blend of organic acids and medium chain fatty acids prevents the inflammatory response and intestinal barrier dysfunction in mice challenged with enterohemorrhagicO157:H7. International Immunopharmacology, 2018, 58:64-71. DOI:10.1016/ j.intimp.2018.03.014.
[13] BALTIC B, STARCEVIC M, DORDEVIC J, MRDOVIC B, MARKOVIC R. Importance of medium chain fatty acids in animal nutrition. 59th International Meat Industry Conference Meatcon 2017. 2017.
[14] 庞培, 田雯, 刘志强, 范觉鑫, 龚金秋, 肖淑华. 中链脂肪酸的抑菌作用及在断奶仔猪料中应用. 广东畜牧兽医科技, 2019, 44(1): 21-23.
PANG P, TIAN W, LIU Z Q, FAN J X, GONG J Q, XIAO S H. Bacteriostatic action of medium chain fatty acids and its application in weaned piglets.Guangdong Journal of Animal and Veterinary Science, 2019, 44(1): 21-23. (in Chinese)
[15] JACKMAN J A, BOYD R D, ELROD C C. Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation. Journal of Animal Science and Biotechnology, 2020, 11. DOI:10.1186/s40104-020- 00446-1.
[16] 薛永强, 黄志威, 雷志伟, 王新毅. 中短链脂肪酸在无抗饲料中的应用. 饲料研究, 2020, 43(3): 133-136.
XUE Y Q, HUANG Z W, LEI Z W, WANG X Y. Application of short –medium chain fatty acids in non-resistant feed. Feed Research, 2020, 43(3): 133-136. (in Chinese)
[17] ROSSI R, PASTORELLI G, CANNATA S, CORINO C. Recent advances in the use of fatty acids as supplements in pig diets: A review. Animal Feed Science and Technology, 2010, 162(1-2): 1-11. DOI;10. 1016/j.anifeedsci.2010.08.013.
[18] DIERICK N A, DECUYPERE J A, DEGEYTER I. The combined use of whole Cuphea seeds containing medium chain fatty acids and an exogenous lipase in piglet nutrition. Archives of Animal Nutrition, 2003, 57(1): 49-63. DOI:10.1080/0003942031000086626.
[19] 汪加明, 周庆华, 王宏玲, 李飞务. 饲料中添加中链脂肪酸对断奶仔猪生长性能的影响. 猪业科学, 2017, 34(9): 90-91.
WANG J M, ZHOU Q H, WANG H L, LI F W. Effect of adding medium chain fatty acid in feed on growth performance of weaned piglets. Swine Industry Science, 2017, 34(9): 90-91.(in Chinese)
[20] 陆蠡珠. 我国脂肪酸的生产和应用. 精细与专用化学品, 2007, 15(1): 24-28.
LU L Z. Production and application of fatty acids in China. Fine and Specialty Chemicals, 2017, 15(1): 24-28.(in Chinese)
[21] BHATNAGAR A S, KUMAR P K P, HEMAVATHY J, KRISHNA A G G. Fatty acid composition, oxidative stability, and radical scavenging activity of vegetable oil blends with coconut oil. Journal of the American Oil Chemists Society, 2009, 86(10): 991-999. DOI:10.1007/ s11746-009-1435-y.
[22] DAYRIT F M. The properties of lauric acid and their significance in coconut oil. Journal of the American Oil Chemists Society, 2015, 92(1): 1-15. DOI:10.1007/s11746-014-2562-7.
[23] WANG J H, WANG X X, LI J T, CHEN Y Q, YANG W J, ZHANG L Y. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. Asian-Australasian Journal of Animal Sciences, 2015, 28(2): 223-230.
[24] DECUYPERE J A, DIERICK N A. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: concept, possibilities and limitations. An overview. Nutrition Research Reviews, 2003, 16(2): 193-209. DOI:10.1079/nrr200369.
[25] CRUZ-ESTRADA A, RUIZ-SANCHEZ E, CRISTOBAL-ALEJO J, GONZALEZ-COLOMA A, FEANDRES M, GAMBOA-ANGULO M. Medium-chain fatty acids from eugenia winzerlingii leaves causing insect settling deterrent, nematicidal, and phytotoxic effects. Molecules, 2019, 24(9). DOI:10.3390/molecules24091724.
[26] FISCHER C L, DRAKE D R, DAWSON D V, BLANCHETTE D R, BROGDEN K A, WERTZ P W. Antibacterial activity of sphingoid bases and fatty acids against Gram-Positive and Gram-negative bacteria. Antimicrobial Agents and Chemotherapy, 2012, 56(3): 1157-1161. DOI:10.1128/aac.05151-11.
[27] 张希, 杨明, 宋飞, 张辉, 冯凤琴. 脂肪酸及其衍生物的抑菌活性. 浙江大学学报(农业与生命科学版), 2013, 39(02): 155-160.
ZHANG X, YANG M, SONG F, ZHANG H, FENG F Q. Bacteriostatic activities of fatty acids and their derivatives. Journal of Zhejiang University (Agriculture and Life Sciences), 2013, 39(2): 155-160.(in Chinese)
[28] DESBOIS A P, SMITH V J. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Applied Microbiology and Biotechnology, 2010, 85(6): 1629-1642. DOI:10. 1007/s00253-009-2355-3.
[29] YOON B K, JACKMAN J A, VALLE-GONZALEZ E R, CHO N J. Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. International Journal of Molecular Sciences, 2018, 19(4). DOI:10.3390/ ijms19041114.
[30] 蒋增良, 张辉, 杜鹃, 冯凤琴. 月桂酸单甘油酯抑菌机理、影响因素及其复配体系的抑菌特性. 中国食品学报, 2016, 16(3): 146-151.
JANG Z L, ZHANG H, DU J, FENG F Q.Antibacterial mechanism and influence factors of glycerol monolaurate and antibacterial properties of its combinations. Journal of Chinese Institute of Food Science and Technology, 2016, 16(3): 146-151. (in Chinese)
[31] SCHLIEVERT P M, PETERSON M L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One, 2012, 7(7). DOI:10.1371/journal.pone.0040350.
[32] KUMAR P, LEE J-H, BEYENAL H, LEE J. Fatty acids as antibiofilm and antivirulence agents. Trends in Microbiology, 2020. DOI:10. 1016/j.tim.2020.03.014.
[33] MESSENS W, GORIS J, DIERICK N, HERMAN L, HEYNDRICKX M. Inhibition ofby medium-chain fatty acids in ansimulation of the porcine cecum. Veterinary Microbiology, 2010, 141(1-2): 73-80.DOI:10.1016/j.vetmic.2009.08. 002.
[34] LOPEZ-COLOM P, CASTILLEJOS L, RODRIGUEZ-SORRENTO A, PUYALTO M, JOSE MALLO J, MARIA MARTIN-ORUE S. Efficacy of medium-chain fatty acid salts distilled from coconut oil against two enteric pathogen challenges in weanling piglets. Journal of Animal Science and Biotechnology, 2019, 10(1). DOI:10.1186/ s40104-019-0393-y.
[35] HULANKOVA R, BORILOVA G.combined effect of oregano essential oil and caprylic acid againstO157:H7,and. Acta Veterinaria Brno, 2011, 80(4): 343-348. DOI:10. 2754/avb201180040343.
[36] 祁姣姣, 朱剑锋, 周海泳, 胡学生, 王创, 刘紫芊, 胡文锋. 由中链脂肪酸与植物精油为主要成分组成的复合型酸化剂抑菌性能的研究. 猪业科学, 2018, 35(01): 109-113.
QI J J, ZHU J F, ZHOU H Y, HU X S, WANG C, LIU Z X, HU W F. Study on the antibacterial properties of compound acidizing agents composed of medium chain fatty acids and plant essential oils.Swine Industry Science, 2018, 35(01): 109-113. (in Chinese)
[37] KIM S A, RHEE M S. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids againstO157:H7. Applied and Environmental Microbiology, 2013, 79(21): 6552-6560. DOI:10.1128/ aem.02164-13.
[38] 王蕊香, 那木吉拉银花. 断奶仔猪发生应激原因与防控措施. 畜牧兽医科学(电子版), 2020, (4): 47-48.
WANG R X, NAMUJILA, YING H. Causes and prevention measures of stress in weaned piglets. Graziery Veterinary Sciences (Electronic Version), 2020, (4): 47-48. (in Chinese)
[39] KIM S A, RHEE M S. Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, beta-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) againstO157:H7. Food Control, 2016, 60:447-454. DOI:10.1016/j. foodcont.2015.08.022.
[40] LILLEHOJ H, LIU Y, CALSAMIGLIA S, FERNANDEZ- MIYAKAWA M E, CHI F, CRAVENS R L, OH S, GAY C G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Veterinary Research, 2018, 49. DOI:10.1186/ s13567-018-0562-6.
[41] SUIRYANRAYNA M V A N, RAMANA J V. A review of the effects of dietary organic acids fed to swine. Journal of Animal Science and Biotechnology, 2015, 6. DOI:10.1186/s40104-015-0042-z.
[42] THORMAR H, HILMARSSON H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents.Chemistry and Physics of Lipids, 2007, 150(1): 1-11.DOI: 10.1016/j.chemphyslip.2007.06.220.
[43] DESBOIS A P, MEARNS-SPRAGG A, SMITH V J. A fatty acid from the diatom phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Marine Biotechnology, 2009, 11(1): 45-52. DOI:10.1007/s10126- 008-9118-5.
[44] FISCHER C L, BLANCHETTE D R, BROGDEN K A, DAWSON D V, DRAKE D R, HILL J R, WERTZ P W. The roles of cutaneous lipids in host defense. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids, 2014, 1841(3): 319-322. DOI:10.1016/j. bbalip.2013.08.012.
[45] DRAKE D R, BROGDEN K A, DAWSON D V, WERTZ P W. Thematic review series: Skin lipids - Antimicrobial lipids at the skin surface. Journal of Lipid Research, 2008, 49(1): 4-11. DOI:10.1194/ jlr.R700016-JLR200.
[46] KENDALL A C, NICOLAOU A. Bioactive lipid mediators in skin inflammation and immunity. Progress in Lipid Research, 2013, 52(1): 141-164. DOI:10.1016/j.plipres.2012.10.003.
[47] FISCHER C L. Antimicrobial activity of host-derived lipids. Antibiotics-Basel, 2020, 9(2). DOI:10.3390/antibiotics9020075.
[48] RICKETTS C R, SQUIRE J R, TOPLEY E, LILLY H A. Human skin lipids with particular reference to the self-sterilising power of the skin. Clinical Science, 1951, 10(1): 89-111.
[49] ZHOU Z, HUANG J, HAO H, WEI H, ZHOU Y, PENG J. Applications of new functions for inducing host defense peptides and synergy sterilization of medium chain fatty acids in substituting in-feed antibiotics. Journal of Functional Foods, 2019, 52: 348-359. DOI:10.1016/j.jff.2018.11.028.
[50] KOOPMAN J S. Milk-fat and gastrointestinal illness. American Journal of Public Health, 1984, 74(12): 1371-1373. DOI:10.2105/ajph. 74.12.1371.
[51] SPRONG R C, HULSTEIN M F, VAN DER MEER R. High intake of milk fat inhibits intestinal colonization ofbut not ofin rats. Journal of Nutrition, 1999, 129(7): 1382-1389.
[52] MISHRA B, WANG G. The importance of amino acid composition in natural AMPs: an evolutional, structural, and functional perspective. Frontiers in Immunology, 2012, 3.DOI:10.3389/fimmu.2012.00221.
[53] VAN DIJK A, HEDEGAARD C J, HAAGSMAN H P, HEEGAARD P M H. The potential for immunoglobulins and host defense peptides (HDPs) to reduce the use of antibiotics in animal production. Veterinary Research, 2018, 49. DOI:10.1186/s13567-018-0558-2.
[54] HANCOCK R E W, HANEY E F, GILL E E. The immunology of host defence peptides: beyond antimicrobial activity. Nature Reviews Immunology, 2016, 16(5): 321-334.DOI:10.1038/nri.2016.29.
[55] LIM C H, PUTHIA M, BUTRYM M, TAY H M, LEE M Z Y, HOU H W, SCHMIDTCHEN A. Thrombin-derived host defence peptide modulates neutrophil rolling and migration in vitro and functional responseScientific Reports2017, 7. DOI:10.1038/s41598- 017-11464-x.
[56] HILCHIE A L, WUERTH K, HANCOCK R E W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nature Chemical Biology, 2013, 9(12): 761-768. DOI:10. 1038/nchembio.1393.
[57] MANSOUR S C, PENA O M, HANCOCK R E W. Host defense peptides: front-line immunomodulators. Trends in Immunology, 2014, 35(9): 443-450. DOI:10.1016/j.it.2014.07.004.
[58] YEUNG A T Y, GELLATLY S L, HANCOCK R E W. Multifunctional cationic host defence peptides and their clinical applications. Cellular and Molecular Life Sciences, 2011, 68(13): 2161-2176. DOI:10.1007/ s00018-011-0710-x.
[59] ZHANG S, CAI H, CAO D, DENG J, JIA J, LI J, MING F, ZHAO P, MA M, LIANG Q, ZENG M, ZHANg L. Recombinant plasmids containing CpG with porcine host defense peptides (PR- 39/pBD-1) modulates the innate and adaptive intestinal immune responses (including maternal-derived) in piglets. International Immunopharmacology, 2019, 70: 467-476.DOI:10.1016/j.intimp.2019. 03.007.
[60] 张萌萌, 姜宁, 张爱忠, 张晨雪. 饲料添加剂影响内源性抗菌肽表达和免疫调节机制. 动物营养学报, 2019, 31(1): 90-96.
ZHANG M M, JIANG N, ZHANG A Z, ZHANG C X. Feed additives affect endogenous antimicrobial peptide expression and immune regulation mechanism.Chinese Journal of Animal Nutrition, 2019, 31(1): 90-96. (in Chinese)
[61] ZENG X, SUNKARA L T, JIANG W, BIBLE M, CARTER S, MA X, QIAO S, ZHANG G. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS One, 2013, 8(8). DOI:10.1371/journal.pone.0072922.
[62] JIANG W, SUNKARA L T, ZENG X, DENG Z, MYERS S M, ZHANG G. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides, 2013, 50:129-138. DOI:10.1016/j.peptides.2013.10.008.
[63] BECHINGER B, GORR S U. Antimicrobial peptides: Mechanisms of action and resistance. Journal of Dental Research, 2017, 96(3): 254-260. DOI:10.1177/0022034516679973.
[64] 陈永宏, 罗芳, 陶金忠, 王晶. 营养物质对动物内源性宿主防御肽表达的调节作用. 畜牧兽医学报, 2020, 51(8): 1775-1783.
CHEN Y H, LUO F, TAO J Z, WANG J. Regulation of nutrients on the expression of endogenous host defense peptide in animals. Acta Veterinaria et Zootechnica Sinica, 2020, 51(8): 1775-1783. (in Chinese)
[65] CHEUNG G Y C, FISHER E L, MCCAUSLAND J W, CHOI J, COLLINS J W M, DICKEY S W, OTTO M. Antimicrobial peptide resistance mechanism contributes to staphylococcus aureus infection. Journal of Infectious Diseases, 2018, 217(7): 1153-1159. DOI:10. 1093/infdis/jiy024.
[66] DENG Z, WANG J, LYU W, WIENEKE X, MATTS R, MA X, ZHANG G. Development of a cell-based high-throughput screening assay to identify porcine host defense peptide-inducing compounds. Journal of Immunology Research, 2018. DOI:10.1155/2018/5492941.
[67] PAPAMANDJARIS A A, MACDOUGALL D E, JONES P J H. Medium chain fatty acid metabolism and energy expenditure: Obesity treatment implications. Life Sciences, 1998, 62(14): 1203-1215. DOI:10.1016/s0024-3205(97)01143-0.
[68] CHIANG S H, PETTIGREW J E, CLARKE S D, CORNELIUS S G. Limits of medium-chain and long-chain triacylglycerol utilization by neonatal piglets. Journal of Animal Science, 1990, 68(6): 1632-1638.
[69] SCHOENFELD P, WOJTCZAK L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. Journal of Lipid Research, 2016, 57(6): 943-954.10.1194/jlr.R067629.
[70] SCHONFELD P, WOJTCZAK A B, GEELEN M J H, KUNZ W, WOJTCZAK L. On the mechanism of the so-called uncoupling effect of medium-chain and short-chain fatty-acids. Biochimica Et Biophysica Acta, 1988, 936(3): 280-288. DOI:10.1016/0005-2728(88) 90003-5.
[71] GEBHARDT J T, THOMSON K A, WOODWORTH J C, DRITZ S S, TOKACH M D, DEROUCHEY J M, GOODBAND R D, JONES C K, COCHRANE R A, NIEDERWERDER M C, FERNANDO S, ABBAS W, BURKEY T E. Effect of dietary medium-chain fatty acids on nursery pig growth performance, fecal microbial composition, and mitigation properties against porcine epidemic diarrhea virus following storage. Journal of Animal Science, 2020, 98(1). DOI:10. 1093/jas/skz358.
[72] MONTGOMERY M K, OSBORNE B, BROWN S H J, SMALL L, MITCHELL T W, COONEY G J, TURNER N. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. Journal of Lipid Research, 2013, 54(12): 3322-3333. DOI:10.1194/jlr. M040451.
[73] ISHIZAWA R, MASUDA K, SAKATA S, NAKATANI A. Effects of different fatty acid chain lengths on fatty acid oxidation-related protein expression levels in rat skeletal muscles. Journal of Oleo Science, 2015, 64(4): 415-421. DOI:10.5650/jos.ess14199.
[74] DING J, LOIZIDES-MANGOLD U, RANDO G, ZOETE V, MICHIELIN O, REDDY J K, WAHLI W, RIEZMAN H, THORENS B. The peroxisomal enzyme L-PBE is required to prevent the dietary toxicity of medium-chain fatty acids. Cell Reports, 2013, 5(1): 248-258. DOI:10.1016/j.celrep.2013.08.032.
[75] 王钰飞, 齐岩, 铃田靖幸, 陈燕军, 优克刚, 安福生, 薛廷伍. 中链脂肪酸在新生仔猪上的研究与应用. 动物营养学报, 2015, 27(7): 1997-2004..
WANG Y F, QI Y, YASUYUKI S, CHEN Y J, KATSUGO Y, AN F S,XUE T W. Research and application of medium-chain fatty acids in neonatal piglets. Chinese Journal of Animal Nutrition, 2015, 27(7): 1997-2004. (in Chinese)
[76] PANYAKAEW P, BOON N, GOEL G, YUANGKLANG C, SCHONEWILLE J T, HENDRIKS W H, FIEVEZ V. Effect of supplementing coconut or krabok oil, rich in medium-chain fatty acids on ruminal fermentation, protozoa and archaeal population of bulls. Animal, 2013, 7(12): 1950-1958.DOI:10.1017/s1751731113001766.
[77] KHOSRAVINIA H. Effect of dietary supplementation of medium- chain fatty acids on growth performance and prevalence of carcass defects in broiler chickens raised in different stocking densities. Journal of Applied Poultry Research, 2015, 24(1): 1-9. DOI:10.3382/ japr/pfu001.
[78] 王建军, 王恬. 中链脂肪酸的生物学特性及其在动物生产中的应用. 动物营养学报, 2011, 23(7): 1073-1078.
WANG J J,WANG T. Medium-chain fatty acids and their application in animal production. Chinese Journal of Animal Nutrition, 2011,23(27):1073-1078. (in Chinese)
[79] HANCZAKOWSKA E, SWIATKIEWICZ M, NATONEK- WISNIEWSKA M, OKON K. Medium chain fatty acids (MCFA) and/or probiotic Enterococcus faecium as a feed supplement for piglets. Livestock Science, 2016, 192:1-7. DOI10.1016/j.livsci.2016. 08.002.
[80] HANCZAKOWSKA E, SZEWCZYK A, OKON K. Effects of dietary caprylic and capric acids on piglet performance and mucosal epithelium structure of the ileum. Journal of Animal and Feed Sciences, 2011, 20(4): 556-565. DOI:10.22358/jafs/66213/2011.
[81] HAN Y K, HWANG I L H, THACKER P A. Use of a micro- encapsulated eucalyptus-medium chain fatty acid product as an alternative to zinc oxide and antibiotics for weaned pigs. Journal of Swine Health and Production, 2011, 19(1): 34-43.
[82] HANCZAKOWSKA E, SZEWCZYK A, SWIATKIEWICZ M, OKON K. Short- and medium-chain fatty acids as a feed supplement for weaning and nursery pigs. Polish Journal of Veterinary Sciences, 2013, 16(4): 647-654. DOI:10.2478/pjvs-2013-0092.
[83] KUANG Y, WANG Y, ZHANG Y, SONG Y, ZHANG X, LIN Y, CHE L, XU S, WU D, XUE B, FANG Z. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Animal Feed Science and Technology, 2015, 208: 145-157. DOI:10.1016/ j.anifeedsci.2015.07.010.
[84] CERA K R, MAHAN D C, REINHART G A. Postweaning swine performance and serum profile responses to supplemental medium- chain free fatty-acids and tallow. Journal of Animal Science, 1989, 67(8): 2048-2055.
[85] DEVI S M, KIM I H. Effect of medium chain fatty acids (MCFA) and probiotic () supplementation on the growth performance, digestibility and blood profiles in weanling pigs. Veterinarni Medicina, 2014, 59(11): 527-535. DOI:10.17221/7817- vetmed.
[86] GEBHARDT J T, THOMSON K A, WOODWORTH J C, DRITZ S S, TOKACH M D, DEROUCHEY J M, GOODBAND R D, JONES C K, COCHRANE R A, NIEDERWERDER M C, FERNANDO S, ABBAS W, BURKEY T E. Effect of dietary medium-chain fatty acids on nursery pig growth performance, fecal microbial composition, and mitigation properties against porcine epidemic diarrhea virus following storage. Journal of Animal Science, 2020, 98(1). DOI:10. 1093/jas/skz358.
[87] PAULO F, SANTOS L. Design of experiments for microencapsulation applications: A review. Materials Science & Engineering C-Materials for Biological Applications, 2017, 77:1327-1340. DOI:10.1016/j.msec. 2017.03.219.
[88] ZENTEK J, BUCHHEIT-RENKO S, MANNER K, PIEPER R, VAHJEN W. Intestinal concentrations of free and encapsulated dietary medium-chain fatty acids and effects on gastric microbial ecology and bacterial metabolic products in the digestive tract of piglets. Archives of Animal Nutrition, 2012, 66(1): 14-26. DOI:10.1080/1745039x. 2011.644916.
[89] OMONIJO F A, KIM S, GUO T, WANG Q, GONG J, LAHAYE L, BODIN J-C, NYACHOTI M, LIU S, YANG C. Development of novel microparticles for effective delivery of thymol and lauric acid to pig intestinal tract. Journal of Agricultural and Food Chemistry, 2018, 66(37): 9608-9615. DOI:10.1021/acs.jafc.8b02808.
[90] HOSSAIN M M, JAYARAMAN B, KIM S C, LEE K Y, KIM I H, NYACHOTI C M. Effects of a matrix-coated organic acids and medium-chain fatty acids blend on performance, and in vitro fecal noxious gas emissions in growing pigs fed in-feed antibiotic-free diets. Canadian Journal of Animal Science, 2018, 98(3): 433-442. DOI:10. 1139/cjas-2017-0053.
Functions of Antibacterial and Inducing Defense Peptide Expression of Medium-Chain Fatty Acid and Its Application in Piglet Feeds
YU ZhengWang, ZHOU ZhongXin
Key Lab of Agricultural Animal Genetics/Breeding and Reproduction of Ministry of Education/Department of Animal Nutrition and Feed Science, Huazhong Agriculture University, Wuhan 430070
In recent years, more and more studies have shown that medium-chain fatty acid (MCFAs) resistance to pathogenic bacteria is an important component of innate defense system of mammals, and MCFAs can also induce expressions of endogenous host defense peptides (HDPs) in human, pig and chicken. However, these new functions of MCFAs have not attracted much attention. MCFAs also have a synergistic antibacterial synergistic effect with feeding organic acids or feeding plant essential oils, which can reduce the use of these active substances. In addition, compared with long-chain fatty acids, the addition of MCFAs in the diet can significantly increase the oxygen consumption and mitochondrial respiration rate in the body of animals, but it produces less reactive oxygen species, which is in line with the characteristics of rapid energy supply required by intestinal metabolism and liver metabolism in young animals. Adding low concentration of MCFAs (0.1%-0.5%, mass ratio) to the diet can significantly increase the survival rate of newborn or weaned piglets, the digestibility of crude protein and crude fat as well as the feed conversion rate, regulate the intestinal flora, and improve the intestinal epithelial structure, thus promoting the growth of animals. Based on the above advantages of MCFAs, mixing MCFAs with forage organic acid or plant essential oil to prepare coated particles may be a good way to use it as a substitute for antibiotics in piglets.
medium chain fatty acid; innate defense system; host defense peptide; synergistic antibiotics; piglets

10.3864/j.issn.0578-1752.2021.13.017
2020-05-31;
2021-04-07
“十三五”国家重点研发计划(2016YFD0501210)、中央高校基本科研业务费专项基金资助(2662018BY017)
喻正旺,E-mail:1575800877@qq.com。通信作者周忠新,E-mail:zhongxinzhou@mail.hzau.edu.cn
(责任编辑 林鉴非)

