肌动蛋白结合蛋白FgAbp1参与禾谷镰孢生长、发育和毒素体形成
张承启,王晓妍,陈莉
肌动蛋白结合蛋白FgAbp1参与禾谷镰孢生长、发育和毒素体形成
张承启,王晓妍,陈莉
安徽农业大学植物保护学院/作物有害生物综合治理安徽省重点实验室/植物病虫害生物学与绿色防控安徽普通高校重点实验室,合肥 230036
【】Abp1作为肌动蛋白结合蛋白家族成员之一,在各种真核生物肌动蛋白骨架形成过程中具有核心作用。本研究旨在分析禾谷镰孢()中肌动蛋白结合蛋白FgAbp1在生长发育、对新型杀菌剂氰烯菌酯敏感性以及毒素体形成等生物学过程中的功能。利用融合PCR和酵母空隙修复技术分别构建基因敲除打靶片段和融合荧光蛋白载体,再通过聚乙二醇介导的原生质体转化的方法获取基因缺失突变体ΔFgAbp1和荧光标记菌株。观察比较野生型PH-1、突变体ΔFgAbp1和回补体ΔFgAbp1-C的菌丝生长、有性生殖以及无性繁殖,并测定基因敲除突变体ΔFgAbp1对杀菌剂氰烯菌酯的敏感性。通过融合绿色荧光蛋白,明确FgAbp1在菌丝中的分布情况;利用透射电子显微镜观察分析FgAbp1对细胞中液泡/囊泡形态的影响。在非产毒环境和诱导产毒条件下,通过双荧光共定位分析FgAbp1在禾谷镰孢毒素体形成过程中的作用。禾谷镰孢中,FgAbp1主要呈颗粒状定位于近细胞膜处。在MM培养基中,基因敲除突变体ΔFgAbp1的生长速率与野生型相比降低了15%,但在营养丰富的CM中ΔFgAbp1的生长速率却减慢了38%。突变体ΔFgAbp1在无性繁殖以及有性生殖过程中相较野生型没有明显缺陷。然而在0.5 μg·mL-1氰烯菌酯的作用下,ΔFgAbp1的菌丝生长完全受到抑制,并且分生孢子萌发率显著下降。此外,敲除基因导致细胞中液泡不能正常形成且囊泡增多。在非产毒条件下,FgAbp1与DON毒素合成关键酶Tri1共定位于囊泡中;在诱导产毒条件下,FgAbp1与Tri1则共定位于毒素体中。此外,敲除基因会导致毒素体不能正常形成。肌动蛋白结合蛋白FgAbp1在禾谷镰孢营养生长、发育、氰烯菌酯敏感性以及毒素体形成过程中发挥着重要作用。
禾谷镰孢;FgAbp1;氰烯菌酯;毒素体
0 引言
【研究意义】由禾谷镰孢()引起的小麦赤霉病是小麦上的毁灭性病害,该病害不但会导致小麦严重减产,而且病原菌在小麦粒中可以产生真菌毒素严重危害人和动物健康[1]。禾谷镰孢通常产生的真菌毒素为脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON)、雪腐镰刀菌烯醇(nivalenol,NIV)以及玉米赤霉烯酮(zearalenone,ZEN),而其中又以DON的毒性最大,也是全世界范围内最受关注的真菌毒素之一,很多国家都已经制定了小麦及其制品中DON毒素残留的最高限量标准[2-3]。DON毒素可以作用于动物细胞的核糖体,抑制蛋白质的合成,从而使机体的免疫系统出现障碍,导致血液、生殖以及呼吸系统发生病变甚至诱发癌症[4]。减少食品中DON毒素污染最直接有效的办法就是防控小麦赤霉病的发生,目前农业生产中防控小麦赤霉病的关键措施就是在小麦抽穗扬花期施用杀菌剂[5-6]。氰烯菌酯作为一种新型杀菌剂对镰孢菌具有特异的抑菌活性,其通过作用于肌球蛋白myosin-Ⅰ,从而实现抑菌和控制产毒的机理在一定程度上已经得到了详细的解析[7-8]。肌动蛋白结合蛋白(actin binding protein,Abp1)作为肌球蛋白myosin-Ⅰ重要的互作元件之一[9],研究其在禾谷镰孢生长发育、杀菌剂敏感性以及DON毒素生物合成过程中的作用,对于揭示该病原菌的产毒调控机制具有重要的理论意义。【前人研究进展】Abp1广泛存在于真核生物细胞中,包括真菌、果蝇、寄生虫以及哺乳动物,并且作为桥梁参与肌动蛋白骨架连接的内吞过程[10]。Abp1p首次被研究鉴定是在酿酒酵母()中,通过亲和层析试验发现Abp1p与肌动蛋白功能的正常发挥密切相关[11]。Abp1p蛋白结构特征主要包括N-端肌动蛋白解聚因子同源结构域(N-terminal actin depolymerizing factor homology,ADF-H)[12]、螺旋区域(helical region)、富含脯氨酸(proline-rich region,PRR)的中心区域以及C-端的SH3(Src-homology 3)结构域[13]。Abp1p不与肌动蛋白单体(actin monomer)结合,而是主要通过ADF-H结构域结合肌动蛋白丝(actin filament)[12,14]。Abp1p具有的两个酸性氨基酸残基序列是结合并激活Arp2/3复合体所必需的[14];通过SH3结构域与Ark1p、Scp1p以及Sjl2p等蛋白互作共同参与内吞过程[13,15-17]。与酿酒酵母的Abp1p类似,哺乳动物的同源蛋白mAbp1也是通过ADF-H结构域与肌动蛋白丝结合并参与受体介导的内吞作用[18-19]。不同的是,酵母的Abp1p定位于近细胞膜侧的肌动蛋白块(actin patch)中,而mAbp1通常定位在近细胞核区域,但是当被Rac GTPase激活后,mAbp1被招募到动态的肌动蛋白束(actin cable)上,并精确地定位到Arp2/3复合体中[11,20]。此外,mAbp1还通过与其他多种蛋白互作参与背纹的形成和细胞运动[21-23]。在酵母中,仅仅敲除并不会影响菌体的生长,只是轻微地影响了细胞的内吞过程,但是当参与内吞过程的、或其中的任何一个与同时被敲除则会致死[24-25];过表达会导致酵母细胞中的肌动蛋白骨架出现严重的缺陷[11]。还有研究表明,同时敲除和肌动蛋白形成相关的蛋白激酶时,会导致酵母对温度异常敏感[26]。Abp1作为细胞内吞过程的标志性蛋白,在酵母中参与内吞作用时与Sla2的功能类似[27-28]。在哺乳动物细胞中,mAbp1介导肌动蛋白骨架与内吞作用之间的联系,并充当汇聚多个信号途径的枢纽[18]。在稻瘟病菌()中,MoAbp1与蛋白激酶MoArk1互作,调控病原菌的生长、致病以及内吞过程[29]。【本研究切入点】Abp1普遍存在于真核生物中,虽然在蛋白结构上具有一定的保守性,但其功能在不同的物种中又具有差异性。禾谷镰孢中Abp1同源蛋白由基因(FGSG_01316)编码,尚未有研究报道其在病原真菌次生代谢过程中的作用。【拟解决的关键问题】基于同源重组原理,通过PEG介导的原生质体转化的方法,获得基因敲除突变体ΔFgAbp1以及相关的荧光标记菌株,明确FgAbp1在禾谷镰孢生长发育、对氰烯菌酯敏感性以及毒素体形成等方面的作用,为进一步揭示DON毒素生物合成调控提供一定的理论依据。
1 材料与方法
试验于2019—2020年在安徽农业大学国家农作物品种审定特性鉴定站完成。
1.1 菌株及培养条件
禾谷镰孢野生型菌株PH-1(NRRL 31084)、基因敲除突变体ΔFgAbp1和回补突变体ΔFgAbp1-C、质粒neo-PYF11、pBluescript SK+以及大肠杆菌DH5等均保存在安徽农业大学国家农作物品种审定特性鉴定站。诱导产毒培养基TBI(trichothecene biosynthesis induction medium):1 L,pH 4.5,含有蔗糖30 g、腐胺0.8 g、KH2PO41 g、MgSO4·7H2O 0.5 g、KCl 0.5 g、FeSO4·7H2O 10 mg和微量元素溶液200 μL(100 mL:柠檬酸5 g、ZnSO4·7H2O 5 g、CuSO4·5H2O 0.25 g、MnSO4·H2O 50 mg、H3BO350 mg和NaMoO4·2H2O 50 mg)。试验中涉及到的完全培养基(complete medium,CM)、基本培养基(minimal medium,MM)、马铃薯琼脂培养基(potato dextrose agar,PDA)、分生孢子诱导培养基(carboxymethyl cellulose,CMC)等的配制方法来源于镰孢菌试验手册(laboratory manual)[30]。
1.2 化学试剂和仪器
高保真和普通DNA聚合酶、RNA反转录试剂盒、SYBR Green Ⅰ荧光染料购自南京诺唯赞生物科技有限公司;酵母转化试剂盒购自美国MP Biomedicals公司;潮霉素、酵母质粒提取试剂盒购自北京索莱宝科技有限公司;质粒小量提取试剂盒、胶回收试剂盒、引物以及常规生化试剂均采购于上海生工生物工程有限公司。
移液器、小型台式离心机、低温冷冻离心机产自Eppendorf公司;PCR仪型号:C1000 Touch,Bio-Rad公司;垂直电泳仪(Powerpac HV)和多功能凝胶成像仪(Chemidoc)均为Bio-Rad公司生产;激光共聚焦显微镜Zeiss LSM780,德国;透射电子显微镜JEOL JEM-1230,日本。
1.3 基因敲除及回补
在真菌基因组数据库(https://fungidb.org/fungidb/)中检索出基因(FGSG_01316),并设计引物(表1)。首先,以野生型菌株PH-1的基因组DNA为模板,分别扩增出基因编码区上游、下游序列各约1 000 bp,以质粒pBluescript SK+为模板扩增出潮霉素磷酸转移酶基因()。其次,利用融合PCR(double-joint PCR)技术原理,按照上游--下游的顺序融合成一个完整的重组DNA片段,从而构建出基因敲除打靶片段[31]。最后,借助原生质体转化的方法将重组打靶DNA片段转入野生型菌株PH-1中[32],在含有100 μg·mL-1潮霉素B的PDA平板中筛选转化子,并用表1中引物Abp1-ID-F/Abp1-ID-R鉴定敲除转化子。
利用高保真酶和表1中的引物Abp1-GFP-F/Abp1- GFP-R,连同基因的启动子区至开放阅读框一起扩增,将纯化后的PCR产物与经Ⅰ线性化的质粒neo-pYF11,共转化进入酵母菌株XK1-25细胞中[33-34]。经PCR鉴定并提取阳性克隆酵母中的重组质粒,将其转入大肠杆菌DH5中进行质粒扩繁;提取大肠杆菌质粒,通过PEG介导的原生质体转化,回补基因缺失突变体ΔFgAbp1,通过抗生素G418(100 μg·mL-1)筛选,同时PCR鉴定出回补突变体。
1.4 菌落生长、无性繁殖和有性生殖测定
生长速率测定:用内径为5 mm的打孔器切取已活化于PDA中生长3 d的菌落边缘,菌碟分别接种于含有PDA、MM和CM的9 cm培养皿中,25℃培养3 d后测量菌落直径并拍照,每次重复3块培养皿,试验重复3次。
产孢量及分生孢子形态观测:从新鲜的菌落边缘取5个直径为5 mm的菌碟接种到30 mL CMC培养液中,每个菌株3瓶,25℃,光暗交替12 h,180 r/min摇培3 d。三层擦镜纸过滤收集分生孢子,血球计数板统计产孢量,试验重复3次。分别吸取2.5 μL滤液中的分生孢子和2.5 μL荧光增白剂(calcofluor white,CFW)于洁净的载玻片上吸打混匀,于荧光显微镜下观察分生孢子的形态特征。

表1 本研究所用到的引物
有性生殖测定:将各菌株接种于胡萝卜培养基中进行有性生殖诱导,每个菌株5个重复。25℃普通光照培养箱中待各菌株完全长满整个培养皿,刮净胡萝卜培养基表面的气生菌丝,并在表面均匀涂抹800 μL 0.1% Tween-20,晾干后封口并正置于黑光灯下,25℃培养20 d后拍照。接种针挑取胡萝卜培养基表面的子囊壳于载玻片上,滴加无菌水,加上盖玻片轻轻按压,显微镜下观察子囊及子囊孢子的形态。
1.5 氰烯菌酯敏感性测定
从新鲜的菌落边缘打孔取直径5 mm的菌碟分别接种于含有0.5 μg·mL-1氰烯菌酯和等量二甲基亚砜(DMSO)的PDA培养基中,25℃培养3 d后测量菌落直径并拍照,每次重复3个皿,试验重复3次。观察氰烯菌酯对分生孢子萌发的影响时,用2%的蔗糖水溶液调节分生孢子浓度至5 000个/mL,加入氰烯菌酯使之终浓度为0.5 μg·mL-1并以等量的DMSO作为对照组,25℃黑暗条件下处理5 h,各菌株分别统计300个孢子计算萌发率。
1.6 荧光定位以及透射电镜观察
将新鲜的菌碟分别接种于PDB和TBI培养基中25℃避光,摇培48 h,吸取菌丝于激光共聚焦显微镜下观察红、绿荧光信号,物镜60×1.30 Oil DIC M27,激发波长488、561 nm,采集8位模式的图像。用透射电子显微镜观察细胞中囊泡的方法参考Tang等[35]。
2 结果
2.1 FgAbp1参与禾谷镰孢营养生长
基因敲除突变体ΔFgAbp1在培养基PDA和MM上的生长速率与野生型PH-1、回补突变体ΔFgAbp1-C相比降低了约15%,但在CM培养基中生长时,ΔFgAbp1的生长速率降低了38%。虽然敲除基因在一定程度上减慢了病原菌的生长速率,但是突变体ΔFgAbp1合成色素的能力并没有发生明显变化(图1-a、1-b)。
2.2 ΔFgAbp1无性繁殖和有性生殖能力分析
观察统计诱导产孢培养基CMC中分生孢子的产量以及形态特征,发现突变体ΔFgAbp1与野生型PH-1相比并没有明显差异(图2-a、2-b)。此外,子囊壳和子囊孢子作为重要的初侵染源,在禾谷镰孢侵染小麦的过程中发挥着关键作用[36]。通过有性生殖诱导发现,野生型PH-1、敲除突变体ΔFgAbp1和回补体ΔFgAbp1-C在子囊壳数量、子囊以及子囊孢子的形态特征上没有明显差异(图2-c),说明基因的缺失并不影响禾谷镰孢的无性繁殖和有性生殖过程。
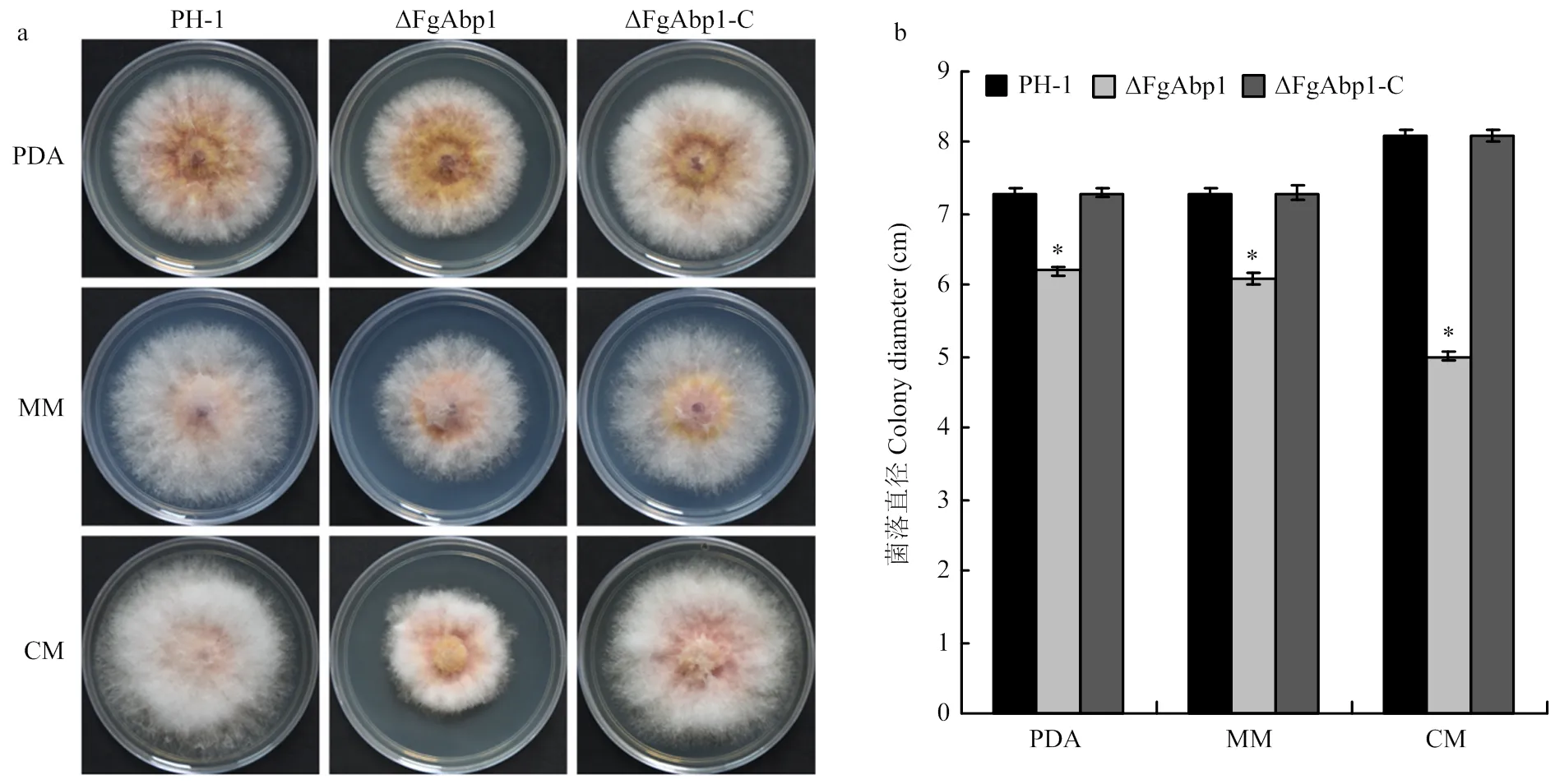
a:野生型PH-1、敲除突变体ΔFgAbp1和回补体ΔFgAbp1-C在PDA、MM和CM中25℃培养3 d的菌落形态Colony morphology of the wild-type PH-1, deletion mutant ΔFgAbp1 and the complemented strain ΔFgAbp1-C on PDA, MM and CM after 3 days of incubation at 25℃;b:统计分析各菌株在PDA、MM和CM中25℃生长3 d后的菌落直径。误差线表示标准差,*:P<0.05 Statistical analysis of each strain colony diameters following growth on PDA, MM and CM at 25℃ for 3 days. Error bars represent standard deviation

a:PH-1、ΔFgAbp1和ΔFgAbp1-C的分生孢子经荧光增白剂染色,标尺20 μm Conidia of PH-1, ΔFgAbp1 and ΔFgAbp1-C were stained with calcofluor white. Bar = 20 μm;b:各菌株在CMC培养液中3 d的产孢量Conidiation of each strain was assayed with 3-day-old CMC cultures;c:PH-1、ΔFgAbp1和ΔFgAbp1-C在胡萝卜培养基中的有性生殖。有性生殖诱导20 d后拍照;插入框中为子囊和子囊孢子Sexual development of PH-1, ΔFgAbp1 and ΔFgAbp1-C grown on carrot agar media. Photos were taken 20 days after sexual induction. Dissecting the perithecia exhibited the asci and ascospores of each strain (inset boxes)
2.3 敲除FgAbp1影响禾谷镰孢对氰烯菌酯的敏感性
将野生型PH-1、突变体ΔFgAbp1和回补体ΔFgAbp1-C分别接种在含有0.5 μg·mL-1氰烯菌酯的PDA平板中时发现,突变体ΔFgAbp1对氰烯菌酯的敏感性显著增加,氰烯菌酯对野生型PH-1的菌丝生长抑制率为75.2%,而相同条件下ΔFgAbp1则几乎不能生长(图3-a、3-b)。通过进一步分析氰烯菌酯对突变体ΔFgAbp1分生孢子萌发的影响,发现在0.5 μg·mL-1氰烯菌酯作用下,ΔFgAbp1分生孢子萌发率显著降低(图3-c、3-d)。但是ΔFgAbp1对苯并咪唑类杀菌剂多菌灵、三唑类杀菌剂戊唑醇的敏感性与野生型相比没有明显差异(数据未显示)。
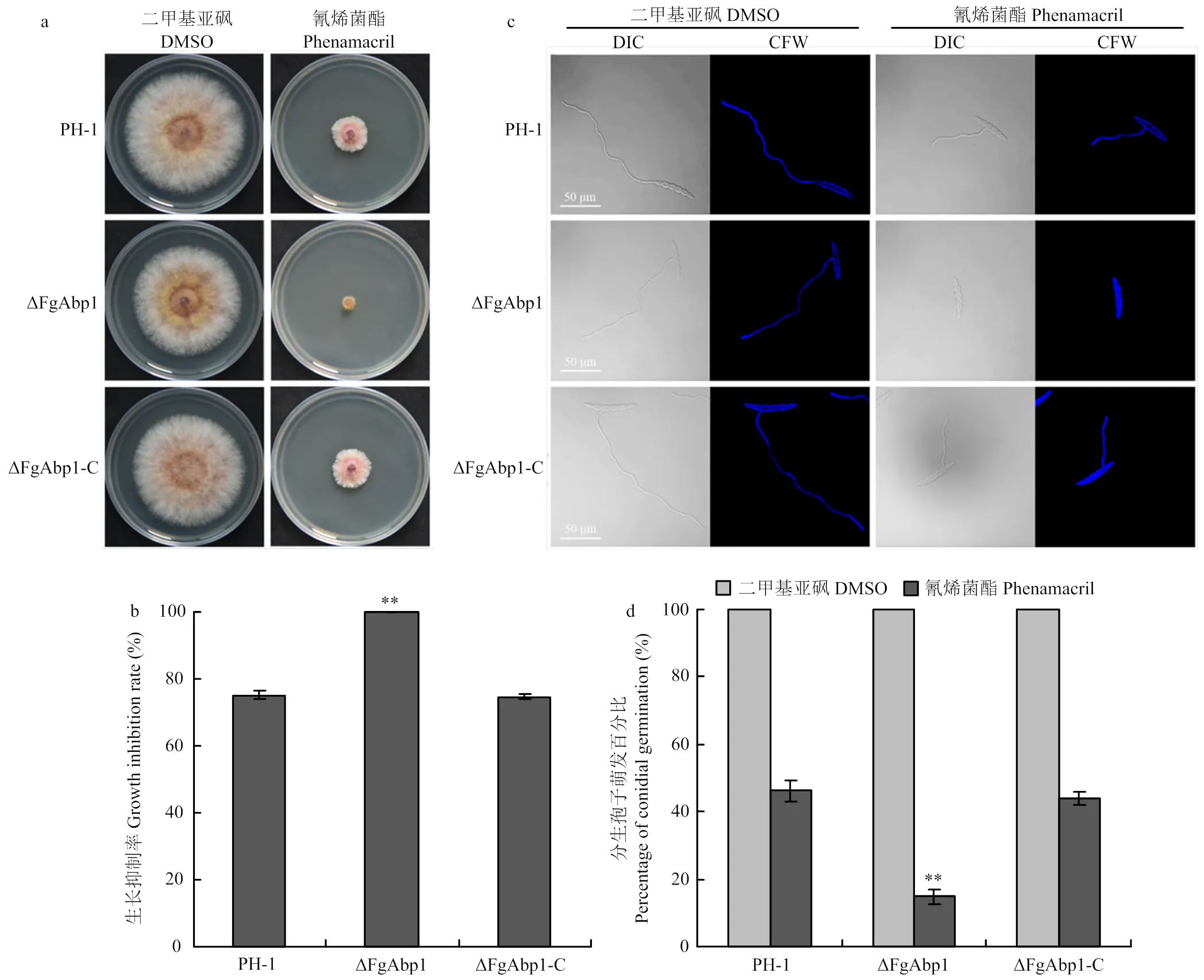
a:PH-1、ΔFgAbp1和ΔFgAbp1-C在含有0.5 μg·mL-1氰烯菌酯的PDA中25℃培养3 d的生长比较。溶剂DMSO作为对照组Comparison of PH-1, ΔFgAbp1 and ΔFgAbp1-C following incubation at 25℃ for 3 days on PDA plates supplemented 0.5 μg·mL-1phenamacril. DMSO was used as a control treatment;b:各菌株在0.5 μg·mL-1氰烯菌酯处理下的生长抑制率。误差线表示标准差,**:P<0.01 The growth inhibition rate of each strain under 0.5 μg·mL-1phenamacril treatment. Error bars represent standard deviation;c:氰烯菌酯对PH-1、ΔFgAbp1和ΔFgAbp1-C分生孢子萌发的抑制作用。激光共聚焦显微镜拍摄微分干涉(DIC)和荧光增白剂(CFW)染色照片。标尺50 μm Inhibitory effect of 0.5 μg·mL-1 phenamacril on conidial germination of PH-1, ΔFgAbp1 and ΔFgAbp1-C. Differential interference contrast (DIC) images of conidia stained with calcofluor white (CFW) were taken with a confocal fluorescence microscope. Bar = 50 μm;d:0.5 μg·mL-1氰烯菌酯处理下各菌株分生孢子的萌发率。误差线表示标准差**:P<0.01 The conidial germination rate of each strain under 0.5 μg·mL-1 phenamacril treatment. Error bars represent standard deviation
2.4 FgAbp1参与囊泡/液泡的形成
通过融合绿色荧光蛋白(green fluorescent protein,GFP)回补基因缺失突变体ΔFgAbp1,激光共聚焦显微镜观察发现回补菌株ΔFgAbp1-C中GFP荧光信号主要呈颗粒状定位于细胞膜附近(图4-a)。为了进一步明确突变体ΔFgAbp1细胞内部结构的变化,利用透射电子显微镜观察发现,野生型菌株PH-1的细胞中液泡形态正常,而基因敲除突变体ΔFgAbp1细胞中则没有大液泡,而是出现了很多囊泡(图4-b)。

a:FgAbp1的亚细胞定位。DIC,微分干涉;GFP,绿色荧光蛋白。标尺10 μm Subcellular localization of FgAbp1. DIC, differential interference contrast. GFP, green fluorescent protein. Bar = 10 μm;b:突变体ΔFgAbp1细胞中形成大量的囊泡和异常的液泡。透射电子显微镜拍摄PH-1和ΔFgAbp1细胞超微结构中液泡/囊泡的形态The mutant ΔFgAbp1 caused a high vesicle number and abnormal vacuole. The ultrastructural morphology of vacuole/vesicle in PH-1 and ΔFgAbp1 was visualized by transmission electron microscopy
2.5 FgAbp1参与毒素体的形成
近年来研究发现,DON毒素生物合成途径中关键氧化酶Tri1定位于一种被称为毒素体(toxisome)的囊泡膜上[37]。在禾谷镰孢中FgAbp1与囊泡/液泡的形成密切相关,为了明确FgAbp1是否参与了毒素体的形成,进行了共定位试验。将FgAbp1融合红色荧光蛋白(red fluorescent protein,RFP),参与DON毒素合成的关键酶Tri1融合GFP。在非诱导产毒培养基PDB中,Tri1-GFP与FgAbp1-RFP共定位于囊泡中(图5-a),但在诱导产毒培养基TBI中,发现Tri1-GFP与FgAbp1-RFP则完全共定位于毒素体膜上(图5-b)。为了进一步证实该结果,将Tri1-GFP分别转入野生型PH-1和突变体ΔFgAbp1中,在诱导产毒条件下,发现PH-1细胞中能产生形态正常的毒素体,而突变体ΔFgAbp1则不能正常形成毒素体(图5-c)。
3 讨论
肌动蛋白作为细胞骨架的重要组成部分,在真核生物中起着至关重要的作用。肌动蛋白在细胞形态、极性的产生和维持,内吞作用和物质运输,细胞的收缩性、运动性以及细胞分裂等生物学过程中发挥功能[38]。但是,肌动蛋白的组装和拆卸,以及它们编织为功能性高级网络的过程,又都受到肌动蛋白结合蛋白的调节[39]。本研究通过基因敲除和回补以及亚细胞定位等方法研究了肌动蛋白结合蛋白FgAbp1在禾谷镰孢中的生物学功能。
基因敲除突变体ΔFgAbp1与野生型相比生长速率有一定程度降低,这与稻瘟病菌中Δ突变体的生长缺陷相类似[29]。然而,酿酒酵母突变体和构巢曲霉()同源基因缺失突变体Δ则并没有明显的生长缺陷[24,40],这说明Abp1 在丝状真菌和酵母中的功能存在一定的分化。肌动蛋白通常在细胞中主要的存在形式有丝状肌动蛋白和斑点状肌动蛋白[9],通过C-端融合GFP发现,禾谷镰孢中FgAbp1主要呈颗粒状定位于近细胞膜侧或弥散在细胞质中,这种现象的出现与细胞中肌动蛋白的形态相吻合。研究已发现,无论是在酵母、哺乳动物还是丝状真菌中,Abp1都直接参与了细胞内吞过程[24,29,40-41]。突变体ΔFgAbp1细胞中液泡/囊泡的形态异常,很可能是因为敲除基因后导致菌体内吞过程受阻所致,因为内吞过程需要囊泡/液泡的参与。在真核生物中,肌球蛋白myosin-Ⅰ与Abp1互作共同参与肌动蛋白的组装以及内吞过程[9,42],对镰孢菌特异的新型杀菌剂氰烯菌酯的作用靶标即为肌球蛋白myosin-Ⅰ(FgMyo1)[8,43]。最新研究表明,FgMyo1与肌动蛋白互作调控禾谷镰孢毒素体的形成进而影响DON毒素的生物合成[7,44]。笔者发现突变体ΔFgAbp1专一性地对氰烯菌酯表现敏感,可能是由于ΔFgAbp1中肌动蛋白骨架受损,影响了肌球蛋白FgMyo1正常的功能,间接导致ΔFgAbp1表现出对氰烯菌酯的敏感性。有趣的是,在非产毒环境中FgAbp1与DON毒素生物合成过程中关键酶Tri1共定位于囊泡中;而在诱导产毒条件下,FgAbp1与Tri1共定位于毒素体膜上,并且的缺失会导致毒素体不能正常形成,该结果表明FgAbp1在禾谷镰孢毒素体的形成过程中发挥着重要作用。关于FgAbp1调控毒素体形成的机制有待于进一步研究。

a:PDB培养液中生长48 h后Tri1-GFP和FgAbp1-RFP共定位情况。标尺5 μm Co-localization of Tri1-GFP with FgAbp1-RFP in liquid PDB medium for 48 h. Bar = 5 μm;b:TBI培养基中诱导48 h后,Tri1-GFP和FgAbp1-RFP共定位于毒素体。标尺5 μm Tri1-GFP and FgAbp1-RFP co-localized in toxisomes after 48 h incubation in TBI medium. Bar = 5 μm;c:TBI培养基中诱导48 h后,PH-1和ΔFgAbp1菌丝中毒素体的形成状态。Tri1-GFP指示毒素体的形成。标尺5 μm The toxisome formation patterns in the mycelia of PH-1 and ΔFgAbp1 after 48 h incubation in TBI medium. The toxisome formation was visualized using Tri1-GFP as the indicator. Bar = 5 μm
4 结论
FgAbp1参与禾谷镰孢的营养生长,但不影响其无性繁殖和有性生殖。更为重要的是,FgAbp1特异性地参与了禾谷镰孢对杀菌剂氰烯菌酯的敏感性,并且与毒素体的形成密切相关。
[1] DEAN R, VAN KAN J A L, PRETORIUS Z A, HAMMOND- KOSACK K E, DI PIETRO A, SPANU P D, RUDD J J, DICKMAN M, KAHMANN R, ELLIS J, FOSTER G D. The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 2012, 13(4): 414-430.
[2] AUDENAERT K, VANHEULE A, HOFTE M, HAESAERT G. Deoxynivalenol: a major player in the multifaceted response ofto its environment. Toxins, 2013, 6(1): 1-19.
[3] BIANCHINI A, HORSLEY R, JACK M M, KOBIELUSH B, RYU D, TITTLEMIER S, WILSON W W, ABBAS H K, ABEL S, HARRISON G, MILLER J D, SHIER W T, WEAVER G. DON occurrence in grains: a north American perspective. Cereal Foods World, 2015, 60(1): 32-56.
[4] PESTKA J J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Archives of Toxicology, 2010, 84(9): 663-679.
[5] 陈云, 王建强, 杨荣明, 马忠华. 小麦赤霉病发生危害形势及防控对策. 植物保护, 2017, 43(5): 11-17.
CHEN Y, WANG J Q, YANG R M, MA Z H. Current situation and management strategies of Fusarium head blight in China. Plant Protection, 2017, 43(5): 11-17. (in Chinese)
[6] 史建荣, 刘馨, 仇剑波, 祭芳, 徐剑宏, 董飞, 殷宪超, 冉军舰. 小麦中镰刀菌毒素脱氧雪腐镰刀菌烯醇污染现状与防控研究进展. 中国农业科学, 2014, 47(18): 3641-3654.
SHI J R, LIU X, QIU J B, JI F, XU J H, DONG F, YIN X C, RAN J J. Deoxynivalenol contamination in wheat and its management. Scientia Agricultura Sinica, 2014, 47(18): 3641-3654. (in Chinese)
[7] TANG G F, CHEN Y, XU J R, KISTLER H C, MA Z H. The fungal myosin I is essential fortoxisome formation. Plos Pathogens, 2018, 14(1): e1006827.
[8] ZHANG C Q, CHEN Y, YIN Y N, JI H H, SHIM W B, HOU Y P, ZHOU M G, LI X D, MA Z H. A small molecule species specifically inhibitsmyosin I. Environmental Microbiology, 2015, 17(8): 2735-2746.
[9] BEREPIKI A, LICHIUS A, READ N D. Actin organization and dynamics in filamentous fungi. Nature Reviews. Microbiology, 2011, 9(12): 876-887.
[10] GARCIA B, STOLLAR E J, DAVIDSON A R. The importance of conserved features of yeast actin-binding protein 1 (Abp1p): the conditional nature of essentiality. Genetics, 2012, 191(4): 1199-1211.
[11] DRUBIN D G, MILLER K G, BOTSTEIN D. Yeast actin-binding proteins: evidence for a role in morphogenesis. Journal of Cell Biology, 1988, 107(6): 2551-2561.
[12] LAPPALAINEN P, KESSELS M M, COPE M J, DRUBIN D G. The ADF homology (ADF-H) domain: a highly exploited actin-binding module.Molecular Biology of the Cell, 1998, 9(8): 1951-1959.
[13] STOLLAR E J, GARCIA B, CHONG P A, RATH A, LIN H, FORMAN-KAY J D, DAVIDSON A R. Structural, functional, and bioinformatic studies demonstrate the crucial role of an extended peptide binding site for the SH3 domain of yeast Abp1p. Journal of Biological Chemistry, 2009, 284(39): 26918-26927.
[14] GOODE B L, RODAL A A, BARNES G, DRUBIN D G. Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. Journal of Cell Biology, 2001, 153(3): 627-634.
[15] FAZI B, COPE M, DOUANGAMATH A, FERRACUTI S, SCHIRWITZ K, ZUCCONI A, DRUBIN D G, WILMANNS M, CESARENI G, CASTAGNOLI L. Unusual binding properties of the SH3 domain of the yeast actin-binding protein Abp1: structural and functional analysis. Journal of Biological Chemistry, 2002, 277(7): 5290-5298.
[16] STEFAN C J, PADILLA S M, AUDHYA A, EMR S D. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Molecular and Cellular Biology, 2005, 25(8): 2910-2923.
[17] HAYNES J, GARCIA B, STOLLAR E J, RATH A, ANDREWS B J, DAVIDSON A R. The biologically relevant targets and binding affinity requirements for the function of the yeast actin-binding protein 1 Src-homology 3 domain vary with genetic context. Genetics, 2007, 176(1): 193-208.
[18] KESSELS M M, ENGQVIST-GOLDSTEIN A E Y, DRUBIN D G, QUALMANN B. Mammalian Abp1, a signal-responsive F-actin- binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. Journal of Cell Biology, 2001, 153(2): 351-366.
[19] MISE-OMATA S, MONTAGNE B, DECKERT M, WIENANDS J, ACUTO O. Mammalian actin binding protein 1 is essential for endocytosis but not lamellipodia formation: functional analysis by RNA interference. Biochemical and Biophysical Research Communications, 2003, 301(3): 704-710.
[20] KESSELS M M, ENGQVIST-GOLDSTEIN A E Y, DRUBIN D G. Association of mouse actin-binding protein 1 (mAbp1/SH3P7), an Src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Molecular Biology of the Cell, 2000, 11(1): 393-412.
[21] CORTESIO C L, PERRIN B J, BENNIN D A, HUTTENLOCHER A. Actin-binding protein-1 interacts with WASp-interacting protein to regulate growth factor-induced dorsal ruffle formation. Molecular Biology of the Cell, 2010, 21(1): 186-197.
[22] FENSTER S D, KESSELS M M, QUALMANN B, CHUNG W J, NASH J, GUNDELFINGER E D, GARNER C C. Interactions between Piccolo and the actin/dynamin-binding protein Abp1 link vesicle endocytosis to presynaptic active zones. Journal of Biological Chemistry, 2003, 278(22): 20268-20277.
[23] HAN J, KORI R, SHUI J W, CHEN Y R, YAO Z B, TAN T H. The SH3 domain-containing adaptor HIP-55 mediates c-Jun N-terminal kinase activation in T cell receptor signaling. Journal of Biological Chemistry, 2003, 278(52): 52195-52202.
[24] HOLTZMAN D A, YANG S, DRUBIN D G. Synthetic-lethal interactions identify two novel genes,and, that control membrane cytoskeleton assembly in. Journal of Cell Biology, 1993, 122(3): 635-644.
[25] TUO S, NAKASHIMA K, PRINGLE J R. Role of endocytosis in localization and maintenance of the spatial markers for bud-site selection in yeast. Plos One, 2013, 8(9): e72123.
[26] COPE M J, YANG S, SHANG C, DRUBIN D G. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. Journal of Cell Biology, 1999, 144(6): 1203-1218.
[27] WESP A, HICKE L, PALECEK J, LOMBARDI R, AUST T, MUNN A L, RIEZMAN H. End4p/Sla2p interacts with actin-associated proteins for endocytosis in. Molecular Biology of the Cell, 1997, 8(11): 2291-2306.
[28] AGHAMOHAMMADZADEH S, SMACZYNSKA-DE ROOIJ I I, AYSCOUGH K R. An Abp1-dependent route of endocytosis functions when the classical endocytic pathway in yeast is inhibited. Plos One, 2014, 9(7): e103311.
[29] LI L W, ZHANG S P, LIU X Y, YU R, LI X R, LIU M X, ZHANG H F, ZHENG X B, WANG P, ZHANG Z G.Abp1, a MoArk1 kinase-interacting actin binding protein, links actin cytoskeleton regulation to growth, endocytosis, and pathogenesis. Molecular Plant-Microbe Interactions, 2019, 32(4): 437-451.
[30] NICHOLSON P. Thelaboratory manual. Plant Pathology, 2007, 56(6): 1037.
[31] YU J H, HAMARI Z, HAN K H, SEO J A, REYES-DOMINGUEZ Y, SCAZZOCCHIO C. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genetics and Biology, 2004, 41(11): 973-981.
[32] PROCTOR R H, HOHN T M, MCCORMICK S P. Reduced virulence ofcaused by disruption of a trichothecene toxin biosynthetic gene. Molecular Plant-Microbe Interactions, 1995, 8(4): 593-601.
[33] BRUNO K S, TENJO F, LI L, HAMER J E, XU J R. Cellular localization and role of kinase activity of PMK1 in. Eukaryotic Cell, 2004, 3(6): 1525-1532.
[34] CHEN Y, ZHENG S Y, JU Z Z, ZHANG C Q, TANG G F, WANG J, WEN Z Y, CHEN W, MA Z H. Contribution of peroxisomal docking machinery to mycotoxin biosynthesis, pathogenicity and pexophagy in the plant pathogenic fungus. Environmental Microbiology, 2018, 20(9): 3224-3245.
[35] TANG G F, ZHANG C Q, JU Z Z, ZHENG S Y, WEN Z Y, XU S, CHEN Y, MA Z H. The mitochondrial membrane protein FgLetm1 regulates mitochondrial integrity, production of endogenous reactive oxygen species and mycotoxin biosynthesis in. Molecular Plant Pathology, 2018, 19(7): 1595-1611.
[36] TRAIL F. For blighted waves of grain:in the postgenomics era. Plant Physiology, 2009, 149(1): 103-110.
[37] MENKE J, DONG Y H, KISTLER H C.Tri12p influences virulence to wheat and trichothecene accumulation. Molecular Plant-Microbe Interactions, 2012, 25(11): 1408-1418.
[38] WINDER S J, AYSCOUGH K R. Actin-binding proteins. Journal of Cell Science, 2005, 118(4): 651-654.
[39] DOS REMEDIOS C G, CHHABRA D, KEKIC M, DEDOVA I V, TSUBAKIHARA M, BERRY D A, NOSWORTHY N J. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiological Reviews, 2003, 83(2): 433-473.
[40] ARAUJO-BAZAN L, PENALVA M A, ESPESO E A. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in. Molecular Microbiology, 2008, 67(4): 891-905.
[41] QUALMANN B, KESSELS M M, KELLY R B. Molecular links between endocytosis and the actin cytoskeleton. Journal of Cell Biology, 2000, 150(5): F111-F116.
[42] MATSUO K, HIGUCHI Y, KIKUMA T, ARIOKA M, KITAMOTO K. Functional analysis of Abp1p-interacting proteins involved in endocytosis of the MCC component in. Fungal Genetics and Biology, 2013, 56: 125-134.
[43] ZHENG Z T, HOU Y P, CAI Y Q, ZHANG Y, LI Y J, ZHOU M G. Whole-genome sequencing reveals that mutations in myosin-5 confer resistance to the fungicide phenamacril in. Scientific Reports, 2015, 5: 8248.
[44] CHEN Y, KISTLER H C, MA Z H.trichothecene mycotoxins: biosynthesis, regulation, and management. Annual Review of Phytopathology, 2019, 57: 15-39.
The actin binding protein FgAbp1 is involved in growth, development and toxisome formation in
ZHANG Chengqi, WANG Xiaoyan, CHEN Li
School of Plant Protection, Anhui Agricultural University/Anhui Province Key Laboratory of Integrated Pest Management on Crops/Key Laboratory of Biology and Sustainable Management of Plant Diseases and Pests of Anhui Higher Education Institutes, Hefei 230036
【】Abp1 is one of the actin binding proteins that plays a central role in actin cytoskeleton of diverse eukaryotic organisms. The objective of this study is to analyze functions of the actin binding protein FgAbp1 in growth and development, sensitivity to the novel fungicide phenamacril and toxisome formation in.【】Targeted gene deletion construct and fluorescent protein fusion vectors were generated by double-joint PCR and budding yeast gap repair system, respectively. Then, the mutant ΔFgAbp1 and fluorescently labeled strains were obtained using polyethylene glycol (PEG) mediated protoplast transformation. Mycelia growth, sexual/asexual reproduction and sensitivity to phenamacril of wild type PH-1, the mutant ΔFgAbp1 and complemented strain ΔFgAbp1-C were investigated. Localization of FgAbp1 in hyphae was examined through fusion green fluorescent protein. Transmission electron microscopy was carried out to assay the role of FgAbp1 in vacuole/vesicle morphology. Under noninducing medium and DON biosynthesis induction conditions, the role of FgAbp1 in the toxisome formation ofwas performed by dual fluorescence colocalization assay.【】FgAbp1 is primarily localized near the cell membrane in patches of. In MM medium, the growth rate of gene knockout mutant ΔFgAbp1 was reduced by 15% compared with the wild type. But in the nutrient-rich CM, the growth rate of ΔFgAbp1 was decreased by 38%. The mutant ΔFgAbp1 had no obvious defects in sexual and asexual reproduction in comparison with the wild type, while the mycelial growth of ΔFgAbp1 was completely inhibited and the conidia showed significant reduction of germination rate with 0.5 μg·mL-1phenamacril treatment. Moreover,deletion resulted in a high vesicle number and a block of normal vacuole formation. During growth in a toxinnoninducing condition, FgAbp1 and the DON biosynthetic key enzyme Tri1 co-fluoresced in vesicles. Unexpectedly, FgAbp1 and Tri1 cellular co-localized in toxisomes under DON biosynthesis inducing conditions. Furthermore, disruption ofresulted in abnormal toxisomes.【】The actin binding protein FgAbp1 plays an important role in vegetative growth, development, phenamacril tolerance and toxisome formation in
;FgAbp1; phenamacril; toxisome

10.3864/j.issn.0578-1752.2021.13.006
2020-10-11;
2020-10-28
国家自然科学基金(31701744)
张承启,E-mail:zhcq@ahau.edu.cn。通信作者陈莉,E-mail:chenlii@ahau.edu.cn
(责任编辑 岳梅)

