Quercetin Increased Protein Utilization and Decreased Nitrogen Excretion in Broilers by Activating TOR Signaling Pathway
Xiao Feng-lin, Mao Yan-jun, Ying Lin-lin, Wang Mi, Wang Shan-shan, Wang Bo, and Li Yao
College of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
Abstract: The study was conducted to investigate the effect and mechanism of dietary quercetin supplementation on protein utilization of Arbor Acres (AA) broilers. A total of 240 1-day-old AA broilers were randomly allocated to four treatments with six replicates,comprising 10 broilers each replicate (60 broilers per treatment). Birds were fed either a corn-soybean meal basal diet without quercetin(control) or a basal diet supplemented with 0.2, 0.4 or 0.6 g of quercetin per kg feed, and the trial lasted 42 days. Dietary quercetin supplementation tended to increase the apparent metabolic rate of protein (p=0.076) and the content of serum albumin (p=0.062)in AA broilers. Compared with the control, dietary quercetin supplementation increased the contents of protein in breast muscle(p<0.05) and in thigh muscle (p=0.053). In addition, quercetin up-regulated mRNA expression of insulin-like growth factor 1 (IGF-1),phosphatidylinositol 3-kinase (PI3K), target of rapamycin (TOR), ribosomal protein S6 kinase 1 (S6K1), eukaryotic translation initiation factor 4E (eIF4E), eukaryotic translation initiation factor 4G (eIF4G), eukaryotic elongation factor 2 (eEF2) and eukaryotic translation initiation factor 4B (eIF4B) genes and down-regulated mRNA expression of eukaryotic elongation factor 2 kinase (eEF2K) and eukaryotic initiation factor 4E binding protein1 (4E-BP1) genes in breast muscle, thigh muscle and liver of AA broilers (p<0.05). The present results suggested that dietary quercetin supplementation enhanced protein utilization in broilers by activating TOR signaling pathway.
Key words: Arbor Acres broiler, phosphatidylinositol 3-kinase, protein utilization, gene expression, target of rapamycin signaling pathway
Introduction
Protein is an essential nutrient for broilers, which provides the necessary raw materials for body's nutrition and metabolism. Proteins in feed may be degraded by digestive enzymes in gastrointestinal tract, which produces a variety of peptides and amino acids that provide important nitrogen sources for organisms and intestinal microorganisms. Peptides and amino acids, which may act as signaling molecules, are nutrients that regulate the metabolism of intestinal epithelium and body. Therefore, the levels of protein utilization in feed importantly affect the metabolic stability and intestinal homeostasis of broilers. The low utilization of protein wastes these resources and increases nitrogen emission. Nitrogen compounds are responsible for groundwater pollution, due to nitrate leaching, eutrophication of the surface water and soil acidification. The European Union has already implemented laws to regulate environmental pollution,especially emission of nitrogen compounds, which are considered to be pollutants that critically affect life on earth. Oenemaet al. (2007) found that nearly 52%of the nitrogen excreted in animal housing systems is used as a plant nutrient, whereas 48% is released into the environment through animal wastes. Thus, the task of developing strategies to reduce nitrogen emissions is urgent. A strategy is to reduce nitrogen excretion from livestock by increasing protein utilization (Médaet al., 2011). In addition, increasing protein utilization efficiency may also alleviate global food shortages and reduce the cost of feeding.
Supplementation of feed additives optimizes protein utilization and ameliorates the negative effects of excessive nitrogen excretion in livestock. Antibiotics are a common additive in livestock feed, which had been widely used in increasing protein utilization since the 1940s (Castanon, 2007). However, antibiotics are poorly absorbed in the intestine of livestock, which causes environmental pollution after excretion (Raiet al., 2016). Therefore, it is essential for researchers to find an environmentally friendly antibiotic alternative to improve protein utilization.
Flavonoids are a potentially safe feed additive for increasing protein utilization in animal production.Ginkgo bilobaextract, which is rich in flavonoids,significantly increases the apparent metabolic rate of protein inArbor Acres(AA) broilers (Renet al.,2018). Other studies showed that flavonoids increase the contents of protein in the meat of livestock (Nuhu,2010; Kambohet al., 2018). In addition, some studies suggested that flavonoids promote protein utilization in livestock by activating TOR signaling pathway (Daviset al., 2002; Kimball and Jefferson, 2004; Zhanget al., 2013; Zenget al., 2014; Lianget al., 2015; Wanget al., 2015; Cursinoet al., 2016). Quercetin, a flavonoid that is found in fruits and vegetables, contains a basic flavonoid structure of 15 carbon atoms that are arranged in the three rings (C6-C3-C6), exhibits unique biological properties that improve physical performance including anti-carcinogenic, anti-inflammatory,antiviral activities, etc (Wanget al., 2018; Liet al.,2016). However, few studies are conducted on the potential of quercetin as a feed additive to improve protein utilization and reduce nitrogen excretion in AA broilers and the mechanism by which quercetin works. The objective of this study was to investigate the mechanism and the effect of quercetin on protein utilization and nitrogen excretion in AA broilers.
Materials and Methods
This study was carried out according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Northeast Agricultural University(Protocol Number: 11928). The surgery was performed under sodium pentobarbital anesthesia, and all the efforts were made to minimize suffering.
Birds, diets and experimental treatment
A total of 240 AA broilers (1-day-old) were obtained from a commercial facility (Yinong Poultry Limited Company, Harbin, China). Birds were randomly assigned to four experimental treatments including six replicates of 10 birds each replicate. Water and experimental diets were providedad libitum.A 23L : 1D illumination schedule was provided for broilers in the first 3 days after hatching. Then reduced the illumination time by 2 h • wk-1until the illumination period of 9 h • d-1was reached. Moreover,the illumination intensity was 30 lx from 0 day to 7 days of the age, 10 lx from 7 to 22 days of the age and 3 lx from 22 days to 42 days of the age. The room temperature was maintained at 34℃ for the first 3 days, and then the temperature was reduced to 22℃ by 2℃ per week, until the end of the experiment.The broilers were vaccinated against infectious bronchitis, Newcastle disease and infectious bursal disease.
Experimental diets were based on corn-soybean meal, with four concentrations of quercetin: 0.0, 0.2,0.4, and 0.6 g of quercetin per kg of feed. Broiler feeding was divided into two phases: the starter phase from 1 day to 21 days and the grower phase from 22 days to 42 days. The basal diet was formulated to meet the nutritional requirements suggested by NY/T33-2004 (China, 2004) (Table 1). Diets containing quercetin were mixed in a basal diet, and quercetin dihydrate powder with 97% purity was purchased from Sigma-Aldrich Company (St. Louis, MO).
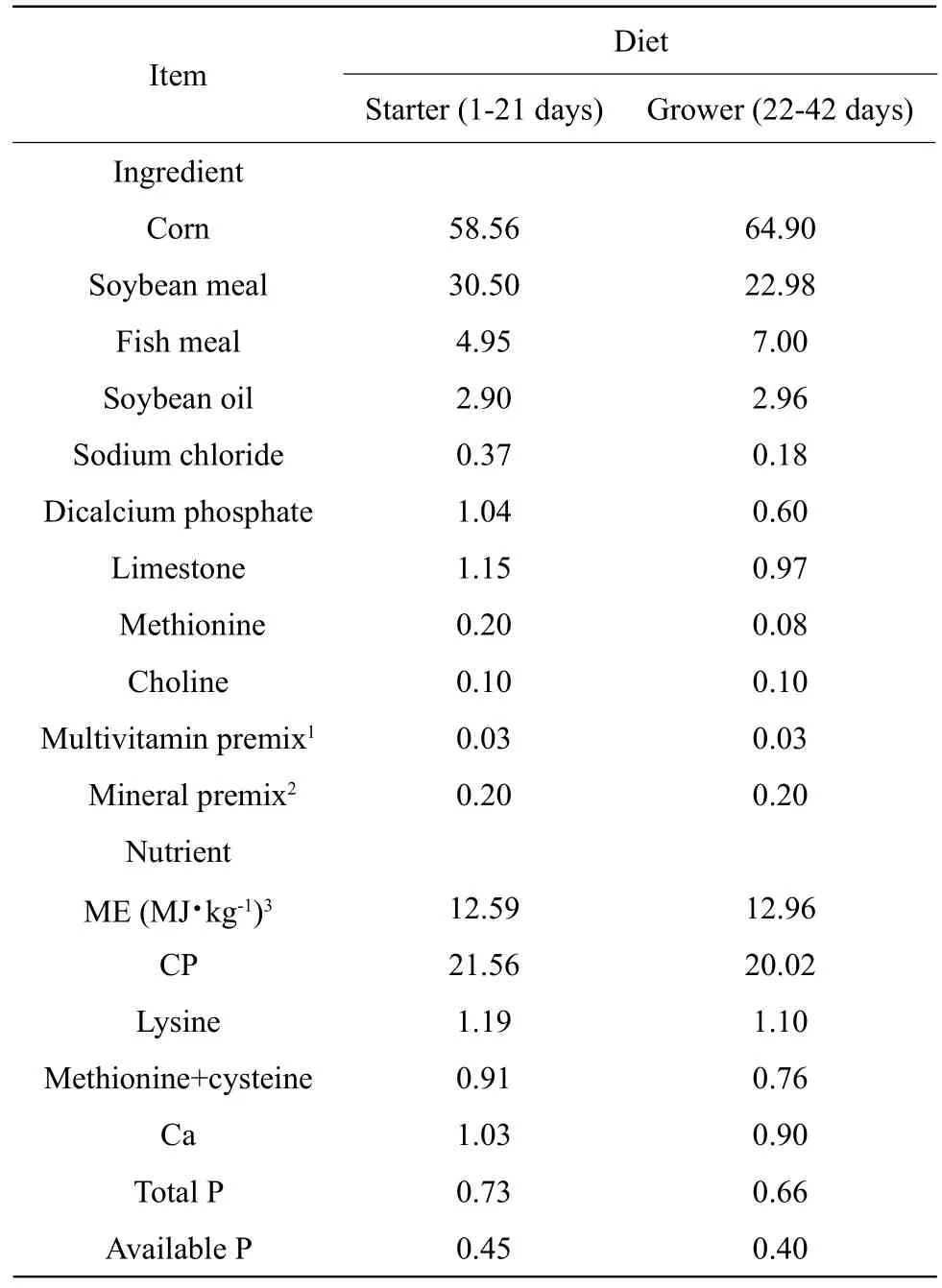
Table 1 Calculated composition of basal diets and nutrient contents (%, air-dry basis)
Sampling
On 21 and 42 days of the age, the average daily gain(ADG) and average daily feed intake (ADFI) of broilers were measured. During the trial, mortality was checked daily and weighed to adjust the feed conversion ratio (FCR). On 38 days of the age, one broiler was randomly selected from each replicate with similar body weight. Feces of each replicate were collected and weighed daily, and the feed intake was recorded for 5 days. At the end of the rearing period,the 24 broilers from each group were dissected. Blood samples were collected in heparinized tubes and were quickly centrifuged (3 000 r • min-1for 20 min)to separate serum. Serum was stored at –20℃, while breast muscle, thigh muscle and liver were stored at–80℃ for further analyses.
Apparent metabolic rate of protein
The feed samples were mixed from the upper, middle and lower parts of the feedbags, and 200 g of the mixture was taken by the "sample quartering" method.Then, the feed sample was crushed and sieved using 40 mesh sieves for subsequent analysis. A nitrogen metabolism test was carried out by means of the total feces collection method. Two broilers were randomly selected from each replicate, and the daily feed intake was accurately recorded on the basis of the replicate.During the metabolism test, the feed samples and all the feces of each treatment were collected and pollutants including feathers and dandruff were removed. After that, all of the collected feces were weighed, and 10% hydrochloric acid was added to the nitrogen fixation. The feces were placed in the labeled aluminum box, heated for 15 min at 105℃, reduced immediately to 65℃, and finally dried to constant weight. Then, the feces were crushed and sieved using 40 mesh sieves for subsequent analysis. The crude protein of feed and feces were analyzed using a Kjeldahl Auto 8400 (Foss Tecator AB, Copenhagen,Denmark) according to the methods of the Association of Official Analytical Chemists (AOAC) (Horwitzet al., 1980). Each sample was analyzed in triplicate. The apparent metabolic rate of protein was calculated by using the following formula:
Apparent metabolic rate of protein (%)=(nitrogen content in feed-nitrogen content in feces/nitrogen content in feed)×100%
Blood parameters
On 42 days of the age, blood samples were collected in anticoagulant tubes and quickly centrifuged (3 000 r • min-1for 20 min) to separate the serum from the wing vein before slaughtering the broilers after 12 h of fasting. The contents of the total proteins, albumin and urea nitrogen in serum were determined using the total proteins, albumin and urea assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Protein content in muscle
At the end of the experiment, one chicken was randomly selected for each replicate and then was slaughtered after blood sampling. Breast muscle and thigh muscle samples were collected in tubes and were stored at –20℃ and –80℃, respectively. The protein contents of breast muscle and thigh muscle were assayed using the total protein kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Real-time quantitative polymerase chain reaction (RT-qPCR)
Expression of 11 target genes and the house-keeping geneβ-actin in breast muscle, thigh muscle and liver were determined using RT-qPCR (Table 2). The total RNA was extracted using TRIzol Reagent (Invitrogen,Carlsbad, CA) from 100 mg tissue samples according to the manufacturer's instructions. The extracted total RNA was reversely transcribed into cDNA using PrimeScript RT Reagent Kit (TaKaRa Biotechnology, Dalian, China). Quantification of cDNA by RT-qPCR was performed using the ABI Prism 7500 sequence detection system (Roche, Mannheim,Germany) with an initial denaturation at 95℃ for 30 s followed by 40 cycles of 95℃ for 5 s and 60℃ for 34 s. The 10 μL reaction mixture contained 5 μL of SYBR Premix ExTaq, 0.2 μL of ROX Reference Dye II, 0.2 μL of each primer (10 μmol • L-1), 3.4 μL of sterilized double-distilled water, and 1 μL of template DNA. Each sample was subjected to RT-qPCR in duplicate, and the cycle threshold value of the duplicates was used for subsequent calculations. The 2−ΔΔCtmethod was used withβ-actin as the reference transcript for relative gene expression. Moreover, melting curve and sequence analyses were performed for each product to verify specificity of amplification (Linet al., 2016).

Table 2 PCR primers of target of rapamycin signaling pathway
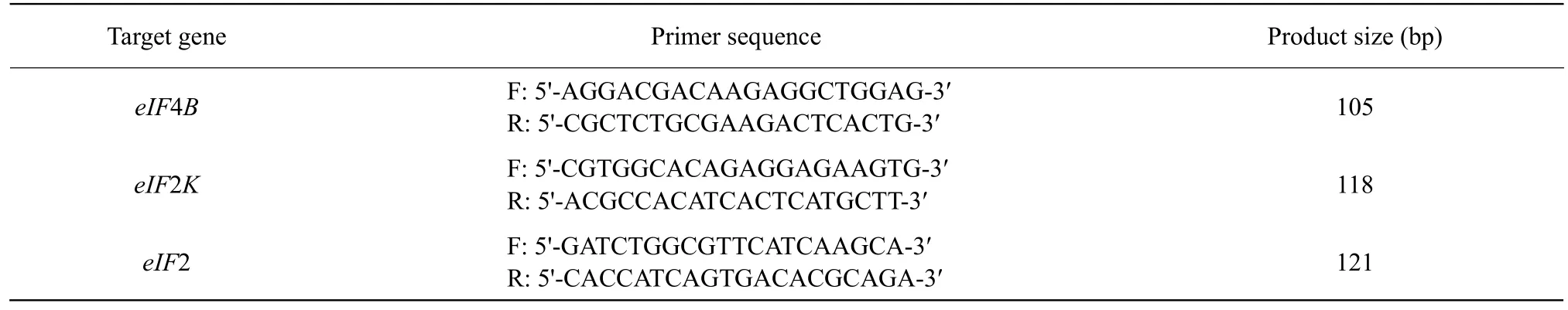
Continued
Statistical analysis
A completely randomized experimental design was employed in this study. Chickens slaughtered were randomly selected from each replicate. The data were subjected to one-way analysis of variance in a completely randomized design with four treatments and six replicates for each treatment using SPSS 20.0 (IBM, Armonk, NY). The treatment means and the standard error of the means were tested using orthogonal polynomial contrasts for the evaluation of the linear and quadratic effects of the dietary quercetin supplementation. The experimental data were tested by variance homogeneity and multiple comparisons were made by Duncan's method. Statistical significance was established atp<0.05 and trends were discussed at 0.05<p<0.10.
Results
Effects of quercetin on growth performance
There was no significant difference in the average daily gain, the average daily feed intake and the feed conversion rate among groups (p>0.05) (Table 3).
Effects of quercetin on apparent metabolic rate of protein
Compared with the control, quercetin supplementation tended to increase the apparent metabolic rate of protein in AA broilers (p=0.076) (Table 4).
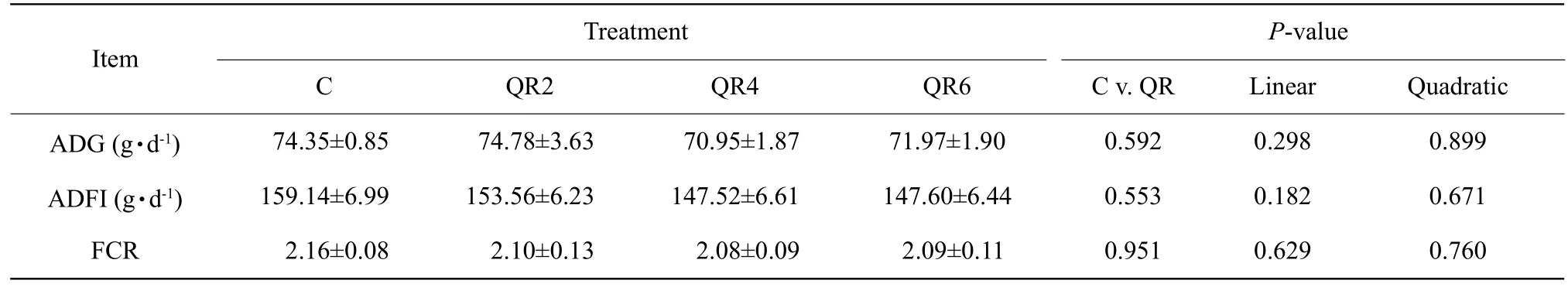
Table 3 Effect of quercetin on growth performance of Arbor Acres broilers
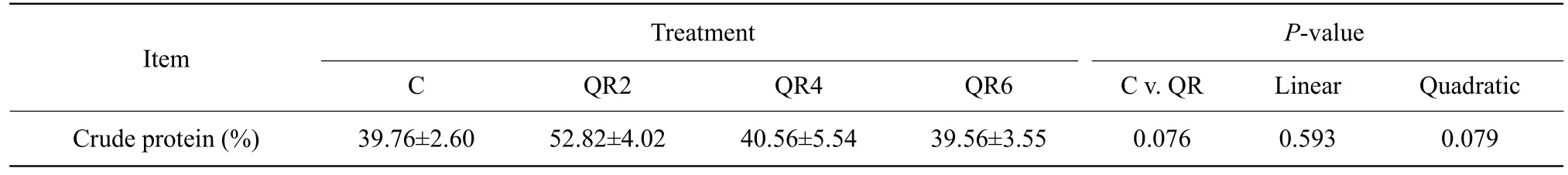
Table 4 Effect of quercetin on apparent metabolic rate of crude protein in Arbor Acres broilers
Effects of quercetin on blood parameters related to protein metabolism
Compared with the control, quercetin supplementation tended to increase the content of serum albumin(p=0.062), while quercetin treatments did not significantly affect the contents of serum total proteins and urea nitrogen (p=0.454 andp=0.813, respectively)(Table 5).
Effects of quercetin on protein content of skeletal muscle
Compared with the control, quercetin supplementation increased the content of protein in breast muscle(p<0.05) and in thigh muscle (p=0.053) (Table 6).
Effects of quercetin on expression of genes related to target of rapamycin signaling pathway
Compared with the control, quercetin supplementation significantly up-regulated mRNA expression ofPI3K,TOR, ribosomal protein S6 kinase 1 (S6K1), eukaryotic elongation factor 2 (eEF2), eukaryotic translation initiation factor 4B (eIF4B), eukaryotic translation initiation factor 4E (eIF4E) and eukaryotic translation initiation factor 4G (eIF4G) (p<0.05), whereas it significantly down-regulated mRNA expression of eukaryotic initiation factor 4E binding protein1 (4E-BP1)and eukaryotic elongation factor 2 kinase (eEF2K)(p<0.05) in the livers of AA broilers (Table 7).
Compared with the control, quercetin supplementation tended to up-regulate mRNA expression of insulin-like growth factor 1 (IGF-1) and protein kinase B (PKB/AKT) (p=0.059 andp=0.054, respectively)and significantly up-regulated mRNA expression ofeIF4B,eEF2,eIF4EandeIF4G(p<0.05), whereas it significantly down-regulated mRNA expression of 4E-BP1 (p<0.05) in thigh muscle of AA broilers(Table 8).

Table 5 Effect of quercetin on content of serum protein in Arbor Acres broilers
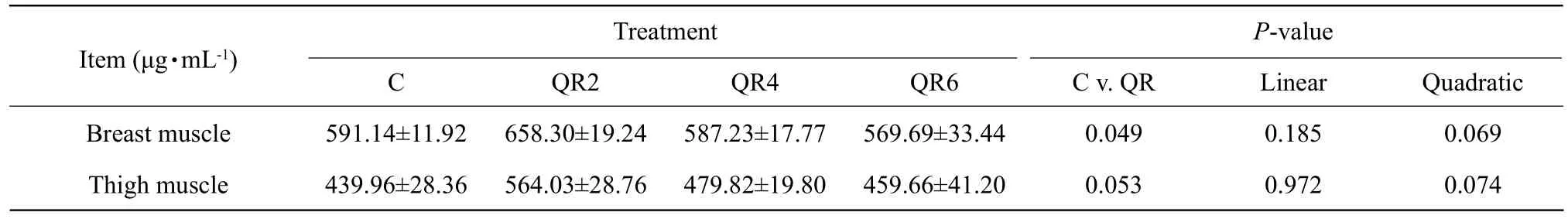
Table 6 Effect of quercetin on protein content of skeletal muscle in Arbor Acres broilers
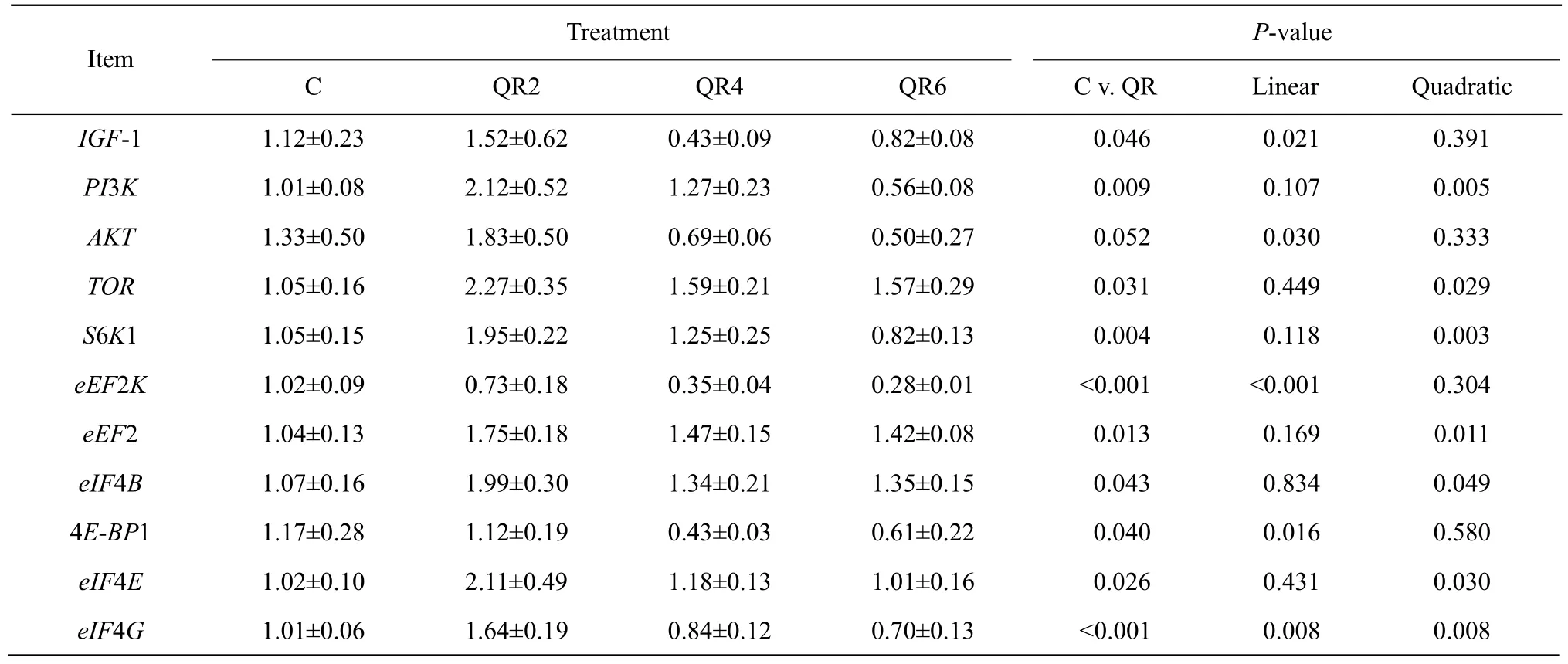
Table 7 Effects of quercetin on gene expression relating to target of rapamycin signaling pathway in livers of Arbor Acres broilers

Table 8 Effects of quercetin on gene expression relating to target of rapamycin signaling pathway in thigh muscle of Arbor Acres broilers
Compared with the control, quercetin supplementation significantly up-regulated mRNA expression ofIGF-1, phosphatidylinositol 3-kinase (PI3K),AKT,TOR,S6K1,eEF2,eIF4B,eIF4EandeIF4G(p<0.05), whereas quercetin supplementation significantly down-regulated mRNA expression ofeEF2K(p<0.05) in breast muscle of AA broilers(Table 9).
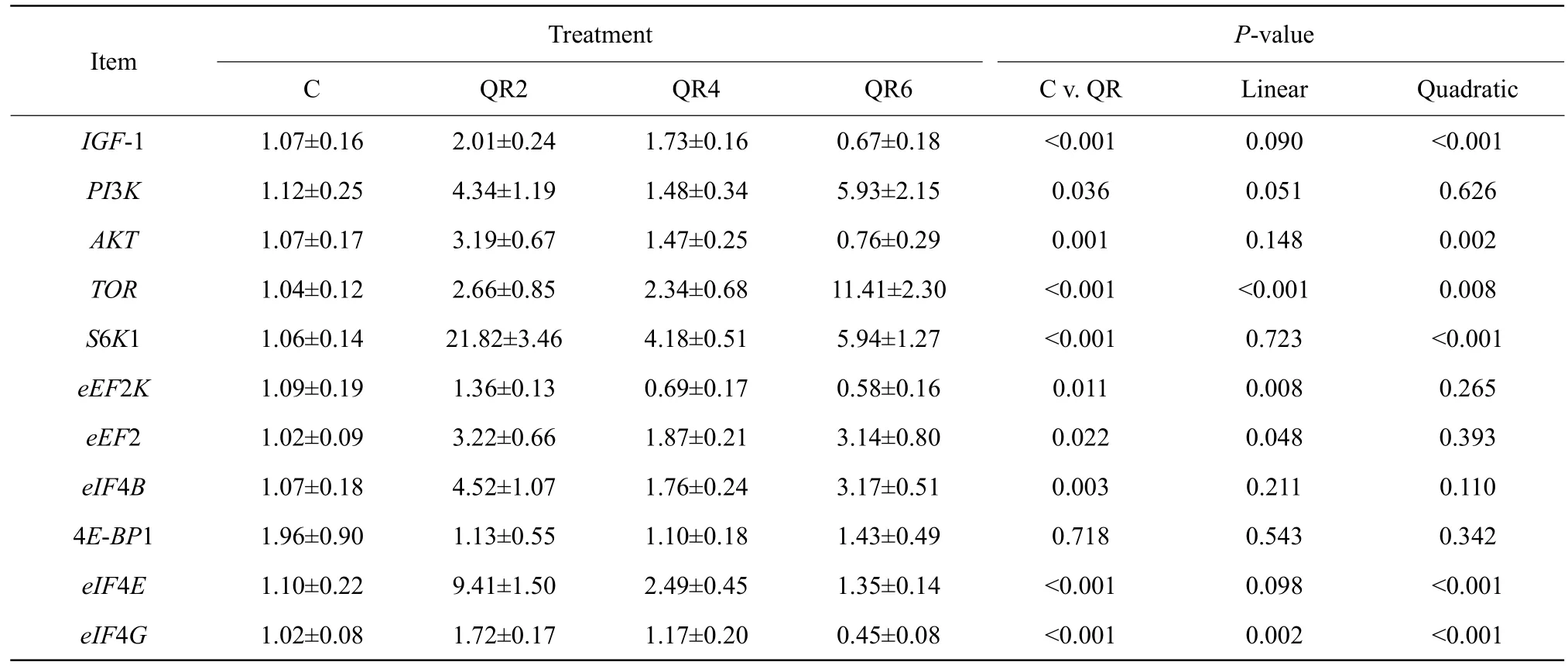
Table 9 Effects of quercetin on gene expression relating to target of rapamycin signaling pathway in breast muscle of Arbor Acres broilers
Discussion
Effect of quercetin on protein utilization in Arbor Acres broilers
The utilization of protein is one of the key indicators reflecting protein deposition in broilers. On the one hand, improvement of protein utilization might reduce feed costs and alleviate food shortages. On the other hand, it might increase protein deposition in animals and improve meat quality, thereby reducing ammonia emissions and nitrogen pollution. The apparent metabolic rate of protein might reflect the utilization of protein in body. Renet al. (2018) found that 0.4 and 0.8 g • kg-1Ginkgo bilobaextract significantly increased the apparent metabolic rate of protein in the experimental diets of AA broilers. Zenget al. (2014)reported that 100 mg • kg-1Forsythia suspensaextract significantly increased the apparent metabolic rate of protein in the trial diets of male AA broilers.These results suggested that flavonoids significantly improved protein utilization. The present results showed that quercetin supplementation increased the apparent metabolic rate of protein in AA broilers. This result was consistent with the above mentioned results,which increased the apparent metabolic rate of protein.This further confirmed that quercetin improved protein utilization, just like other flavonoids.
Effects of quercetin on protein deposition in Arbor Acres broilers
Skeletal muscle and blood were the main locations of protein deposition in poultry, and the amount of deposition was directly related to the quality of animal products and protein metabolism of bodies. Serum albumin was a functional protein that was synthesized in liver daily and was released into blood, which accounted for more than half of all the components in serum and carried a variety of endogenous and exogenous substances, including thiol groups, nitric oxide, bilirubin, inorganic ions, hormones and drugs(Arques, 2018; Arques and Ambrosi, 2011). Serum albumin had many biological activities, such as maintaining of osmotic pressure, anticoagulant, anti-platelet aggregation, anti-oxidation, anti-inflammation, etc(Arques and Ambrosi, 2011; Zhang and Frei, 2002;Wiedermann, 2007; Rocheet al., 2008; Anrakuet al.,2013; Lamet al., 2013; Paaret al., 2017). In addition,serum albumin was also decomposed into amino acids for body metabolism. Therefore, serum albumin was an important index for maintaining body health and stabilizing the internal environment. Although data regarding the dietary effects of flavonoids on the content of albumin were not available, previous studies examining flavonoid-rich plants had addressed their dietary effects on albumin. Kaliaet al. (2017) reported that the supplementation of 100, 150, 200, 300, 400 and 800 mgPrunus armeniacaseed extract • kg-1body weight in chickens significantly increased serum albumin. Moreover, adding propolis to broiler feed also increased the content of albumin (Kalafovaet al.,2016). These results suggested that flavonoids improved the contents of serum albumin in broilers, thereby maintaining body homeostasis, enhancing body metabolism and ameliorating performance. The present results showed that quercetin supplementation increased contents of albumin in AA broilers, which was supported by other flavonoids studies.
Protein was an important part of tissues and organs in bodies. Increasing the contents of protein in skeletal muscle reduced nitrogen emissions and improved the qualities of livestock and poultry products (Leeet al., 2010). Flavonoid-rich Moringa leaf increased the protein contents of rabbit meat (Nuhu, 2010).Supplementation of soy and citrus flavonoids significantly increased the protein contents of breast muscle in broilers (Kambohet al., 2018). The abovementioned studies suggested that flavonoids increased protein deposition in skeletal muscle of livestock and poultry,promoted animal development and improved meat quality. In the present study, quercetin supplementation increased the protein contents of breast and thigh muscles, which further confirmed that quercetin promoted protein deposition in muscles of broilers.
In a word, quercetin increased the contents of protein in breast and thigh muscles of broilers, and also increased contents of serum albumin. These results indicated that quercetin improved protein utilization partlyviapromoting protein metabolism and increasing protein deposition in skeletal muscles of broilers.
Effects of quercetin on target of rapamycin signaling pathway in Arbor Acres broilers
The target of the rapamycin signaling pathway was a key pathway to control the growth and development of somatic cells (Tobias and Michael, 2000). Amino acids, energy and growth factors activated TOR signaling pathway, including upstream and downstream signaling pathways (Loewithet al., 2002;Gingraset al., 2001). PI3K/AKT/TOR signaling pathway was one of the most critical upstream signaling pathways. The downstream pathways of TOR signaling pathway included 4E-BP1, S6K1 and other signaling factors, and the target of the rapamycin signaling pathway controlled the process of ribosome formation and protein synthesis in cells by regulating the phosphorylation status of S6K1 and 4E-BP1(Avruchet al., 2006). TOR signaling pathway regulated amino acid transporters in intestinal epithelial cells, which promoted amino acid absorption and utilization (Zhanget al., 2013; Zhanget al., 2014).Moreover, it regulated the binding of mRNA and ribosomes, initiated translation and induced protein synthesis in muscle cells (Kimball and Jefferson,2004).
The target of rapamycin signaling pathway was highly sensitive to insulin-like growth factor 1 (IGF-1).IGF-1 might regulate the synthesis of intracellular proteins and growth of muscle fiber by regulating TOR signaling pathway. Daviset al. (2002) found that the total protein contents in skeletal muscles were increased by 25%-60% after the injection of 20 mg • kg-1IGF-1 per hour in the 7-day-old piglets,which indicated thatIGF-1 stimulated expression ofTORgene and then activated TOR signaling pathway to regulate the protein levels of skeletal muscles. Some studies had also shown thatepimediumflavonoids stimulated expression ofIGF-1 (Lianget al., 2015).Wanget al. (2015) reported that the proper dose of rutin activated PI3K/AKT/mTOR signaling pathway.Cursinoet al. (2016) showed that the flavonoidrich plantDeguelia duckeanasignificantly increased mRNA expression of eEF2 and eIF4E proteins. It suggested that flavonoids played an estrogen-like role, competed with the estrogen receptor and then affected the secretion of signal factors (IGF-1), thus activating TOR signaling pathway, regulating the phosphorylation status of downstream signaling factors, and finally enhancing the protein translation,elongation and synthesis in cells.
Two downstream cell-signaling molecules of TOR signaling pathway were S6K1 and 4E-BP1. Activated TOR might regulate the transcription and translation of mRNA in two directions by regulating the phosphorylation levels of 4E-BP1 and S6K1.Moreover, the regulatory effects of TOR on the phosphorylation of the two proteins of 4E-BP1 and S6K1 occurred in parallel. TOR regulated the function of the translation initiation complex and the syntheses of ribosome proteins and translational regulatory proteins by regulating the phosphorylation of 4E-BP1 and S6K1, respectively. 4E-BP1 and its downstream factors mainly regulated the protein translation process. Hypophosphorylated 4E-BP1 bound to eIF4E to inhibit mRNA translation, whereas hyperphosphorylation of 4E-BP1 was separated from eIF4E, which bound to eIF4G and initiated mRNA translation. S6K1 regulated the translation process of some ribosome proteins and translational regulatory proteins. Activated S6K1 affected the syntheses of intracellular proteins by regulating mRNA translation process of the three proteins (eIF4B, S6 and eEF2K)(Gingraset al., 2001). Eukaryotic elongation factor 2 kinase (eEF2K) might catalyze the phosphorylation of eEF2 and decrease the binding capacity of eEF2 and ribosomes, thus hindering the extension of peptide chain, while activated S6K1 reduced expression of eEF2K mRNA and then inhibited the effect of eEF2K on eEF2. In this study, quercetin up-regulated mRNA expression ofIGF-1,PI3K,TOR,S6K1,eIF4E,eIF4G,eEF2,eIF4Band down-regulated that ofeEF2Kand 4E-BP1. Quercetin activated TOR signaling pathway,thereby regulating mRNA expression ofSK61,4E-BP1 and its downstream signal molecules, and ultimately increasing protein syntheses in the livers and protein deposition in muscles. In addition, it was surprising that the expression of partial signal factors in TOR signaling pathway was decreased by increased quercetin in breast, thigh muscles and livers of broilers, which probably resulted from the dual effect of quercetin. In other words, quercetin at a low dose,similar to other flavonoids, might play an estrogenic role, whereas quercetin at a high dose might act as anti-estrogenic activity in bodies.
Conclusions
In conclusion, dietary quercetin supplementation increased protein utilization through activating TOR signaling pathway.
 Journal of Northeast Agricultural University(English Edition)2021年2期
Journal of Northeast Agricultural University(English Edition)2021年2期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Identification of QTLs Associated with Resistance to Pseudomonas syringae pv. Glycinea in Soybean (Glycine max (L.) Merr)
- Development of an Artificial Diet for Effective Oral Delivery of dsRNA to Soybean Pod Borer, Leguminivora glycinivorella (Lepidoptera: Tortricidae)
- Grain Yield and Nitrogen Use Efficiency of Hybrid Rice in Response to High Plant Density and Nitrogen Rate
- Index Design and Comprehensive Evaluation of Germplasm Resources of Fruits Based on Mathematical Model of AHP and FCE: Sterculia nobilis Smith as a Case
- Effects of Interaction of Soil Moisture and Organic Matter on Powdery Mildew Disease and Growth of Heracleum moellendorffii Hance
- Comparative Analysis of Bacillus thuringiensis Vip3Aa57 and Vip3Aa62 Insecticidal Activities
