Comparative Analysis of Bacillus thuringiensis Vip3Aa57 and Vip3Aa62 Insecticidal Activities
Shen Ya-wen, Li Shuai, Wang Jing, Wang Jian, Cui Jun, Ding Ming-yue, Yang Xiao-xue, and Gao Ji-guo
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
Abstract: Bacillus thuringiensis (Bt) exhibits strong insecticidal activity and is harmless to non-target organisms such as human and animals. Bt becomes the most commonly used environment-friendly insecticidal microorganism. However, the insecticidal activities of different Bt strains variy significantly. Therefore, it is particularly important to compare the insecticidal activities of different strains and explore their insecticidal effector mechanisms to expand Bt insecticidal spectrum and enrich transgenic resources. Here, the insecticidal activities of Vip3Aa57 and Vip3Aa62 strains, carrying vegetative insecticidal protein-encoding genes that were inserted into the expression vector pET-21b and transformed into Escherichia coli Rosetta (DE3) strain, expressing 88 ku protein, were compared. Vip3Aa57 protein reportedly displayed body weight inhibition effect on Spodoptera exigua without affecting Heliothis armigera while Vip3Aa62 protein was known to have strong insecticidal activity against S. exigua (LC50=5.124 ng • mg-1). A low H. armigera activity (LC50=870.1 ng • mg-1) was observed. The paraffin sectioning results showed that Vip3Aa57 protein affected S. exigua midgut cell morphology. The laser confocal microscopic imaging results showed that Vip3Aa57 bound to receptors in the midgut without damaging the midgut tissue morphology. This study would be conducive for making full use of Bt strains in the soil to compare the insecticidal activities of different Vip insecticidal genes. It could thus provide significant help in revealing the underlying insecticidal mechanisms of Vip3Aa insecticidal genes, developing new insecticidal proteins and delaying pest resistance problems.
Key words: Bacillus thuringiensis (Bt), Vip, insecticidal activity, laser confocal microscopy
Introduction
Bacillus thuringiensis(Bt) is an insect pathogen,belonging to the gram-positiveBacillusgenus(Leopoldoet al., 2014; Raymodet al., 2010). Bt spores are formed during a later growth stage in the process of full crystal production, whose main components in insecticidal are crystal proteins (ICPs) or delta-endotoxin (Adanget al., 2014). Bt mainly relies on insecticidal proteins to kill pests and it is the most widely used insecticidal microorganism in the world.Recently, with the advancement of technology, the gradual purification from strains or metabolites, a variety of auxiliary proteins (Helper proteins), such as P19, P20 and P21, as well as several kinds of Vips and secret insecticidal proteins (Sips) and their insecticidal effects, becomes increasingly significant.
Different from Bt ICPs, Vips do not normally form insecticidal toxins with sporulation crystals. Unlike ICPs, produced in the late growth period of Bt, Vips secretion starts during the vegetative growth period,reaching a peak in the early growth period of stable growth. Vips exist in a soluble protein form in the culture medium and are susceptible to temperature changes, as heat treatment at 95℃ would lead to their complete deactivation (Baraneket al., 2015). It has also been reported that Vip3A amino acid sequence does not exhibit relevant homology with that of Cry protein, and Vips exhibit a wide insecticidal spectrum with a significantly high insecticidal activity and specificity for a variety ofLepidopteraagricultural and forestry pests in the familyNoctuidae, such asAgrotis ipsilon,Spodoptera exiguaandSpodoptera litura Fabricius(Baraneket al., 2015; Lobnaet al.,2011; Joaquín and Iñigo, 2017; Chakrounet al., 2016;Yuet al., 1997). Vip3Aa is the most heavily researched broad-spectrum insecticidal protein with the largest number of Vips and relatively high toxicity against important agricultural pests (Wuet al., 2008; Chenet al., 2017). In terms of research and application,Vip3Aa has significant potential as a new insecticidal agent. In this study, molecular methods were applied to separate Bt strain as experimental materials.Moreover, two Vip3Aa protein types were expressed,purified and determined their biological activities,while their insecticidal activities were verified using immunohistochemistry, followed by light and confocal microscopic imaging. This study provided a valuable scientific basis for potentially expanding the insecticidal spectrum of Bt strains and delaying pest resistance.
Materials and Methods
Target gene expression and protein purification
The recombinant plasmids of Vip3Aa57 and Vip3Aa62 preserved in the laboratory were transformed intoEscherichia coliRosetta (DE3) strain for expression,these recombinant strains were cultured and induced overnight in a shaker at 16℃ and 180 r • min-1. The bacteria were collected after centrifugation at 4℃ and 8 000 r • min-1for 3 min.
The pellet was suspended in 20 tendency • L-1Tris-HCl buffer and the bacteria were crushed with ultrasonication (complete crushing) for 10 min. The supernatant was centrifuged at 12 000 r • min-1and 4℃ for 10 min and the proteins were purified using SDS-PAGE protein electrophoresis, following Ni column method. First, the column was rinsed with 5 volumes of distilled water, then added 0.5 volumes of 0.1 mol • L-1nickel solutions, and rinsed again with 5 volumes of distilled water. Next, the column was balanced with 10 volumes of the binding buffer. Flow passage fragments were collected at a flow rate of 1-4 mL • min-1. And then, the column was washed using 10 volumes of binding buffer to collect the washed fragments. Elution was performed with 5 volumes of elution buffer. The elution fragments were collected in aliquots of 1 mL to avoid the dilution of the elution products. Finally, SDS-PAGE protein electrophoresis and the ImageJ software were used to analyze SDSPAGE map and quantify the protein.
Determination of insecticidal activity and data processing
The bioactivities ofS. exiguaandHeliothis armigerawere determined using the feed-mixing method that was divided into the activity preliminary screening and the secondary screening phases. During the preliminary screening, two protein concentrations were established (a high and a low), and each experiment was performed in triplicates. The culture conditions were incubation at 25℃ for 7 days using a photoperiod of 8 h light and 14 h darkness. After 7 days, the numbers of dead and living insects were counted, and the health data were calculated using SPSS software,along with LC50and death rates.
Paraffin sectioning
The dehydrated tissues were transferred into a mixture of 100% alcohol and xylene (volume ratio of 1 : 1).After 20 min, the mixture was replaced by a pure xylene solution for 30-40 min. The transparent tissues were intruded into a mixture of xylene and melted paraffin (volume ratio of 1 : 1) for 30 min. Then, the melted pure paraffin was transplanted for 30 min and embedded. Next, the embedded paraffin block was removed from the embedding box, slightly dissolved,and fixed on the wooden block. The paraffin blocks were trimmed into isosceles trapezoids and sliced using a paraffin slicer. The paraffin sections were placed and spread on a glass slide with distilled water, and then dried in a 37℃ constant-temperature oven. The dried sections were dewaxed with xylene for 5 min and 100% alcohol+xylene (volume ratio of 1 : 1) for 5 min. Next, a 90%-80%-70%-50% ethanol gradient rehydration was used for 5 min at each step to evaporate water. Sections were stained with hematoxylineosin for 5 min and observed under a microscope.
Laser confocal microscopy
Immunostaining and confocal microscopy was used to observe the cell cultures at 60%-80% confluency,grown in culture dishes adapted for confocal laser microscopy. After the treatment, the cells were washed three times with PBS deleted ligands and fixed with 4% paraformaldehyde for 37℃ and 30 min. For the co-localization experiments, cells were permeabilized using 0.2% of Triton X-100 and immunostained with the primary and the secondary antibodies diluted in 5% of skimmed milk powder. Cellular cortical actin and nuclei were labeled for 30 min with fluorescein isothiocyanate (FITC)-phalloidin (Sigma) and DAPI(Sigma), respectively.
Results
Expression of Vip3Aa57 and Vip3Aa62 genes and protein purification
After the recombinant plasmid-containing expression strain was induced by isopropyl thiogalactoside(IPTG) and the protein was tested using SDS-PAGE,an electrophoresis band was found at approximately 88 ku. This result was consistent with the expected target protein size, indicating that the target proteins Vip57 and Vip62 could be efficiently expressed by the expression vector pET21b inE. coliRosetta (Fig. 1).The extracted protein was purified using affinity chromatography. It could be seen from Fig. 1 that the elution solution containing 500 mmol • L-1imidazole could fully elute the target protein at a one-fold column volume elution level. With the increase of the elution multiple, the corresponding target protein concentration decreased successively, and the concentration was already very low at a four-fold column volume elution.
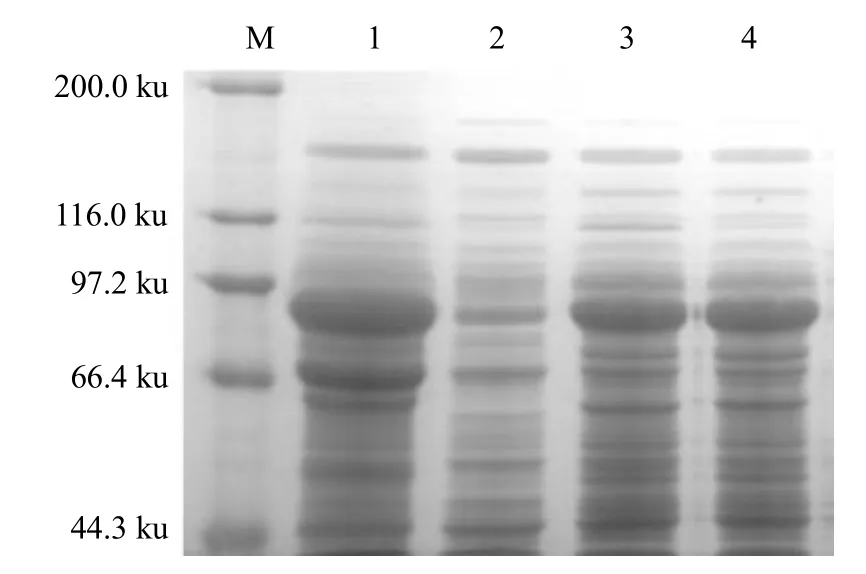
Fig. 1 Expression product analyses of Vip3Aa57 and Vip3Aa62
Fig. 2 Vip3Aa57 protein affinity chromatography purification
Determination of insecticidal activity in vivo
Preliminary screening
Proteins induced by Vip3Aa57 and Vip3Aa62 were extracted, and the protein concentrations were quantified using the ImageJ software developed by the National Institutes of Health, and the bioactivity of the proteins at a determined concentration was examined.The tested insects were the newly-hatchedS. exiguaandH. armigeralarvae. The insects, in which the signs of an insecticidal effect was observed in the preliminary screen, were re-screened. Five different concentration gradients were selected for the bioactivity measurements during the re-screening and each experiment was performed in triplicates. BothS. exiguaandH. armigerahad 20-20 worms in each group. The results are shown in Tables 1-3. Vip3Aa57 protein had no lethal effects either onS. exiguaorH. armigeraat concentrations of 50 and 5 ng • mg-1, but it inhibited the body weight gain ofS. exigua, which was more obvious at the higher concentration. The body weight gain inhibition rate was 96.3 and 2.1% protein concentrations of 50 and 5 ng • mg-1, respectively.
S. exiguamortality at Vip3Aa62 concentrations of 50 and 5 ng • mg-1was 84% and 28%, respectively,and the corrected mortality was 83.06% and 23.76%,respectively. The mortality of the bollworm was 30%and 15%, respectively, and the corrected mortality was 22.22% and 5.56%, respectively.
Table 1 Vip3Aa57 inhibited weight gain in S. exigua
Table 2 Preliminary bioassay of Vip3Aa62 and Vip3Aa57 on S. exigua
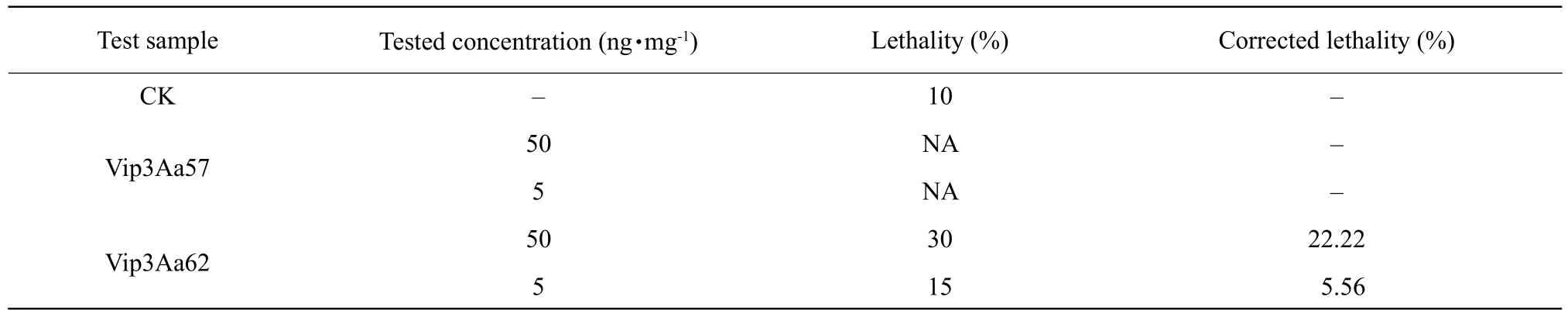
Table 3 Preliminary bioassay of Vip3Aa57 and Vip3Aa62 on H. armigera
It could be seen from these results that Vip3Aa57 did not have any significantly lethal effect either onS. exiguaorH. armigera, but it inhibited weight gain inS. exigua. Vip3Aa62 exhibited higher insecticidal activity againstS. exiguathanH. armigera.
Secondary screening
The above-described Vip3Aa57 and Vip3Aa62 protein bioactivities were determined by establishing a gradient at five different concentrations of 50,25, 15, 5 and 1 ng • mg-1in the secondary screening.For each group, the experiments were performed in triplicates with 20 larvae both in the case ofS. exiguaandH. armigera. LC50was calculated using the SPSS software. The results of the secondary screening are shown in Tables 4 and 5. The results showed that Vip3Aa57 had no lethal effect either onS. exiguaorH. armigera, but significantly inhibited weight gain inS. exigua, with a decreasing weight gain inhibition rate at lower concentrations. LC50of Vip3Aa62 protein in the newly-hatchedS. exiguaandH. armigeralarvae was 5.124 ng • mg-1, and above 870.1 ng • mg-1, respectively, indicating that Vip3Aa62 had a considerable insecticidal effect onS. exigua.
Table 4 Vip3Aa57 inhibited weight gain in S. exigua
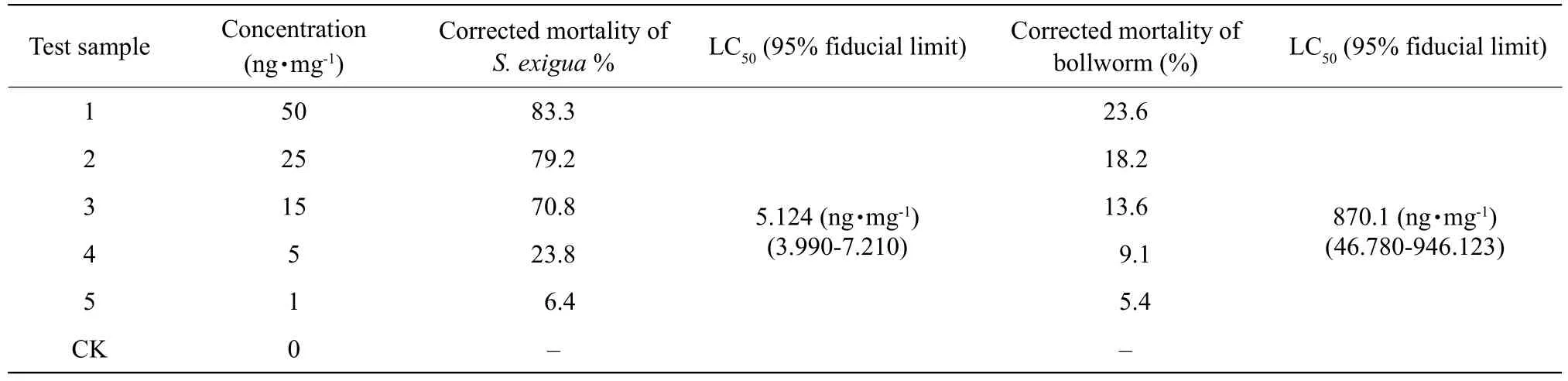
Table 5 Secondary bioassay results of Vip3Aa62 against S. xigua and H. armigera
Paraffin sectioning
The bioassay showed that Vip3Aa57 protein significantly inhibited weight gain inS. exigua.S. exigualarvae fed with toxic proteins for 7 days, then their midgut tissues were examined under an anatomic microscope and processed for paraffin sectioning(Fig. 3). Under the microscope, the intestinal tissues in the control group were intact, while the intestinal cells in the experimental group were significantly enlarged and demonstrated morphological changes and damage.Previous studies had shown that Vip3Aa protein was activated by protease secreted into the intestinal fluid. In the process of binding to the mast cells,the binding of Vip3Aa and certain cell membrane receptor protein might cause membrane perforation or trigger a signaling pathway, eventually leading to the death of the worm (Sena and Hernandez-Rodriguez,2009; Abdelkefi-Mesrati,et al., 2009). However, this experiment showed no widespread perforation in the experimental group, which might be the reason why the insects did not die while their weight gain was inhibited.
Laser confocal microscopic imaging
The cells and nuclei were visualized using laser confocal microscopy (Fig. 4). The results showed that Vip3Aa57 bound to the nucleus to some extent did not damage the cell surface. Such a result could also explain why Vip3Aa57 protein inhibited weight gain without exhibiting lethality.
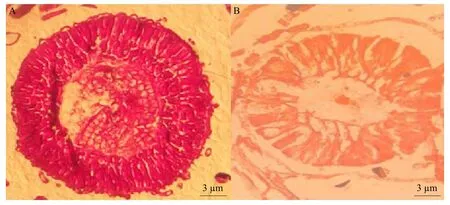
Fig. 3 Paraffin sections of midgut tissues in Spodoptera exigua larvae
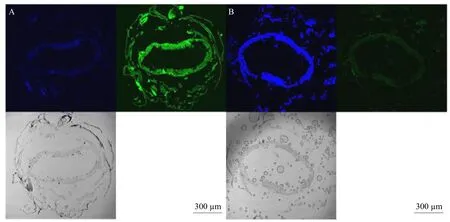
Fig. 4 Confocal laser imaging results
Discussion
Vips showed strong insecticidal activity against a variety ofLepidopteraspecies and did not have interactive resistance with Cry insecticidal proteins.Thus, the study of Vips could contribute to expand the insecticidal spectrum (Gouffonet al., 2011; Chakrounet al., 2014; Chakrounet al., 2016). The purification of the target proteins was an important experimental step,and there were multiple different purification methods according to different protein characteristics. Common purification methods, such as affinity chromatography using ligand-specific recognition, ion exchange taking advantage of different ionic charges and gel filtration,were based on molecular weight, hydrophobic and reverse chromatography relied on the difference in hydrophobicity. Affinity chromatography was a common method of protein purification, based on the principle that a protein could be reversibly bound to a specific ligand on the column. This technique was widely used in the selection of purification methods,due to its simple operation, high selectivity, high loading and high specificity. In this study, the target proteins with six histidine tags for purification were labeled and then an 88 ku protein with a purity of 90%and a concentration of 1.5 mg • mL-1was obtained. It could be concluded that the purpose of the purification had been achieved.
The effector mechanism of Vip3 behind insect lethality was intensely studied. Vip3 entered the digestive system of the insects in the form of soluble protein at the optimal pH. Next, it entered the midgut and formed proteins of 22, 33, 45 and 62 ku through protease activity, the 62 ku protein being the core member.
Certain experiments showed that the full-length Vip3Aa could not cause lesions in the midgut cells of tobacco hawkmoth, and only the enzymaticallydigested 62 ku core protein could combine with the receptor proteins of the midgut epithelial cells to form ion channels and subsequent perforations in the plasma membrane, inducing apoptosis and eventually leading to the death of the insects (Andréet al., 2016). The biological activity determination results of this study showed that Vip3Aa57 significantly inhibited weight gain inS. exigua, but not inH. armigera. The results of the paraffin sectioning showed that although Vip3Aa57 did not cause extensive damage in the midgut tissue ofS. exigua, the tissue morphology itself was damaged,which might be the cause of weight gained inhibition rather than death. Finally, Vip3Aa62 had a significant insecticidal effect onS. exigua, whereas it affectedH. armigeramarginally.
Conclusions
The biological activity assay showed that Vip3Aa57 protein exhibited a significant weight suppression effect onS. exigua, but did not affectH. armigerasignificantly. Vip3Aa62 protein displayed a considerable insecticidal activity onS. exiguawith a LC50of 5.124 ng • mg-1, whereas it affectedH. armigeramarginally with an LC50of 870.1 ng • mg-1. Affinity chromatography was used to purify Vip3Aa57 protein.The target protein was eluted adequately using one column volume elution of an eluent containing 500 mmol • L-1of imidazole and it was observed that the corresponding target protein concentration decreased with the increase of the elution multiple.
The results of the paraffin sectioning showed that certain morphological of midgut tissue cell changed,which could lead to body weight gain inhibition inS. exigua. The laser confocal microscopic imaging results showed that Vip3Aa57 protein could bind to the nucleus to some extent without damaging it.
 Journal of Northeast Agricultural University(English Edition)2021年2期
Journal of Northeast Agricultural University(English Edition)2021年2期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Wild-type MIC Distribution and Epidemiological Cut-off Value and Resistant Characteristics of Colistin Against Escherichia Coli from Chickens
- Quercetin Increased Protein Utilization and Decreased Nitrogen Excretion in Broilers by Activating TOR Signaling Pathway
- Effects of Altitude Change on Nutritional Quality and Elymus nutans Regularity in Qinghai-Tibet Plateau
- Comprehensive Evaluation of Processing Quality of Tibetan Native Hulless Barley Variety by Factor Analysis
- Effects of Interaction of Soil Moisture and Organic Matter on Powdery Mildew Disease and Growth of Heracleum moellendorffii Hance
- Index Design and Comprehensive Evaluation of Germplasm Resources of Fruits Based on Mathematical Model of AHP and FCE: Sterculia nobilis Smith as a Case
