Development of an Artificial Diet for Effective Oral Delivery of dsRNA to Soybean Pod Borer, Leguminivora glycinivorella (Lepidoptera: Tortricidae)
Wang Guo-yue, Wang Nan, Li Ming-yue, Xin Jun-jie, Qin Yu-shi, Meng Fan-li, , Yuan Qiang, Wang Xiao-yun, and Zhang Xiao-ming
1 Key Laboratory of Soybean Biology in the Chinese Ministry of Education, College of Agriculture, Northeast Agricultural University, Harbin 150030, China
2 College of Agriculture, Northeast Agricultural University, Harbin 150030, China
3 Division of Soybean Breeding and Seeds, Soybean Research & Development Center, CARS (Key Laboratory of Biology and Genetics & Breeding for Soybean in Northeast China, Ministry of Agriculture), Northeast Agricultural University, Harbin 150030, China
Abstract: RNA interference (RNAi) technology is a promising crop protection strategy against agricultural pests. The soybean pod borer(SPB), Leguminivora glycinivorella (Mats.), is a serious pest of soybean in northeastern Asia. The neonate larvae of SPB are endophagous,initially feeding on the inner layer of the pod and then on the immature soybean seeds, a behavior that makes them difficult to rear. Therefore,a suitable artificial diet is desirable to facilitate RNAi research. Seven artificial diets and soybean pods were used for rearing SPB neonate larvae. The survival and mean weight of the larvae reared on artificial diet 6 (A6, included 2 g soybean flour and 10 g R3 immature soybean pod powder) were found to be significantly higher than those for larvae reared on all other diets. Subsequently, A6 diet was prepared in DEPC-treated water to obtain a RNase-free artificial diet (RF). The survival and mean weight of the larvae were not significantly different between A6 and RF diets. Feeding neonate larvae with RF-SpbP0 dsRNA (ribosomal protein P0) silenced SpbP0 gene, resulting in arrested development and increased mortality. The results of this study suggested that the newly developed A6 or RF diets could rear SPB neonate larvae and help towards developing an effective method for oral delivery of dsRNA to SPB larvae.
Key words: soybean pod borer, artificial diet, RNA interference
Introduction
The soybean pod borer (SPB),Leguminivora glycinivorella(Mats.) Obraztsov (Lepidoptera: Tortricidae),is an important economic pest of soybean and has a wide distribution throughout northeastern Asia,including northern China, North Korea, Japan and the Russian Far East (Zhaoet al., 2008). SPB is a univoltine insect, and the mature larvae make cocoons in the soil from October to July and pupate in mid-July.Adults then emerge from mid-July to mid-August(Meng and Ran, 2017). After mating, females oviposit on young bean pods. The hatched larvae enter the pod and feed on the immature beans until developing into mature larvae. This behavior can cause 40% yield loss(Wanget al., 2018). Chemical insecticides have been widely used for nearly 30 years to control SPB (Songet al., 2003). However, the insecticides do not readily penetrate the soybean pods, thus have a limited effect on the larvae once they are inside the pods. Moreover,the emergence period lasts for almost 1 month,prompting farmers to use the pesticide multiple times to control the adults (Meng and Li, 2017). Multiple and long-term pesticide use has not only led to the appearance of insecticide resistance in certain SPB populations, but also has polluted the environment(Chenget al., 2016). Therefore, the development of efficient approaches that do not use pesticides for SPB management is essential.
RNA interference (RNAi) technology shows great potential for crop protection and is becoming a promising tool for integrated pest management (IPM)(Zhanget al., 2017; Zottiet al., 2018). RNAi method is based on an evolutionarily conserved mechanism,referring to a set of molecular processes in which long double-stranded RNA (dsRNA) molecules are processed into 21-25 nt (nucleotides) siRNA (short interfering RNA) molecules (Zhaoet al., 2015). Using siRNA as a guide, RNA-induced silencing complex(RISC) finds and cleaves the target mRNAs, thereby silencing the target gene (Sweverset al., 2013; Zhuang and Hunter, 2012; Dueck and Meister, 2014; Azlanet al., 2016). Feeding with dsRNA molecules (RNAi or plant-mediated RNAi) has been exploited to control insect pests of agriculturally important crops in recent years. When dsRNA that is targeting essential genes is ingested by pests, down regulation of these genes by RNAi pathway may result in arrested development or death (Zottiet al., 2018; Meister and Tuschl, 2004).However, RNAi efficiency varies among different insects. Some insects exhibit a robust RNAi response,with long-lasting effects that may even become hereditary. Some insects show lower or inefficient RNAi responses, with gene silencing often being only temporary (Jogaet al., 2016; Menget al., 2018).Several factors are involved in the observed differential RNAi efficiencies, including the target gene selected, the length of dsRNA, the concentration of dsRNA, RNAi machinery within the target pest and the efficiency of dsRNA delivery method(Rodrigueset al., 2018; Rodrigueset al., 2017).
In SPB, the silencing of Spbtry1 (serine protease),SpbP0 (ribosomal protein P0) and Spb18S, using RNAi or transgenic plant-mediated RNAi, has been reported previously (Meng and Ran, 2017; Wanget al.,2018; Meng and Li, 2017). SPB exhibits a robust systemic RNAi response, and systemic RNA interference deficient (SID) transmembrance, channel-mediated,and scavenger-receptor-mediated endocytosis are involved in the response (Menget al., 2018). However,for RNAi-based control of SPB to be effective,the optimal target gene(s) and method of dsRNA delivery need to be further investigated. However,the availability of high-quality experimental larvae is limited mainly due to the feeding behavior of SPB larvae, which develop inside soybean pods until maturing, and the lack of an alternative diet for largescale rearing in the laboratory.
Oral delivery is an efficient method for administering dsRNA and rapidly screening potential target genes (Rodrigueset al., 2017). However, the nuclease is included in the diet used, which degrades dsRNA molecules mixed into the artificial diet. This often leads to the oral delivery of dsRNA having a reduced RNAi effect compared with directly injecting dsRNA(Penget al., 2018). Therefore, a nuclease-free artificial diet for the effective delivery of dsRNA needs to be developed for evaluating dsRNA machinery and the effects of RNAi on candidate target genes. The aims of this study were to develop an artificial diet suitable for rearing SPB neonate larvae in the laboratory and to develop an oral delivery method that would allow screening and selecting the most efficient candidate genes for reducing larval development and increasing larval mortality.
Materials and Methods
Experimental insects
SPB larvae were collected from a naturally infested field at the experimental station of Northeast Agri-cultural University in Harbin City, Heilongjiang Province, China. The population was maintained on its host-plant soybean at 80%-90% relative humidity,in an 18 : 6 h light: dark cycle, and at 26℃ until they emerged as adults. The adult insects were provided with a 5% honey solution as food and allowed to oviposit on young bean pods. The eggs were collected daily, and the resulting neonate larvae were used in the following experiments.
Preparation of new artificial diet and RNasefree artificial diet
The ingredients of all the diets are shown in Table 1.Dongnong 47 soybean seeds and R3 immature soybean pods were provided by the Key Laboratory of Soybean Biology in the Chinese Ministry of Education. Corn flour and carrots were purchased from a local market.Vitamin mixture (Minshen Parma, Hangzhou, China)and other ingredients (industrial-grade products) were ordered from Harbin CBIO Bioscience Technology Co., Ltd.
The new artificial diet was prepared in two steps.Firstly, the carrot was thinly cut with a planer. Carrot slices, R3 immature soybean pods and soybean seeds were baked in an oven at 95℃ for 30 min, then 65℃overnight and ground to a fine powder in a 250 mL stainless steel blender. The soybean flour, R3 immature soybean pod powder, corn flour and carrot flour were sterilized, respectively. Secondly, the major nutritional and antimicrobial ingredients and agar: soybean flour,R3 immature soybean pod powder, corn flour, carrot flour, brewer's dried yeast, caster sugar, potassium sorbate, dimethyl 2-phenylmalonate, cholesterol and agar were combined with 67 mL of water in the proportions shown in Table 1 for each diet (A1-A7)and stirred for 5 min. They were autoclaved at 121℃and 15 psi for 15 min together, and then cooled to approximately 60℃. The second part of the diet,including ascorbic acid, the vitamin mixture, linoleic acid and 2.5 mL of distilled water were added to the mixture. After mixing all the ingredients and cooling to 25℃, the solution was stored at 4℃.

Table 1 Ingredients of artificial diet for rearing larvae of L. glycinivorella (SPB)
The first step of RNase-free artificial diet was the same as for the normal artificial diet, as described in the previous paragraph. In the second step, the major nutritional and antimicrobial ingredients and agar were combined with 67 mL of 1% DEPC-treated water, stirred for 5 min separately, and then stored at 4℃ for 24 h to remove any nuclease. Finally, they were autoclaved at 121℃ and 15 psi for 15 min together and cooled to approximately 60℃. The second part of the diet, including ascorbic acid, the vitamin mixture and linoleic acid were dissolved in 2.5 mL of sterilized 1% DEPC-treated water, and then added to the mixture. After mixing all the ingredients and cooling to 25℃, the solution was stored at 4℃. This was RNase-free artificial diet (RF) used in the following experiments.
Experiment 1: Evaluation of new artificial diet
Seven kinds of the artificial diet were prepared by changing the nutritional composition as shown in Table 1.Twenty-four neonate larvae were transferred to a 24-well cell culture plate and reared with either one of the artificial diets or soybean pods. The artificial diet and soybean pod were replaced every 3 days. SPB body weight and mortality were recorded, when the larvae reached the fourth instar. The best performing diet from this experiment was used in the following experiment.
Experiment 2: Evaluation of RNase-free artificial diet
Twenty-four neonate larvae were transferred to a 24-well cell culture plate and reared with RF or A6.Both RF and A6 were replaced every 3 days. SPB body weight and mortality were recorded every 3 days,until the fourth instar. Each experiment was replicated 10 times.
Experiment 3: dsRNA feeding bioassays
Based on the earlier study (Meng and Li, 2017), the ribosomal protein P0 (Spbp0) was selected as gene silencing in SPB by feeding them specific dsRNA.Spbp0 dsRNA was synthesized using the Promega T7 RiboMAX Express Large-Scale RNA Production System (Promega, WI, USA), according to the manufacturer's protocols, using the primers Spbp0-T7-F and Spbp0-T7-R (Table 2).
Table 2 Primers of dsRNA synthesis and qRT-PCR
In order to study the effect of RF diet on dsRNA stabilization and RNAi slicing effect, SPB larvae were fed on dsRNA as the followings. The neonate larvae were provided with Spbp0 dsRNA in RF diet or A6.dsRNA was carefully mixed with the artificial diet by hand. The final concentration of dsRNA in the diet was 10 μg • mL-1. Larvae were treated with the same concentration of green fluorescent protein (GFP)dsRNA, and RF diet or A6 without dsRNA was as a negative control. Each treatment and the control were carried out on 24 larvae and replicated in triplicate. The larvae were reared at 26℃, 80%-90% humidity, and 16 : 8 h light : dark cycle for 6 days. dsRNA artificial diet was replaced with a new one every 3 days.SPB body weight and mortality were recorded every 3 days. Some feeding larvae were randomly selected,frozen in liquid nitrogen, and kept at –80℃ for future using.
Quantitative real-time PCR (qRT-PCR)
The total RNA was isolated from the larvae using the High Pure RNA Tissue Kit (DNase I) (CWBIO,Beijing, China). cDNA synthesis was carried out using the TIANScript RT Kit (Tiangen, Beijing, China) from 500 ng of RNA, the results of which were used as a template for the gene expression studies. The expres-sion analyses of the target gene were conducted using the SYBR Green Supermix kit (Bio-Rad, Hercules,CA, USA). RT-qPCR reactions were performed on a Roche LightCycler®480 real-time PCR system (Roche,Basel, Switzerland) using the following cycling conditions: 95℃ 5 min; 40 cycles at 95℃ 30 s, 60℃ 15 s,72℃ 45 s; 95℃ 1 min, and 55℃ 1 min. At the end of each qRT-PCR experiment, a melt curve was generated to check for primer-dimer formation. qRT-PCR analysis contained three biological replicates, each having three technical replicates (Meng and Li, 2017). SPBβ-actin was used as a reference gene and the 2−ΔΔCtmethod was used to calculate the relative expression levels of the target gene in the samples compared to the controls.
Statistical analyses
All the data in this study were presented as mean±SE. Significant differences were determined by oneway analysis of variance followed by least significant difference tests for mean comparisons. The statistical analyses were performed with the SAS 9.21 software(SAS Institute, Cary, NC, USA).
Results
Experiment 1: Evaluation of new artificial diet
The data on the survival and weight of SPB larvae reared on seven artificial diets and on soybean pods are shown in Fig. 1. The survival rates of larvae fed on A5, A6 and A7 diets were significantly higher than those of the larvae fed on soybean pods, and the survival rates of those fed A6 diet were the highest(Fig. 1A). There were no statistically significant differences among SPB larvae body-weight reared on the seven artificial diets or soybean pods (Fig. 1B).

Fig. 1 Survival and mean weight of SPB larvae reared on seven artificial diets and soybean pods
Evaluation of RNase-free artificial diet
The results of Experiment 1 showed that 77.08% of neonate larvae reached the final instar, when fed A6 diet as neonates. The composition of A6 diet was the most appropriate for rearing SPB neonate larvae.However, the natural ingredients of A6 diet included RNase, which was not suitable for oral delivery of dsRNA. RNase of A6 was removed by treating with 1% DEPC-treated water, resulting in RF diet. To evaluate RF diet, the survival rates and the weight of SPB were measured during the last instar. There was no significant difference for survival rate or weight between larvae fed with A6 diet and those fed with RF diet (Fig. 2).
Oral dsRNA feeding bioassay
Three days after the neonate larvae were fed RF mixed with Spbp0 dsRNA,Spbp0 gene transcript significantly decreased in those larvae compared to the control larvae. This decrease in Spbp0 transcript level continued until the sixth day (Fig. 3A). The larval survival rate decreased after 3 days in RF Spbp0 dsRNA-fed group. The final survival rate in the larvae fed RF Spbp0 dsRNA was 38%, and this survival rate was significantly lower than that of the control larvae(Fig. 3B). There was a significant difference in the mean weight among RF Spbp0 dsRNA-fed and the control groups after 6 days (Fig. 3C). These results suggested that the RF diet could deliver Spbp0 dsRNA to SPB larvae and suppress the expression ofSpbp0 gene.
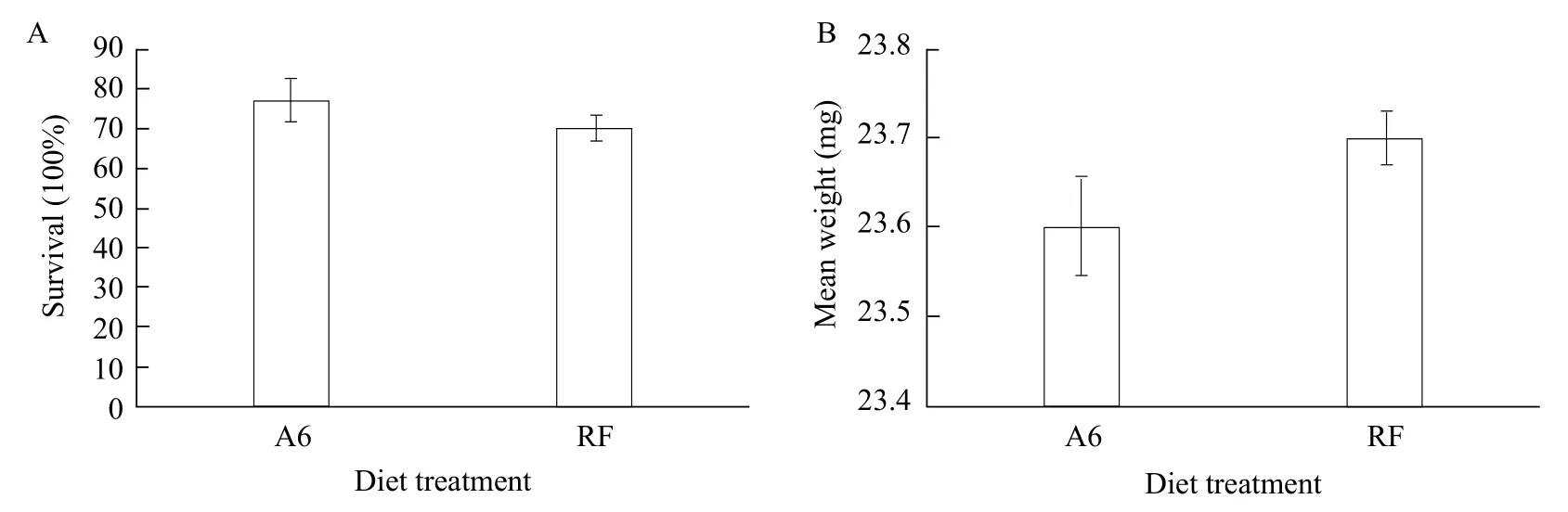
Fig. 2 Survival and mean weights of SPB larvae reared on artificial diet A6 and RNase-free artificial diet (RF)

Fig. 3 Development and survival of SPB larvae fed on RNase-free artificial (RF) diet with Spbp0 dsRNA
Discussion
The results of the present study had demonstrated that SPBL. glycinivorellaperformed well on the artificial diet A6 developed, compared with a range of different artificial diets and the moth's host plant soybean.The survival rate of the larvae fed with A6 diet was higher than those fed soybean pods. Furthermore, the mean weight of A6-fed larvae was similar to that of those fed soybean pods. These results implied that the new artificial diet A6, which comprised a mixture of soybean flour, R3 immature soybean seed powder, R3 immature soybean pod powder, corn flour, carrot flour,caster sugar and brewer's dried yeast could be used as an alternative to soybean pods for rearing purposes(Wanget al., 2013).
The main sources of proteins in A6 artificial diet were the soybean flour, R3 immature soybean seed powder and R3 immature soybean pod powder. The survival rates of larvae fed with A5, A6 and A7 diets were all higher than those fed soybean pods, with the rate of A6-fed larvae being the highest. The main differences among A5, A6 and A7 diets were the proportions of soybean flour and R3 immature soybean pod powder. Subtle differences in diet composition affected neo-nate-larval development (Blancoet al.,2009; Blancoet al., 2008). A ratio of 5 : 1 for R3 immature soybean pod powder to soybean flour was found to be the most suitable for neonate-larval development. The protein content of R3 immature soybean pod powder, including pod wall, was 25.7%,lower than that of the soybean flour (38.56%). SPB neonate larvae initially fed on the inner layer of the pod wall after entering the pod, and then on the immature soybean seed (Wanget al., 2018; Meng and Li, 2017).Thus, the total protein content of the diet was not a major factor affecting neonate-larval development, but rather, the secondary chemistry of the soybean pod wall accurately explained the developmental pattern of neonate larvae (Hervetet al., 2016; Fenget al., 2012; Naseriet al., 2017).
Both microinjection and oral delivery of dsRNA through transgenic soybean had been reported to be valuable methods for achieving RNAi in SPB(Wanget al., 2018; Meng and Li, 2017). However,microinjection could negatively affect the survival of the insect, due to mechanical damage or the shock of sudden higher levels of the injected dsRNA in the hemolymph (Gonget al., 2014; Zhuet al., 2018).Owing to their small size and endophagous habits,it was difficult to carry out microinjections on SPB neonate larvae without affecting larval survival rates.Therefore, a feeding assay based on artificial diets was developed to find a more effective method for orally delivering dsRNA to SPB neonate larvae. A6 diet was prepared in DEPC-treated water to produce RF diet. The survival rates and mean weight of RF-fed larvae were similar to those of A6-fed larvae,suggesting that DEPC-treatment did not affect the nutritional content of A6 diet. Therefore, this RF diet containing Spbp0 dsRNA was used to knock down the expression ofSpbp0 gene. Results from qRTPCR analyses showed that Spbp0 transcript levels in RF-fed larvae significantly decreased by up to 29.9%after feeding 3 days, compared to those of the control group larvae. DEPC-treatment ensured the absence of RNase in RF diet. The survival rate of the larvae fed dsRNA for 6 days was 38%. This survival rate was significantly higher than that of the larvae fed Spbp0 dsRNA (4%) and was similar to that of the larvae fed SpbP0 dsRNA-expressing soybean pods (33%-16.7%)(Meng and Li, 2017). Thus, the delivery of dsRNAviaRNase-free artificial diet was a suitable method for screening a large number of genes or for pest management.
Conclusions
In the present study, a nuclease-free artificial diet was developed for the effective delivery of dsRNA to evaluate dsRNA machinery and the effects of RNAi on candidate target genes. The findings indicated that the newly developed A6 or RF diets could be used to rear SPB neonate larvae in the laboratory and developed an oral delivery method that would allow screening and selecting the most efficient candidate genes for reducing larval development and increasing larval mortality.
 Journal of Northeast Agricultural University(English Edition)2021年2期
Journal of Northeast Agricultural University(English Edition)2021年2期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Identification of QTLs Associated with Resistance to Pseudomonas syringae pv. Glycinea in Soybean (Glycine max (L.) Merr)
- Grain Yield and Nitrogen Use Efficiency of Hybrid Rice in Response to High Plant Density and Nitrogen Rate
- Index Design and Comprehensive Evaluation of Germplasm Resources of Fruits Based on Mathematical Model of AHP and FCE: Sterculia nobilis Smith as a Case
- Effects of Interaction of Soil Moisture and Organic Matter on Powdery Mildew Disease and Growth of Heracleum moellendorffii Hance
- Comparative Analysis of Bacillus thuringiensis Vip3Aa57 and Vip3Aa62 Insecticidal Activities
- Comprehensive Evaluation of Processing Quality of Tibetan Native Hulless Barley Variety by Factor Analysis
