Fabrication and Characterization of Antimicrobial Food Packaging Materials Composed of Konjac Glucomannan, Chitosan and Fulvic Acid
CHEN Xiaohan, PANG Jie,2,3, WU Chunhua,2,3,
(1.Engineering Research Center of Fujian-Taiwan Special Marine Food Processing and Nutrition, Ministry of Education,College of Food Science, Fujian Agriculture and Forestry University, Fuzhou 350002, China;2.State Key Laboratory of Food Safety Technology for Meat Products, Xiamen 361100, China; 3.Key Laboratory of Marine Biotechnology of Fujian Province, Institute of Oceanology, Fujian Agriculture and Forestry University, Fuzhou 350002, China)
Abstract: A konjac glucomannan (KGM)/chitosan (CS) antimicrobial film was prepared by a sol-gel method using fulvic acid as the cross-linking agent.The physical, mechanical, structural and antimicrobial properties of the antimicrobial film was evaluated by rheology, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscope (SEM).Results showed that the introduction of fulvic acid promoted the formation of hydrogen bonds and electrostatic interactions between the functional groups of KGM and those of CS, thereby improving the thermostability and optical properties of the antimicrobial film.Meanwhile, compared with KGM/CS antimicrobial films, the mechanical properties of KGM/CS antimicrobial films added with fulvic acid increased by 16.85 mPa.Water vapor permeability(WVP) of KGM/CS antimicrobial films was 8.65 g/(Pa·s·m) and KGM/CS antimicrobial films added with fulvic acid was 5.25 g/(Pa·s·m).More importantly, the film had good antimicrobial properties and thus could be applied in active food packaging to maintain the quality of foods.
Keywords: konjac glucomannan; chitosan; fulvic acid; antimicrobial properties; active food packaging
Biopolymer-based biodegradable packaging materials,which are extracted from plant, animal, or microbial sources,have received considerable attentions globally due to environmental pollution from petroleum-based plastic food packaging materials and depletion of fossil resources[1-3].Konjac glucomannan (KGM) is a natural polysaccharide extracted from the tubers ofAmorphophallus konjacplants[4].KGM has been widely explored in the preparation of active food packaging films because of its non-toxicity,biodegradability and abundant resources[5-7].In comparison to synthetic plastics, poor physicochemical and antimicrobial properties of native KGM films limited their applications in active food packaging industry[8-9].Chitosan (CS) obtained from the deacetylation of chitin[10], has been reported to exert a wide range of antimicrobial properties due to the polycationic amino groups[11-12].KGM/CS films were successfully prepared by chemical cross-linking methods to enhance the mechanical and antimicrobial properties of wound dressing[13]; however, chemical cross-linking agents are generally toxic and expensive[14], which are impractical for the applications in food packaging materials.
Fulvic acid, as one kind of natural anionic organic compounds, is economical and ubiquitous[15].It is produced by organic material (i.e., plants and microorganisms)degradation, and has been considered to be stable under alkaline and acidic environment[16].It could be used as a natural cross-linker of bio-polymer with higher amount of surface site density and oxygen-containing functional groups,such as hydroxyl group (—OH), carboxyl group (—COOH)and phenolic group (phenolic hydroxyl)[17-18].Fulvic acid can react with the amino group (NH3+) of CS to form ionic bonds,and induce the formation of hydrogen bonding interaction between CS and KGM[19-20].There are many studies on the preparation of films aiming to enhance mechanical strength and antimicrobial activity of KGM films[21-23]; however,limited information was reported on the characterization of the antimicrobial films prepared from KGM/CS blends cross-linked by fulvic acid.
In this study, KGM/CS antimicrobial films were fabricated via the sol-gel approach, and the ratio of fulvic acid on mechanical strength and antimicrobial activities of KGM/CS antimicrobial films was investigated.Furthermore,the physicochemical and structural characteristics of KGM/CS antimicrobial films with and without cross-linked by fulvic acid were compared for better application in food packaging materials.
1 Materials and Methods
1.1 Materials and reagents
KGM (mw: 1 000 kDa; purity: 95%; viscosity:35 000 mPa·s of 1% (m/m) solution) was supplied by San Ai Konjac Food Co.Ltd.(Yibin, Sichuan, China).CS (> 75%degree of deacetylation) and fulvic acid were purchased from Macklin Co.Ltd.(Shanghai, China).Other chemical reagents purchased from Sinopharm Group Chemical Reagent Co.Ltd.(Shanghai, China) were of analytical grade and commercially available.
1.2 Instruments and equipments
MCR301 Rheometer and PP50 Parallel-plates (50 mm diameter and 1 mm gap) Anton Paar Instruments Inc.,Austria; Nicolette 6700 Spectrophotometer Thermo Fisher Scientific Co.Ltd., MA, USA; X-ray Diffractometer Rigaku Co.Ltd., Tokyo, Japan; SU8010 Scanning electron microscopy (SEM) Hitachi Co.Ltd., Tokyo, Japan;Mechanical strength stretching machine Shimadzu Scientific Instruments Co.Ltd., Kyoto, Japan; SDTQ600 Thermogravimetric analyses (TGA) apparatus TA Instruments Co.Ltd., New Castle, Delaware, USA; UV2600 Ultraviolet (UV)-vis spectrophotometer Shimadzu Corporation, Japan.
1.3 Methods
1.3.1 Preparation of KGM/CS films and KGM/CS antimicrobial films
The films were prepared by the solvent casting technique.Briefly, 1 g of KGM powder was dissolved into 100 mL of distilled water (40 ℃) in a water bath shaking incubator under magnetic stirring at 400 r/min for 4 h to obtain KGM film solution.At the same time, CS was dissolved in 1% (V/V) acetic acid in the ratio of 1:100 (m/V)under magnetic stirring for 4 h to obtain CS film solution.Then, 0.2% (m/m, on a dry basis of the weight of KGM) of CS film solution was added into 80 mL KGM film solution,stirring at 400 r/min for 1 h.Fulvic acid (5 mg/mL) was dissolved into deionized water (pH 4, 0.5 mol/L NaCl, 40 ℃),and added in the final film solution to obtain concentrations of 0%, 0.005%, 0.01% and 0.015% (m/m) of KGM (m/m, on a dry basis of the weight of KGM) as KGM/CS, KGM/CS-1,KGM/CS-2, KGM/CS-3, respectively.Then, 25 mL of final film solution was cast on a plastic Petri plate and dried at 45 ℃ for 12 h.The obtained films were conditioned in a chamber at 50%relative humidity and 25 ℃ for 72 h prior to testing.
1.3.2 Characterization of KGM/CS films and KGM/CS antimicrobial films
1.3.2.1 Rheology of film solutions
The rheological properties of the film solutions were determined by using a rheometer.The parallel-plates were used in all rheological tests.The shear rate was set to 0.1—100 s-1, and the temperature to 25 ℃.
1.3.2.2 Fourier transform infrared analysis
The samples were mixed with potassium bromide and then compressed.The Fourier transform infrared (FTIR)spectrum of samples were detected by a spectrophotometer in the range of 600—4 000 cm-1at a resolution of 4 cm-1.
1.3.2.3 X-ray diffraction
The freeze-dried samples of the different ratios were measured by the X-ray diffractometer, equipped with Cu Kα radiation at a scan rate (2 h) of 4 min-1at 40 kV to obtain X-ray diffraction (XRD) pattern data.The intensity of spectrum was recorded in the range of 2θ= 2°—55° and a temperature of 25 ℃.
1.3.2.4 Scanning electron microscopy
The film samples were cut into rectangular strips of 1 cm × 5 cm, which were frozen in solution nitrogen and then separated.They were carried out by SEM to obtain the cross-sectional microstructures, and the detection voltage was 3.0 kV.Before testing, the samples were sputtered with about 20 nm thick gold for 60 s.
1.3.2.5 Antimicrobial test
Staphylococcus aureusandEscherichia coliwere selected as indicator bacteria, and the diameter of inhibition zone was measured to determine the antimicrobial effect of film samples.TheS.aureusandE.coliwere grown over the Luria Bertani(LB) media, and a circular film samples (1 cm in diameter) was adhered to the surface of the medium.After 24 h incubation at 36 ℃, the size of inhibition zone was measured.
1.3.2.6 Mechanical properties
The tensile strength (TS) and elongation to break (EB)of the films were determined with a mechanical strength stretching machine.The samples were cut into rectangular strips of 1 cm × 5 cm, the thickness of each sample was measured with a spiral micrometer, and the average thickness was calculated.The TS test was carried out using a mechanical strength stretching machine, with initial length 3 cm,and test speed at 25 mm/min.After sorting and analyzing the data, the formula was used to calculate TS and EB based on the equation (1) and equation (2) as below, respectively[24].

WhereFis the maximum tension/N;dis the average thickness/mm;l0is the initial length (30 mm).

Wherelis the length of the stretch/mm;l0is the initial length (30 mm).
1.3.2.7 Water vapor permeability
The water vapor permeability (WVP) of the film samples was measured gravimetrically at 25 ℃.Briefly,2 g of anhydrous calcium chloride particles dried for 24 h at 105 ℃, which were poured into weighing bottles (40 mm in diameter and 25 mm in depth) and then covered by the films.All the film samples were balanced at a relative humidity of 75% at 25 ℃.The weight of each bottle was measured at 1 h intervals over 72 h.Measurements were repeated at least 5 times for each film samples in parallel, and WVP was calculated based on equation (3)[24].

Where Δm/Δtis the rate of change of mass over time/(g/s);Ais the effective film area/m2;dis the film thickness/m; Δpis the partial water vapor pressure difference across the film (Δp= 3 282 Pa, 25 ℃).
Now as magicians lose all their power as soon as they are in prison, the King felt himself much embarrassed at being thus at the mercy of those he had so greatly offended
1.3.2.8 Thermogravimetric analysis
The film samples were detected by a TGA apparatus.The samples were heated at the range of 25—600 ℃ at a rate of 10 ℃/min and scanning rate of 30 mL/min under nitrogen atmosphere.
1.3.2.9 Light transmittance
The film samples were cut into rectangular strips of 1 cm × 5 cm.The spectrum was recorded in the range of 200—600 nm under an UV-vis spectrophotometer to obtain a light transmittance pattern.
1.4 Statistical analysis
The data were demonstrated as the mean ± standard deviation.Variance analysis was examined by SPSS 25.0 software statistical analysis system.Duncan’s multiple range test (P< 0.05) was served to compare the differences in the midst of films.
2 Results and Analysis
2.1 Rheological property of KGM/CS film-forming solution and KGM/CS antimicrobial film-forming solutions
Rheological property of KGM/CS solutions was studied to gain a better understanding of the network structure of film solutions.As shown in Fig.1, the incorporation of fulvic acid into KGM/CS film solutions resulted in an increased viscosity, suggesting the network structure of KGM/CS solutions was reinforced.It could be related to the formation of the electrostatic interactions and hydrogen bonds by introducing fulvic acid, and thus the original intermolecular interaction among KGM and CS was destroyed.The incorporation of excessing fulvic acid led to a decrease in the viscosity of antimicrobial film solution.The reduction in viscosity might be due to the neutralization of some positive surface charges of CS by cross linking of excess negatively charged fulvic acid[19,25].It has been found that a decrease in surface charges was related to less repulsive forces between CS polymer chains, hence promoting aggregation[26].Therefore, the incorporation of 0.01% (m/m) of fulvic acid could be confirmed as the best concentration for the sol-gel methods.

Fig.1 Steady rheological properties of KGM/CS film-forming solution and KGM/CS antimicrobial film-forming solutions
2.2 Fourier transform infrared and X-ray diffraction analysis of KGM/CS films and KGM/CS antimicrobial films
The FTIR spectra of all films in the region of 600—4 000 cm-1were presented in Fig.2A.The FTIR spectra of the KGM/CS films showed a broad absorption band at 3 376 cm-1, which was due to O—H stretching and N—H vibrations[24].Moreover, the peaks at 2 924 cm-1indicated the stretching of C—H, and the absorption bands at 1 639 cm-1was related to the overlapping of the amide I groups of CS[24].In addition, the peaks at 1 026 and 825 cm-1were attributed to the stretching vibration of C—H and mannose groups in KGM[27].The peak at 1 723 cm-1could be correlated to carboxyl group and C—O bonds stretching of the intermolecular interaction.
XRD was further performed to verify the influence of fulvic acid on the network interaction of KGM/CS films(Fig.2B).A broad peak at 2θ= 20° was observed in the native KGM films, suggesting that KGM was an amorphous material, which was consistent with previous finding[11].After cross-linking with fulvic acid, the peak intensity was decreased and a broader peak appeared in KGM/CS antimicrobial films, which could be attributed to the new intermolecular interaction between hydrogen bonds.Thus, the organized network of KGM/CS was rearranged by fulvic acid cross linking, and similar XRD patterns were also reported in previous study[28].

Fig.2 FTIR spectra (A) and XRD profiles (B) of KGM/CS films and KGM/CS antimicrobial films
2.3 Scanning electron microscope analysis of KGM/CS films and KGM/CS antimicrobial films
The influence of fulvic acid on the microstructure of KGM/CS films was evaluated, and the cross-section offilms was revealed by SEM images (Fig.3).The cross-sectional microstructures of the KGM/CS films were flat and smooth with homogenous cracks and pores, indicating KGM and CS possessed good compatibility and miscibility.The SEM images were consistent with the previous results[13,24].Comparing with the SEM image of KGM/CS films, the micrographs of KGM/CS-1 and KGM/CS-2 films (cross-linked antimicrobial films by fulvic acid) exhibited more dense cross-sectional microstructures.The dense structure was due to the formation of the hydrogen bonding interactions among KGM and CS induced by fulvic acid[24].Meanwhile,their cross-sectional microstructures were uneven and rugged by cross-linking of fulvic acid[11].The reason could be explained by the electrostatic interaction between carboxyl group of fulvic acid and amino groups of CS during the process[19].With the increase of fulvic acid to 0.015 %(m/m), visible cracks were found in KGM/CS-3 film.This phenomenon could be attributed to less electrostatic repulsion leading to the aggregation of CS polymer chains,and the aggregation could damage the prime uniform three-dimensional network in KGM/CS antimicrobial films[3].In this study, the reinforced intermolecular interaction and dense network microstructure was directly correlated to the effectiveness of KGM/CS antimicrobial films[24].

Fig.3 SEM images of KGM/CS films and KGM/CS antimicrobial films
2.4 Antimicrobial properties of KGM/CS films and KGM/CS antimicrobial films
It is of vital importance for active food packaging films to possess antimicrobial capability.The quality of food could be seriously affected by indicator bacteria resulting in various diseases[17].The inhibition zone of the KGM/CS films and KGM/CS antimicrobial films against two typical indicator bacteriaS.aureusandE.coliwere shown in Table 1.All film samples showed bigger inhibition zone againstS.aureusthanE.coli, which indicated that films showed higher antimicrobial activity against Gram-positive bacteria than Gram-negative bacteria.It was attributed to differences in the cell physiology and metabolism of bacteria[17].
KGM/CS films had the lowest antimicrobial activity with the smallest inhibition zone (P< 0.05).The antimicrobial activity of the KGM/CS film was mainly attributed to the presence of CS.Previous studies have found that thegroups in CS chain could interact with the negatively charged bacterial cell membrane, leading to increased penetrability of membrane and a leakage of intracellular components[29].Compared with KGM/CS films, the addition of fulvic acid enhanced the antimicrobial activity of KGM/CS films.It may be related to the fact that fulvic acid created a weakly acidic film system, which further improved the antimicrobial activity of CS.In addition, fulvic acid possessed broad-spectrum antimicrobial properties[15].Similar results have been reported for potato starch/CS composite films crosslinked with citric acid[30].
The antimicrobial activity might decrease when the addition of fulvic acid was higher than 0.01%.As shown in Table 1, KGM/CS-3 film showed smaller inhibition zone against bothE.coliandS.aureuscompared to the KGM/CS-2 film.It may be related to the fact that the excess fulvic acid reduced the quantity of the free NH+3groups of KGM/CS films, thus the antimicrobial activities were diminished[19].The results of antimicrobial properties confirmed that KGM/CS antimicrobial films could be good candidates in the food industry to prolong the shelf life and maintain the good quality of packaged food.

Table 1 Diameter of inhibition zones of KGM/CS films and KGM/CS antimicrobial films
2.5 Mechanical properties of KGM/CS films and KGM/CS antimicrobial films
Mechanical properties were one of the most important indicators to evaluate food packaging materials.TS and EB of KGM/CS films and KGM/CS antimicrobial films were calculated as listed in Table 2.The TS of KGM/CS films was(40.94 ± 1.40) mPa, and KGM/CS antimicrobial films showed significant higher TS (P< 0.05).The TS of KGM/CS-1 and KGM/CS-2 were (42.61 ± 2.24) and (57.79 ± 4.01) mPa,respectively, indicating the contribution of improved TS by fulvic acid.The reason was probably related to the dense network structure by crosslinking the carboxyl group of fulvic acid with amino groups of CS.Excessing fulvic aciddecreased TS of antimicrobial films (KGM/CS-3), which could be explained that extra carboxyl group (carried by fulvic acid) lowered the degree of crosslinking and intermolecular interactions[11].Meanwhile, the EB showed negative changes in antimicrobial films.The EB of the KGM/CS films was (33.92 ± 0.39)%, and those of KGM/CS antimicrobial films ranged from 21.04% to 28.88%.There was less mobility between polymer chains after crosslinking, resulting in an increase in TS and a decline in EB[31].Therefore, the addition of fulvic acid had a significant effect (P< 0.05) on the mechanical properties of the KGM/CS film samples.

Table 2 Mechanical properties and WVP of KGM/CS films and KGM/CS antimicrobial films
2.6 Water vapor permeability of KGM/CS films and KGM/CS antimicrobial films
WVP represented the vital barrier parameter reflecting the ability of KGM/CS films and KGM/CS antimicrobial films against water vapor.A lower WVP is beneficial for active food packaging in general.As indicated in Table 2,KGM/CS films exhibited significant higher WVP (P <0.05)than KGM/CS antimicrobial films, which was attributed to abundant hydrophilic groups of KGM backbone[17].The WVP of KGM/CS films was (8.65 ± 0.23) g/(Pa·s·m), which was decreased significantly to (5.25 ± 0.21) g/(Pa·s·m)(P <0.05) when the fulvic acid loading increased from 0%to 0.01%.The reason might be caused by more compact structure formed through the cross-linking reaction between KGM/CS and fulvic acid, resulting in higher resistance to the diffusion of water molecules[32].This also confirmed that fulvic acid promoted cross-linking during KGM/CS film formation, which was consistent with SEM results.Similar results were reported on CS/ε-polylysine composite films cross-linked with sodium tripolyphosphate in previous study[11].It is worth noting that the WVP of KGM/CS antimicrobial films increased when the addition of fulvic acid higher than 0.01% (m/m).A possible reason could be due to the excessive fulvic acid leading to a discontinuous internal structure, thus improving the diffusivity of water vapor through the antimicrobial film gaps.
2.7 Thermal properties of KGM/CS films and KGM/CS antimicrobial films
The thermal stability of KGM/CS films and KGM/CS antimicrobial films was tested using TGA, and multi-step decomposition thermograms are presented in Fig.4.The smaller weight loss in the first stage (25—100 ℃) was correlated to moisture evaporation of the films, which was accompanied by the damage of inter-molecular and intra-molecular hydrogen bonds among KGM, CS, and fulvic acid[13].The second stage (100—200 ℃) of weight loss was caused by the decomposition of glycerol used as a plasticizer[17].The largest weight loss in the third stage(200—600 ℃) was associated with the damage of electrostatic interactions among CS and fulvic acid in KGM/CS films.As shown in the curves, no significant difference in weight loss was found when fulvic acid was incorporated into KGM/CS films, but KGM/CS films cross-linked with fulvic acid showed less weight loss than those without fulvic acid.This indicated that the addition of fulvic acid enhanced the thermal stability of the films by crosslinking.Similar results have been found in potato starch/CS composite films cross-linked with citric acid[30].
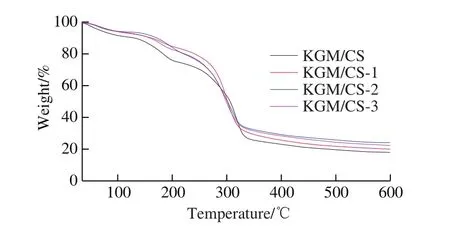
Fig.4 TGA spectra of KGM/CS films and KGM/CS antimicrobial films
2.8 UV-barrier properties of KGM/CS films and KGM/CS antimicrobial films
The UV-barrier property is an important indicator for developing active food packaging, which shows the capability to prevent discoloration, loss of nutrients, and lipid oxidation in the packaged food[17].The transmission of UV and visible light at the wavelength (200—600 nm) of the films is shown in Fig.5.A slight decrease in the UV transmittance (259—366 nm)was observed in KGM/CS antimicrobial films (KGM/CS-1,KGM/CS-2), indicating that KGM/CS antimicrobial films exhibited UV-shielding properties.However, the UV-barrier property decreased sharply when the addition of fulvic acid higher than 0.01%, and KGM/CS-3 films exhibited the lowest transmittance among all antimicrobial films.It has been found out that CS could improve the UV-light absorption of the films, while excess fulvic acid caused the agglomeration ofthe CS[33].Notably, in the visible light region (400—600 nm),the transmittance of all the films samples was between 85%—90%.The results implied that the optical transmittance properties of the KGM/CS films were not affected by the incorporation of fulvic acid.The reason could be due to the good compatibility and dispersibility of fulvic acid in the KGM/CS matrix[24].Moreover, a signature photo of the KGM/CS antimicrobial films could be observed clearly.It further confirmed the good transparency and UV-barrier properties of KGM/CS antimicrobial films, which could be well applied in food packaging applications.

Fig.5 UV-shielding properties of KGM/CS films and KGM/CS antimicrobial films
3 Conclusion
In the current study, KGM/CS antimicrobial films were prepared by solvent casting technique using fulvic acid as cross-linking agent.The influence of fulvic acid on the physicochemical and antimicrobial properties of KGM/CS antimicrobial films was investigated.Results confirmed the incorporation of fulvic acid (0.005%—0.01%m/m) to the KGM/CS films obviously reinforced the KGM/CS blending matrix, improved mechanical strength and thermal stability, and decreased the WVP.Fulvic acid promoted the formation of electrostatic interactions and hydrogen bonds among functional groups of KGM and CS, which was further confirmed by rheological tests, FTIR, XRD and SEM.Moreover, KGM/CS antimicrobial films showed antimicrobial capability againstE.coliandS.aureus.Overall, using economical and non-toxic fulvic acid as the cross-linking agents could significantly improve the antimicrobial and physicochemical properties of KGM/CS membrane, which were the most important indicators to evaluate food packaging materials.Therefore, KGM/CS antimicrobial films broaden prospects in the application of fruit and vegetable packaging.

