可充电镁电池负极改性策略
薛琳琳,吕瑞景,王澳轩,罗加严
(天津大学化工学院,天津300072)
1 Introduction
As industrial technologies continue to evolve,the combustion of fossil fuels has an irreversible impact on the global environment with a general decline even depletion of its reserves. Under this background,batteries have emerged to store energy from sustainable sources. Lithium-ion batteries(LIBs)have been widely used in various portable electronics and electric vehicles[1,2]. As commercially available LIBs are no longer able to meet the growing energy demand,lithium metal batteries(LMBs)have got extensive researches due to high theoretical specific capacity and low electrochemical potential of lithium metal anode[3]. However,the challenges of safety issues,natural abundance,higher cost,etc. have severely hampered its application in rechargeable batteries[4]. On the basis of this,some other metal elements including sodium(Na),potassium(K),magnesium(Mg),zinc(Zn),calcium(Ca)and aluminum(Al)batteries have been extensively investigated in recent years[5-12]. Among these researches,rechargeable magnesium batteries(RMBs)are considered to be one of the most promising energy storage systems for the following advantages:(1)only a bit higher potential(-2.37 V)than Li have been detected for Mg,which ensures a large cell voltage(Table 1);(2)Mg shows approximately twice and even higher volumetric capacity(3832 mA·h/cm3)than those of Li(2066 mA·h/cm3),Na(1128 mA·h/cm3)and K(591 mA·h/cm3)metal anodes;(3)abundance of Mg in the crust is 8th,which directly leads to a significant reduction in cost compared with Li batteries;(4)most importantly,unlike Li,Na,K,Zn and Ca batteries,Mg electrode will not form dendrites during cycling process in most cases,which eliminates the safety concerns of short circuits to a large extent[13,14]. Therefore,owing to its superiority of relatively low reduction potential,high volumetric capacity,low cost and safety assurance,RMBs have become a research hotspot in recent years[15,16].
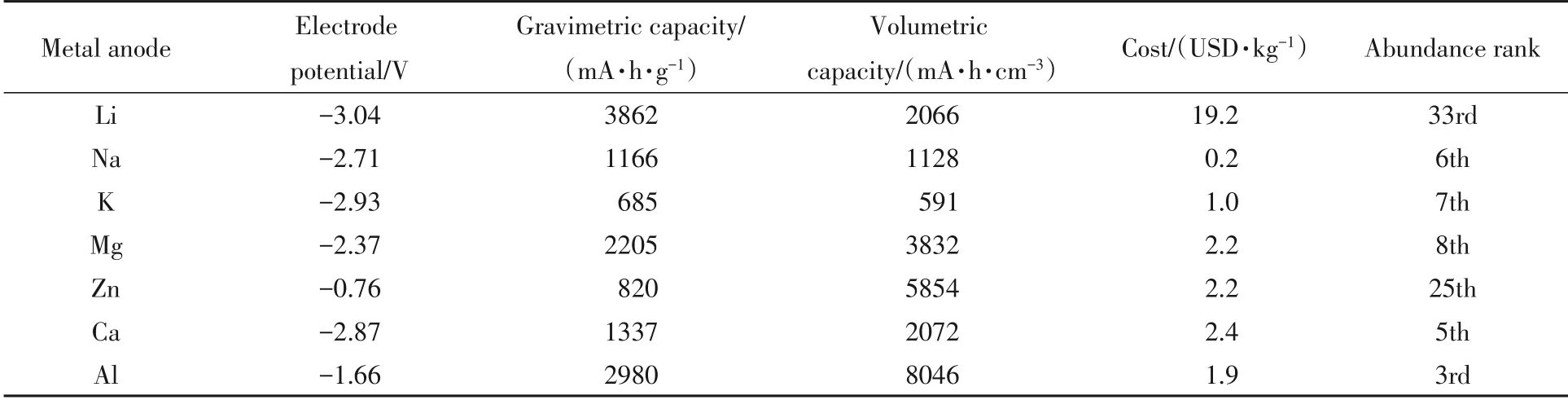
Table 1 Different properties of various metal anodes
Unfortunately,the development of RMBs still faces many challenges. One of the starkest obstacles is the formation of a passivation film on the surface of Mg anode in most conventional electrolytes(as illustrated in Fig.1). This film is insulating to electrons and ions especially to Mg2+,thus leading to irreversible deposition/stripping behavior of Mg[17].
To avoid the formation of passivation film,solvent and solute in the electrolyte must be stable with Mg anode instead of being reduced when getting touched. Commonly,most polar aprotic solvents such as carbonates,lactones,and nitriles,which are readily reduced by Mg should be ruled out. The mainly used organic solvents are ethers like tetrahydrofuran(THF),2-methyltetrahydrofuran(2Me-THF),diethyl ether(DEE),dimethoxyethane(DME)and tetraglyme(TGDME),because they are thermodynamically compatible with Mg metal. However,the low polarity of ethers impedes the ability to dissolve magnesium salts,wherefore it is not less important to study soluble and stable solutes[18,19].
Enormous efforts have been devoted to the research on novel electrolytes to inhibit the formation of passivation film. Nelsonet al.[20]found that the dense passivation film could be avoided in the ether solution of Grignard reagent(R-Mg-X,R is alkyl or aryl,X is halogen),thus enabling sustainable deposition/stripping of Mg. Aurbachet al.[21]manifested the deposition/dissolution process of Mg in Grignard reagent was not a simple electron gain or loss,but accompanied by complex adsorption and desorption processes associated with high impedance. In 2000,Aurbach’s group proposed the first generation of electrolytes based on the reaction between Lewis acid(AlX3-nRn)and Lewis base(R2Mg),which significantly enhanced the stability of Mg anode[22]. Subsequently,further improvements were achieved in dichloride complex(DCC)[23],all phenyl complexes(APC)[24-26],hexamethyldisilazide(HMDS)-based electrolytes[27,28]and boron-based compounds[29-31].
No doubt these organic composite electrolytes have made a breakthrough progress in RMBs. Nevertheless,the expensive price,complex synthesize process,low ionic conductivity,poor compatibility with cathodes,sensitivity to air and moisture,and poor anodic stability limit the application of these electrolytes in practical Mg battery systems. Hence,achieving reversible deposition/stripping of Mg in the electrolytes of simple salts[e. g.,Mg(PF6),Mg(BF4),Mg(TFSI)2and Mg(ClO4)2]may be one of the most feasible ways currently. Unfortunately,as mentioned above,the decomposition of the electrolyte or parasitic reactions between electrolyte and anode will result in the formation of passivating film which comprises of magnesium compounds[e. g.,MgO,Mg(OH)2and MgF2][21,32-35]. The passivation layer hinders Mg2+conduct,leads to large overpotential and limits the output voltage and reversible capacity severely. To date,it must be acknowledged that there is no standard electrolyte that meets all requirements simultaneously for practical application of RMBs.
In parallel with developing the state-of-the-art electrolytes,facilitating modification of anode coming back to conventional electrolytes in RMBs is an alternatively promising avenue. Up till the present moment,the researches concerning anode modification mainly focus on Mg alloy materials and formation of artificial interphase(Fig.1). The main concept underlying the former is that some alloy materials will exhibit lower voltage(less negative redox potential),thus most electrolytes and even some contaminants are less likely to be reduced. The latter mainly mimics the solid electrolyte interphase(SEI)in lithium batteries. Using appropriate synthetic approaches or additives,the ion-conducting and electron-insulating interphase which will widen the electrochemical stability window of electrolytes to some extent is established artificially. In this review,we summarize the two strategies and mechanisms concerning anode modification. Conclusion and perspective for further development of RMBs are proposed based on the analysis and comparison of previous researches.

Fig.1 Schematic of the passivation obstacle and modification strategies for RMBs
2 Alloy Anode Materials for RMBs
Mitigating the issue of incompatibility between anode and most conventional electrolytes for RMBs can be possibly achieved through alloy anodes. For the redox potential of alloy anodes is around 0.2-0.5 V higher than that of Mg metal,conventional electrolytes may not be reduced to form passivation film on the surface[36],which allows utilization of a wider variety of electrolytic solutions that support reversible alloying-dealloying reactions.
In recently years,the research on alloy anodes in RMBs has stimulated a strong interest of researchers.The mainly discussed elements for Mg alloy anodes base on ⅢA,ⅣA,ⅤA(e.g.,Bi,Sn,Ga). Aurbachet al.[37]has listed relevant researches in a clear manner(Fig.2). Herein,the theoretical or experimental results of some representative elements are discussed.

Fig.2 The development of RMBs using alloy anodes based on group ⅢA, ⅣA, ⅤA elements and multi metal alloys[37]
2.1 Bismuth(Bi)-based Alloy Anodes
Bi is considered to be one of the most attractive elements for Mg alloy anodes owing to its high theoretical gravimetric capacity(385 mA·h/g)[38]and excellent Mg2+conductivity(5.9×10-14cm2/S in Bi,3.9×10-10cm2/S in Mg3Bi2)[39]. Based on the alloying reaction(2Bi+3Mg2++6e→Mg3Bi2),Bi-Mg alloy shows no intermediate compounds except for Mg3Bi2[40].
Arthur and coworkers firstly reported the electrochemical behavior of Bi-Mg alloy[36]. Using EtMgCl-Et2AlCl/THF as electrolyte,Bi anode showed a maximum specific capacity of 247 mA·h/g by retaining a specific capacity of 222 mA·h/g at the 100th cycle. Moreover,the CV graph of Bi anode cycled in Mg(TFSI)2/CH3CN indicated that Bi-Mg alloy could be used as anode in conventional electrolytes.
On top of the influence of electrolytes,morphological structures of Bi-Mg alloy anode also affect the electrochemical properties greatly. Shaoet al.[41,42]synthetized Bi nanotubes(Bi-NT)by a hydrothermal reaction method. They evaluated its performance in 0.1 mol/L Mg(BH4)2-1.5 mol/L LiBH4/diglyme electrolyte and speculated the structural transformations during the discharge/charge process furthermore. The testing results indicated that Bi nanotubes anode showed superior performance compared with Bi microparticles(Bi-micro,ca. 100 μm). The migration of Mg2+in Bi-NT was highly reversible and fast for the exhibition of narrower peak separation and lower overpotential than Bi-Micro,thus the specific capacity was higher for Bi-NT[Fig.3(A)and(B)]. As the rate varied from 0.02C to 5C,the performance of Bi-NT was substantially better than Bi-micro,especially at high rates[Fig.3(C)]. In addition,Bi-NT with only 7.7% of capacity fading after 200 cycles exhibited more excellent cycling stability than Bi-micro,which showed capacity decay of 27.5%under the same condition[Fig.3(D)]. The distinct difference above could be attributed to the different structural transformations during the discharge/charge process. Nanotube structures of Bi-NT with hollow space were converted to interconnected nanoparticles during first discharge. These nanoparticles retained the overall morphology reversibly during the subsequent charge and discharge processes,thus achieving insertion/deinsertion of Mg2+without causing structural collapse. However,the preformed Bi-micro without hollow space inside just pulverized and lost the connection due to large volume variations[Fig.3(E)].

Fig.3 Electrochemical performance and illustration of structural transformation for Bi-Mg alloy anode[41]
The studies above provided valuable tests and conclusions about the electrochemical performance and bulk structural transformation of Bi-Mg alloy anodes,but they provided little information on the local structures of anodes and dynamics of Mg2+. In this case,Liuet al.[43]investigated the electrochemical behavior of Bi nanowires as anode materials in RMBs using25Mg solid state NMR spectroscopy. The result demonstrated that the excellent performance of Bi nanowires was in part ascribed to the rapid Mg2+mobility between two Mg sites of Mg3Bi2that displayed fast ionic transport.
Although the uniform insight into the mechanism and pathway for Mg2+diffusion in Bi electrode has not been obtained,these understandings have promoted a firm foundation and inspiration for follow-up researchers(e.g.,Kravchyket al.[38],Heet al.[44],Tanet al.[45],Penkiet al.[46]and Xuet al.[47]),promoting further development of working mechanism and electrochemical properties for Bi-Mg alloy in RMBs.
2.2 Tin(Sn)-based Alloy Anodes
Sn is another promising element for alloy anodes in RMBs with higher abundance and less toxicity than Bi. Singhet al.[48]compared the performance of Sn based anodes with Bi based anodes,and examined the feasibility of Sn-Mg alloy anode in a conventional electrolyte additionally. Result showed that the magnesiation plateau of Sn(Sn+2Mg2++4e→Mg2Sn)was around at 0.15 V(vs. Mg/Mg2+),while magnesiation plateau of Bi was around at 0.23 V. Moreover,the hysteresis between magnesiation and demagnesiation voltage for Bi was much larger than Sn. This might demonstrate the thermodynamic superiority of Sn based anodes.
However,the high hysteresis between magnesiation and demagnesiation capacity suggested that most of Mg2+was not extracted from Mg2Sn,which probably led to pulverization of Mg2Sn during demagnesiation process[Fig.4(A)]. In addition,it was evident that Sn anode displayed higher capacity(903 mA·h/g)than Bi at relatively low rate but dramatic capacity decreases appeared in both magnesiation and demagnesiation process at higher rates. This indicated that Sn anodes suffered from poor kinetics and reversibility[Fig.4(B)].Fortunately,two systems using Mg2Sn as anode and Chevrel phase(Mo6S8)as cathode in conventional electrolyte[0.5 mol/L Mg(TFSI)2/DME]and organohaloaluminate electrolyte displayed similar electrochemical performances,thus highlighting the compatibility of Sn-Mg alloy anode with conventional electrolytes[Fig.4(C)and(D)].

Fig.4 Electrochemical performance and XRD patterns of Sn-Mg alloy anode[48]
Subsequently,Wanget al.[49]investigated the diffusion characteristic of Mg atom intoα-Sn andβ-Sn using first-principles calculation based on density functional theory(DFT). Notably,α-Sn(cubic gray tin)andβ-Sn(tetragonal white tin)are two stable phases at different temperatures. It was reported that the diffusion barriers were 0.395 eV and 0.435 eV for an isolated Mg atom in theα-Sn andβ-Sn,respectively. Moreover,owing to strong binding of Mg-Mg atoms in theβ-Sn,the diffusion barrier decreased in theα-Sn,whereas increased in theβ-Sn with additional Mg atom inserting. This means thatα-Sn is more suitable thanβ-Sn as anode material in RMBs.
Very remarkably,β-Sn has been mostly used for exploring Sn-Mg alloy electrodes,becauseα-Sn is less stable at room temperature. Natheless,the spontaneous features of Sn have been impeded by the slow alloying reaction with Mg at room temperature. Reducing the particle size of Sn is a valid way to facilitate the kinetics of magnesiation and demagnesiation process.
Yaghoobnejad Aslet al.[50]reported a cost-effective avenue through dealloying of bulk Mg2Sn in half-cell to obtain nanostructuredβ-Sn. Herein,the avenue mainly took advantage of stresses arising during demagnesiation of bulk Mg2Sn electrode and the nanostructuredβ-Sn was formedin situwith a size of sub-100 nm[Fig.5(A)]. However,electrochemical test results showed that passive film was likely formed on anode in APC electrolyte,and thus degraded the capacity retention. The fact that only roughly 1/3 of the theoretical capacity was achieved suggested the improved dynamics might be accompanied by capacity sacrifice for nanostructured Sn based anodes to a certain degree[Fig.5(B)].In a later research,Nacimientoet al.[51]studied the performance of micro-(ca. 100 μm)and nano-(ca. 100 nm)particulate Sn electrodes in 0.5 mol/L PhMgCl/THF and 0.5 mol/L EtMgCl/THF electrolytes. Very impressively,micro-Sn electrodes displayed extremely low capacity(<10 mA·h/g)during the whole cycling process[Fig.5(C)]. In contrast,nano-Sn showed an initial capacity of 225 mA·h/g whereas with a severe capacity fading[Fig.5(D)].
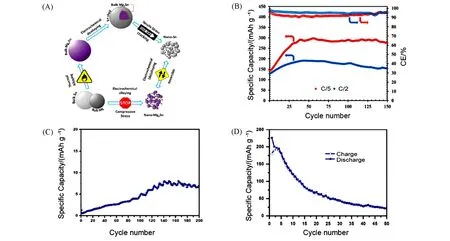
Fig.5 Synthesis process and electrochemical performance of Sn based anode
Obviously,although Sn-Mg anodes in such Grignard electrolytes revealed poor stability,nanodispersion was expected to be a suitable strategy to improve the initial capacity. However,as mentioned in the previous work,nanostructured Sn electrodes experienced relatively low capacity or significant capacity fading,which possibly contributed to the large volume exchange or passive film formation[48,50]. Regrettably,the last two researches introduced above have not studied the behaviors of nanostructured Sn anodes in conventional electrolytes. It thus appears that there are still many details to unveil and improve for further development of nanostructured Sn or other nanomaterials as electrodes in RMBs.
2.3 Other Elements-based Alloy Anodes
The feasibility of Gallium(Ga)[52],Germanium(Ge)[53],Antimony(Sb)[36],Silicon(Si)[54],Lead(Pb)[55],Indium(In)[56],Phosphorus(P)[57]and Aluminum(Al)[58]to serve as alloy anodes for RMBs was also examined. However,further researches to most of these alloys suffer from multiple challenges owing to their sluggish kinetics,poor reversibility and even intrinsic toxicity. For instance,although Mg-Ge anode has been predicted to deliver a high theoretical capacity of 1475 mA·h/g for full magnesiation to the Mg2Ge phase,the insertion of Mg atoms into bulk Ge is difficult and the alloying reaction might be impossible[59]. Mg-In anode could deliver a specific capacity of 425 mA·h/g in Mg-organohaloaluminate electrolytes at a rate of C/100,but showed poor electrochemical performance at higher rate with fast voltage fading during the discharge process.In addition,there is no evidence that it can undergo reversible electrochemical processes in conventional electrolytes[56]. More specific properties of alloy anodes are listed in Table 2.

Table 2 Specific properties of representative alloy anodes
Although some alloyed Mg anodes are compatible with most conventional electrolytes,massive volume expansion(up to 300%)is one of the main factors leading to severe capacity decay and instability[62,63]. Simply put,the sites and diffusion rates of Mg atom are various for alloys,resulting in lattice distortion,inducing significant mechanical stresses and further leading to volume expansion and even collapse of anode materials in the magnesiation and demagnesiation process. Once cracks appear on the anode,new surface need to be generated continuously during the cycling,which consumes the anode material severely and thus result in the capacity attenuation overall batteries.
On base of this,instead of solid-solid transformation,Wanget al.[61]exploited the solid-liquid phase transition between Mg2Ga5(solid)and Ga(liquid)near room temperature to achieve an unprecedented cycling life at a high rate of 3C. The alloy of Ga-Mg phase could be changed from liquid to solid by lowering the temperature or changing the composition[Fig.6(A)]. Authors used electrochemical methods at a constant temperature(40 ℃)and pressure(atmospheric pressure)to add Mg atoms to liquid Ga,resulting in the formation of solid Mg2Ga5alloy(5Ga+2Mg2++4e→Mg2Ga5). In the reverse process,the removal of Mg from this solid Mg2Ga5at 40 ℃led to the formation of liquid Ga,which meant that no material fragmentation occurred and the entire Ga/Mg2Ga5material system could continuously achieve self-healing through a reversible liquid-solid phase conversion[Fig.6(B)]. In addition,there was no any significant stress in the magnesification and demagnesification process of the Ga-Mg alloy according to the result of FEM simulations,which indicated that the Ga/Mg2Ga5system possessed decent performance. Tested in APC electrolyte,the performance of Mg2Ga5anode at 40 ℃was far better than that at 20 ℃. Owing to the self-healing ability of the Ga/Mg2Ga5system,Mg2Ga5alloy anode displayed remarkable long cycle-life(with 212.7 mA·h/g after 1000 cycles)[Fig.6(C)].Further,the compatibility of Mg2Ga5with conventional electrolytes was investigated using acetonitrile(AN),propylene carbonate(PC)and dimethoxyethane(DME)as the solvents in combination with Mg(TFSI)2and Mg(ClO4)2as the salts,and good compatibility was found in Mg(TFSI)2/AN[Fig.6(D)].
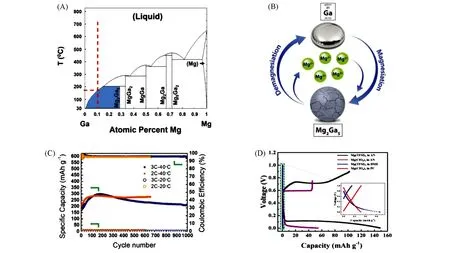
Fig.6 Mechanism and electrochemical performance of Ga-Mg alloy electrodes[61]
In a broad sense,using appropriate alloy anodes might open a way combining the advantages of Mg and alloy elements together to develop RMBs further. However,although some alloys are compatible with conventional electrolytes,most of them display sluggish diffusion kinetics,poor coulombic efficiency or toxic characteristic,which makes obstacles for practical applications and calls for calls for further researches.
3 Artificial Interphase for RMBs
Aside from the design of appropriate alloy anodes,an alternative strategy to tackle the passivation problem of Mg anode is to engineer a Mg2+-conducting and electron-insulating artificial interphase that modifies anode and constrains further parasitic reactions between Mg and electrolyte,thus enabling highly reversible behavior of Mg in most conventional electrolytes.
Two main ways to modify anode with artificial interphase have been reported in recent years. The one is constructing an artificial protective coating on anode surface through various reactions in advance. The other is forming interphase layerin situby changing the components of conventional electrolytes like adding appropriate reagents. Representative researches will be introduced from these two aspects.
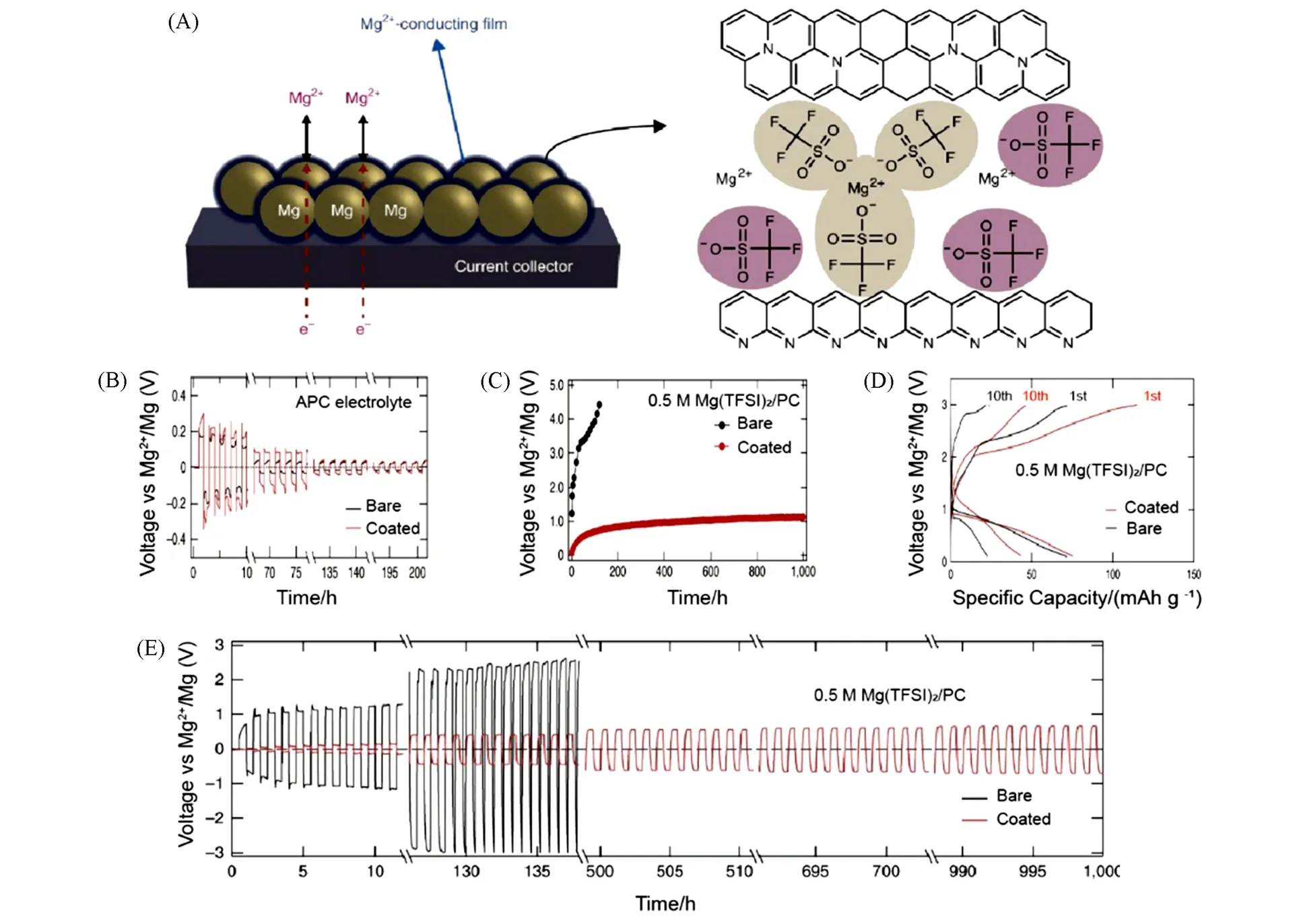
Fig.7 Structure of the interphase and electrochemical performance of coated or bare Mg anodes[64]
3.1 Artificial Interphase Formed in Advance
Sonet al.[64]engineered an artificial Mg2+-conducting interphase on the Mg anodeviasurface reaction in advance firstly,which decoupled the anodic and cathodic requirements for electrolytes and displayed reversible deposition/stripping progress in carbonate electrolytes. The interface film(ca. 100 nm)was made from thermal-cyclized polyacrylonitrile[cPAN with a molecular formula of(C3H3N)n]and Mg trifluoromethanesulfonate[Mg(CF3SO3)2],which contained a pyridine-based PAN matrix hybridized with a network of multicoordinated Mg(CF3SO3-)units[Fig.7(A)]. Reversible deposition/stripping reactions were achieved for both bare and coated Mg anode,whereas the deposition overpotential for coated Mg electrodes was slightly higher compared with bare Mg in the first 100 h due to the lower Mg2+conductivity in the artificial interphase than that in the APC electrolyte[Fig.7(B)]. However,as cycling goes on,the uncoated Mg electrode exhibited extremely high and steeply increased overpotential because of the passivation formed by the reduction-vulnerable electrolyte[0.5 mol/L Mg(TFSI)2/PC]. In stark contrast,the artificial layer coated Mg electrode displayed remarkable performance for 1000 h with relatively stable overpotential[Fig.7(C)and(E)]. It appeared that the artificial polymeric interphase not only successfully prevented the reduction of reduction-vulnerable solvents with Mg,but also promoted Mg2+transport. Assembling the full cell with V2O5as the cathode,the reversibility and cycling stability were improved significantly for the system using coated Mg as anode[Fig.7(D)].
It should be emphasized that this work demonstrated the feasibility of constructing high-energy RMBs with high-voltage oxide cathodes and oxidation-resistant carbonate electrolytes. Hence,a dilemma faced in RMBs,that is,the conflict between an ethereal organometallic electrolyte and the desired oxidation stability can be resolved. However,the steps for synthesizing the interphase are awfully complex under rigorous condition.

Fig.8 Morphology characterization and electrochemical performance of pristine and Sn based Mg anodes[65]
In a follow-up work,Luoet al.[65]proposed a facile and safe method to obtain Sn-based artificial interphase with fast ionic transport feature based on the research of Sn-Mg alloy. Mg metal undergone ion-exchange reaction with SnCl2directly at room temperature to form a modified layer before cycling. Sn and Mg2Sn acted as fast pathway for Mg2+and the insulating component MgCl2/SnCl2provided the necessary potential gradient to avoid Mg deposition on the surface of the modified layer[Fig.8(A)]. The morphology of Mg electrodeposition with Sn-based artificial layer in Mg(TFSI)2/DME electrolyte was further investigated by SEM and EDS mapping. Non-dendritic and particle-like morphology of electrodeposited magnesium was observed at the current density of 0.05 mA/cm2,while an irreversible deposition/stripping behavior was exhibited at 2 mA/cm2for the pristine Mg anode[Fig.8(B)]. In contrast,a relatively uniform and smooth Mg deposition on modified Mg was obtained[Fig.8(C)],and the laminar Mg was deposited under the coating layer with about 5 μm thickness[Fig.8(D)],to which corresponding EDS mappings further testify[Fig.8(E)]. The modified Mg/Mg symmetrical cell could efficiently cycle for a long time(1400 h)at current density up to 6 mA/m2with a significantly lower deposition/stripping overpotential(ca. 0.2 V)in conventional ether electrolyte. Matched with TiS2as cathode,the cycling stability and capacity of modified Mg anode were significantly improved compared to the pristine Mg anode[Fig.8(F)and(G)].

Fig.9 Formation and operating mechanism of MgF2 interphase and electrochemical performance of fresh or coated Mg anodes[66]
In the same year,Caoet al.[66]reported a chemically inert magnesium fluoride(MgF2)interphase formed through reaction of Mg surface with hydrofluoric acid(HF)[Fig.9(A)]. Fluorine element was uniformly distributed in the artificial layer with <200 nm thickness on Mg foil,which indicated the MgF2interphase was formed evenly after HF treatment[Fig.9(E)]. Interestingly,though MgF2was known as Mg2+insulator,it allowed Mg2+diffusion through its porous and mechanically rigid structure in this research[67]. Mg2+penetrated through MgF2film which hindered further reaction between electrolyte(herein,APC electrolyte)and Mg mental during deposition and stripping process[Fig.9(B)]. This was contrast with the behavior of bare Mg anode in APC electrolyte. Specifically,the byproducts from reduction of electrolyte covering on bare Mg blocked the transport of Mg2+at high overpotentials[Fig.9(C)and(D)]. Tested in symmetrical system,bare Mg electrode experienced high and increased hysteresis even up to 200 mV after cycling for 60 h. On the contrary,the Mg anode coated with MgF2interface displayed relatively low and stable hysteresis around 25 mV during the whole cycling,demonstrating the small impedance because of artificial interphase[Fig.9(F)]. Further tests were conducted in full cell with Bi as cathode. The charge capacity of fresh Mg decreased from 416 mA·h/g to 232 mA·h/g after 50 cycles. However,the capacity was 342 mA·h/g and remained 286 mA·h/g for anode coated with MgF2after 50 cycles,exhibiting better capacity retention and kinetics of Mg2+migration compared to fresh Mg[Fig.9(G)and(H)].
Though it was demonstrated that improved compatibility between Mg metal and the electrolyte could be achieved through the formation of MgF2layer,the electrochemical properties of coated Mg anode in conventional electrolytes were not studied in this work regretfully. Most importantly,compared with the usage of SnCl2reagent mentioned above,using HF reagent would probably pose significant safety threat.
3.2 Artificial Interphase Formed in Situ
Artificial interphase discussed above was formed on the surface of anode by chemically treating based on various reactions before battery assembly. Another approach is to add precursor into electrolytes,establishing the artificial Mg2+-conducing interphasein situ.
Wang and coworkers proposed that adding a small concentration of iodine(≤50×10-3mol/L)to the conventional electrolyte[Mg(TFSI)2/DME]facilitated the formation of Mg2+-conducting solid MgI2layerin situ[68].The overpotential for reversible deposition/stripping of Mg was significantly reduced owing to MgI2interphase,and decreased with the concentration of iodine additive increasing. It was clear that deposition/stripping overpotential decreased to 0.05 and-0.17 V with 50 mmol/L iodine in 0.5 mol/L Mg(TFSI)2/DME[Fig.10(A)and(B)]. Meanwhile,the electrochemical impedance spectroscopy(EIS)indicated a new interphase with small impedance emerged due to the addition of iodine,thus enhancing kinetics of Mg2+migration[Fig.10(C)—(F)].
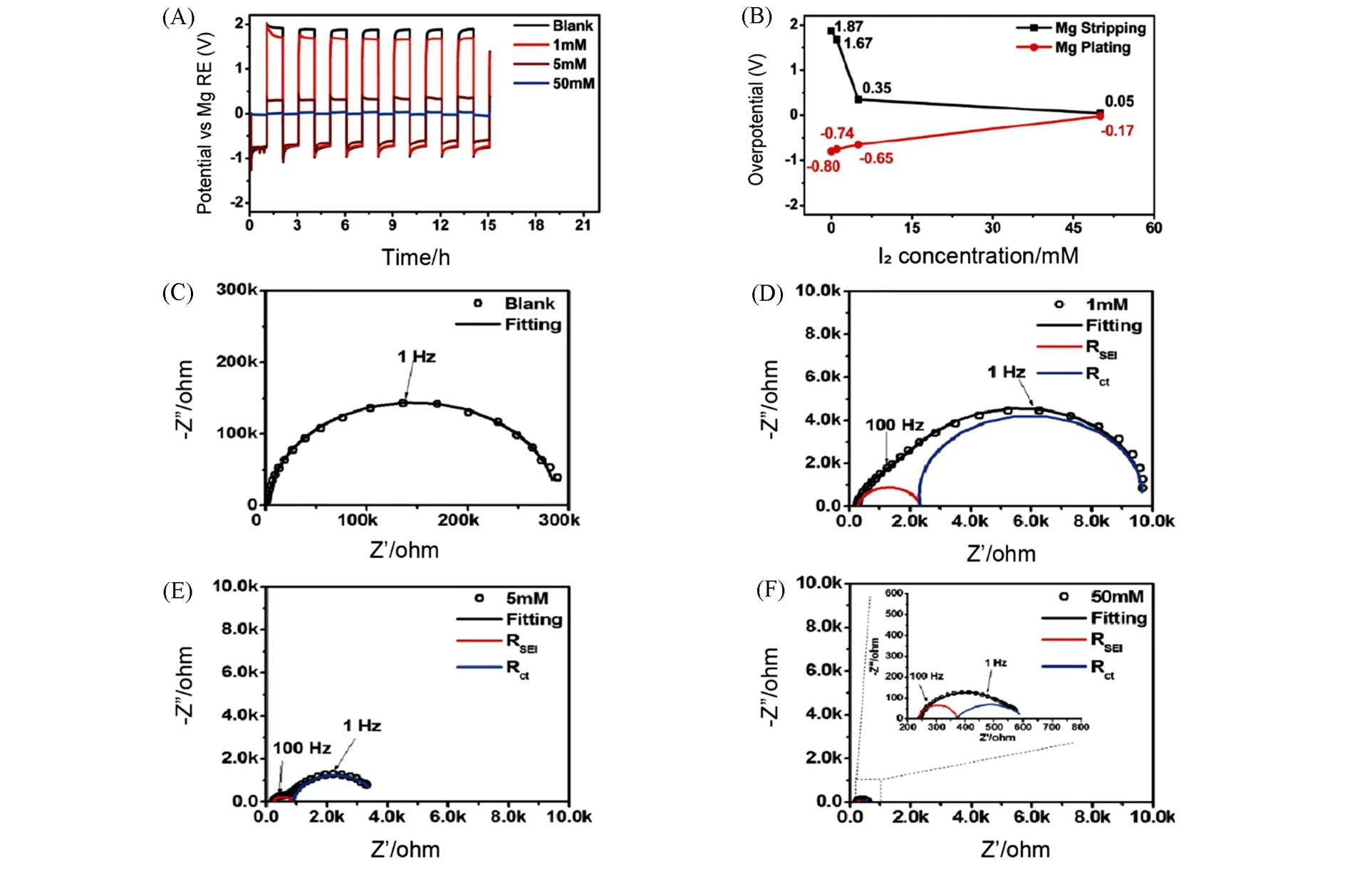
Fig.10 Electrochemical performance of Mg anodes in electrolytes with or without I2 additive[68]
This work firstly converted passive film formed on Mg anode into activate layer by adding I2to conventional electrolyte and light on the effects of additives. More details about the morphology features of Mg anode affected by additives would be appreciated for further mechanism study.

Fig.11 Operating mechanism of interphase and electrochemical performance of anodes in electrolytes with or without GeCl4 additive[69]
Instead of I2with severe corrosivity,Luoet al.[69]reported a Ge-based artificial layer formed on the surface of Mg metalin situby adding GeCl4into Mg(TFSI)2/DME electrolyte as the reaction(Ge4++2Mg→Ge+2Mg2+)occurred. Compared to the breakableex-situformed interfacial layer,a self-repairing process would occur when the artificial protection film ruptured abruptly on account of excess additives in electrolyte[Fig.11(A)—(C)]. Moreover,Mg,Ge and few Cl elements were distributed uniformly on the surface layer proved by EDX mapping analysis[Fig.11(D)and(E)]. The symmetric system with modified electrolyte displayed exceptionally stable and low overpotential(ca. 250 mV)cycling for over 1000 h at current density of 0.02 mA/cm2,which ascribed to unhindered Mg2+diffusion in the formed artificial layer. On the contrary,the Mg electrode in blank electrolyte experienced a high overpotential and reduced lifetime[Fig.11(F)]. Even if the current density increased to 0.02 mA/cm2,the Mg anode in GeCl4-containing electrolyte still exhibited remarkable reversibility without pronounced voltage fluctuation over 350 h[Fig.11(G)].
It was speculated that the built-in electric field in Ge and its compounds might regulate the deposition of Mg during cycling process. Further characterization indicated that the location of deposited Mg layer was underneath the protective film. The insight was important to further explore the working principle of artificial interphase formedin situ.
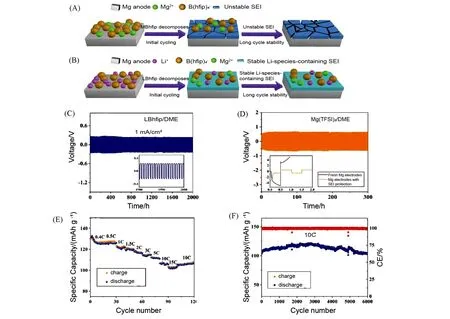
Fig.12 Mechanism of SEI formation and electrochemical performance of anodes in MBhfip/DME or LBhfip/DME electrolyte[70]
Inspired by ion-conducting solid electrolyte interphase(SEI)in Li batteries,Cuiet al.[70]constructed a stable artificial interphase on Mg anode by the partial decomposition of a pristine Li electrolyte during the electrochemical process. In MBhfip/DME electrolyte(0.5 mol/L Mg[B(hfip)4]2dissolved in DME),the decomposition product of MBhfip formed a loose and unstable SEI on the surface of Mg anode,leading to instability for long cycles. On the contrary,in LBhfip/DME electrolyte(0.5 mol/L Li[B(hfip)4]dissolved in DME),LBhfip was decomposed to form a more stable SEI containing Li. Simultaneously,the exfoliated Mg2+from Mg anode could be directly introduced into electrolyte during the initial electrochemical cycles,forming a hybrid Mg2+/Li+electrolyte and thus ensuring reversible and stable deposition/stripping of Mg for a long time[Fig.12(A)and(B)]. In symmetric cell,the stable polarization potential was achieved,which attributed to the outstanding long-term stability and superior Mg2+conductivity of LBhfip/DME electrolyte[Fig.12(C)]. More importantly,the Mg anode removed after cycling in the LBhfip/DME electrolyte was well compatible with Mg(TFSI)2/DME electrolyte,which cycled for 300 cycles without pronounced overpotential enhancement,suggesting the superior protective effect of artificial interphase[Fig.12(D)]. Further,the full cell of Mo6S8/Mg in LBhfip/DME electrolyte was assembled to investigate cycling performance. The system still showed a discharge capacity of 101.3 mA·h/g and average Coulombic efficiency over 99%after 6000 cycles at 10C[Fig.12(E)and(F)].
The ingenuity of this work might lie in using a kind of Li electrolyte instead of adding reagents to electrolyte,thus without concerns about solubility and concentration of additives.
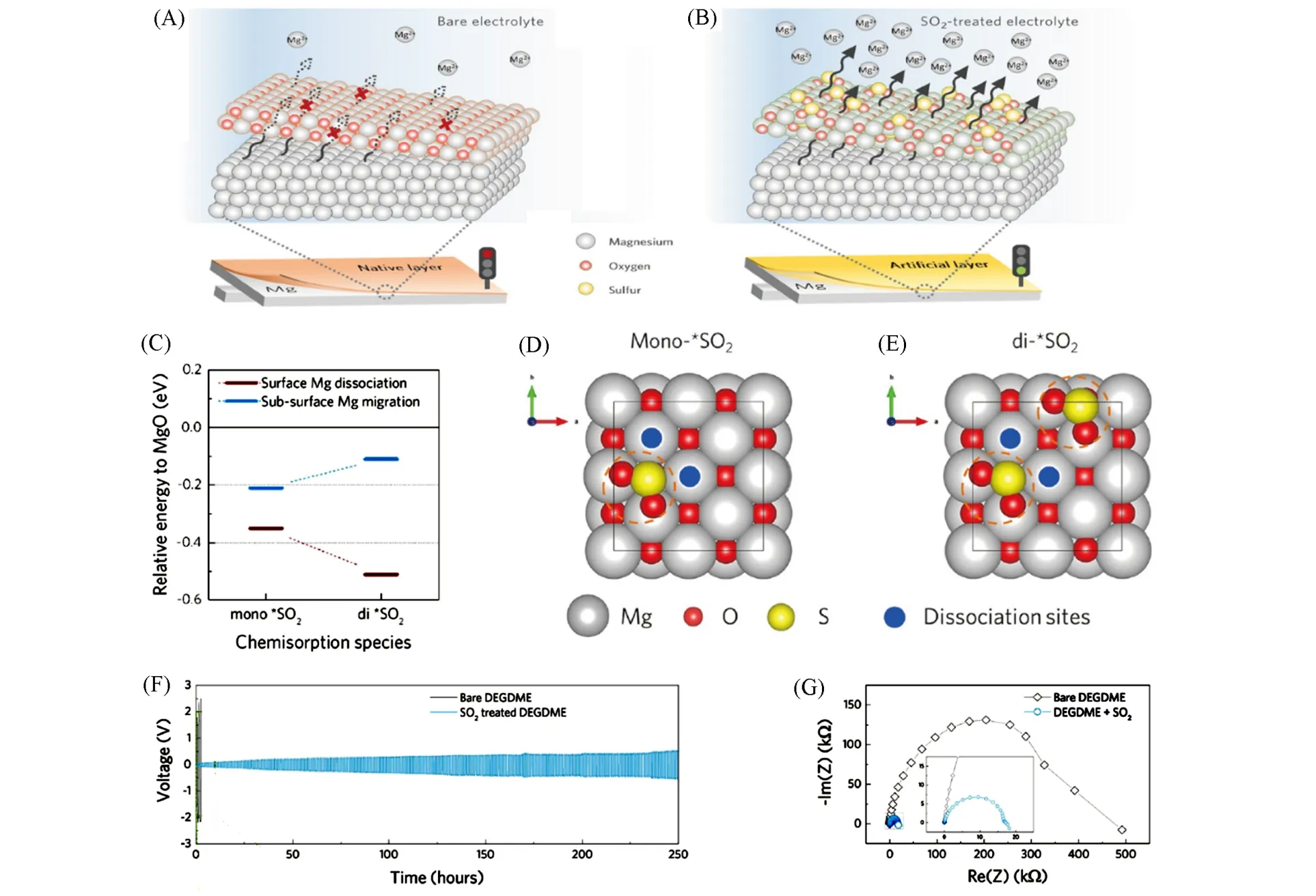
Fig.13 Operating mechanism of the tailored interphase on Mg anode and electrochemical performance in conventional electrolytes[71]
Recently,taking inspiration from the tunability of the MgO which had been widely used as an effective trapper of environmental pollution gases including NOxand SOx,Parket al.[71]proposed a new strategy to tailor passivation film into Mg2+-conducting interphase(MgSO3-dominant structure)viasimple chemisorption of SO2on Mg anode[Fig.13(A)and(B)]. Depending on the SO2chemisorption structure(mono- and di-)on the MgO surface,DFT calculations suggested that the dissociation energy of Mg atom for mono-SO2and di-SO2decreased by 0.35 eV and 0.51 eV respectively. The migration progress of Mg atom was found to be energetically favorable by -0.21 eV and -0.11 eV compared with the reference energy from the MgO(001)surface[Fig.13(C)—(E)]. Thus,it indicated that the chemical tuning of the interphase could markedly lower the dissociation energy and migration barriers of Mg2+. Compared with the large polarization of bare cells,symmetric cells treated with SO2could undergo reversible Mg deposition and stripping with a reasonable overpotential over 250 h at a constant current rate of 0.01 mA/cm2in conventional electrolyte of 0.5 mol/L Mg(TFSI)2in diethylene glycol dimethyl ether(DEGDME)[Fig.13(F)]. Additionally,the charge-transfer resistance could be reduced significantly from 500 kΩ to 20 kΩ through SO2chemisorption progress,which was consistent with the difference of voltage hysteresis[Fig.13(G)].
Compared with coating using precursors that results in uneven formation of the film with difficulty in controlling the thickness and composition,this gas-doping process is more facile and ensures the relatively conformal coating for Mg metal anode.
There is no doubt that the artificial interphase formed on the Mg metal surface has resolved notorious passivation problem partly. Herein,we compare the artificial interphase formed in advance andin situfurther(Fig.14). As mentioned above,constructing an artificial protective coating on anode surface in advance constrains parasitic reactions to the most extent without direct contact between Mg and electrolytes. However,the interphase may be not uniform and long-term cycling at high current density may affect or even destroy the preformed artificial interphase due to its loose structure or poor contact with the surface of Mg. For interphase formedin situ,although more conformal and uniform interphase that possesses the property of self-repairing on account of excess precursors in electrolytes would be obtained,it is difficult to control thickness and composition of the interphase. Beyond that,the precursor added in electrolytes may affect the electrochemical behavior of Mg,and thus the choice and concentration of precursor need to be optimized meticulously. In short,artificial interphase with decent chemical stability,mechanical integrity and intrinsic uniformity still need to be investigated.

Fig.14 Comparison of artificial interphase formed in advance and in situ
4 Conclusions and Perspective
With the researches focusing on electrolytes encountering the bottleneck,modification of anodes to inhibit the formation of passivation film in most conventional electrolytes is becoming the research hotspot for RMBs. As discussed above,two main strategies concerning anode modification are designing alloy anode materials and constructing artificial interphase.
Alloy anodes undoubtedly expand the selection range of electrolytes in RMBs due to its compatibility with most conventional electrolytes. However,it is a double-edged sword because the relatively low reaction activity of Mg alloying/dealloying with most elements. It limits the kinetics of Mg2+diffusion,and in addition,the extensive volume variations during alloying/dealloying process make implementation of the alloy anodes commercially difficult. Most importantly,the capacity of RMBs will not be implemented adequately using Mg alloy as anodes.
Hence,facilitating reversible deposition/stripping of Mg using Mg metal anode may be one of the main directions for further development of RMBs in the long term. Great efforts should be devoted to the following aspects.
(1)Establish conformal and uniform artificial interphase with the features of appropriate thickness,feasible ion-conductivity,intrinsic uniformity,high chemical and mechanical stability. The artificial interphase formed in advance might experience mechanical cracks and fractures due to its loose structure or poor contact with the surface of Mg during cycling process. The thickness,composition and ion-conductivity ofin situformed interphaseviamost inorganic additives are difficult to control. Thus,using polymer like PAN[64],PEC[72],PVA[73],etc. to engineer elastic and self-repairing interphasein situmay be a promising strategy meeting the requirements mentioned above.
(2)Design the structures of Mg metal anode by changing particulate size and constructing scaffolds.Employment of ultrasmall Mg nanoparticles should be a reasonable strategy toward decreasing the thickness of the passivating surface films in the case of the same amount of passivating species,thus increasing ion diffusion in the anodes[74,75]. Conceivably,imitating structure design in LMBs[76],constructing 2D or 3D scaffolds for Mg anode may reduce the local current density,facilitate deposition/stripping behavior,and even regulate the morphology of artificial interphase when combined with interphase establishing strategies.
(3)Exploit compatible,accessible and low-cost electrolytes to control the interphase reactions with Mg metal anode. Using appropriate electrolytes in LMBs to form ion-conducting SEI is an ingenious avenue for Mg metal anode[70]. On top of it,further exploiting polymer electrolytes[77]and even solid electrolytes[78]is expected to mitigate detrimental decomposition reactions and promote Mg2+diffusion in Mg anode.
In conclusion,it is anticipated that more ingenious approaches concerning anode modification will emerge for the further studies in RMBs and even provide promising avenues for other multivalent metal anodes facing the similar obstacles.
This paper is supported by the National Natural Science Foundation of China(No. 51872196),the Natural Science Foundation of Tianjin,China(No. 17JCJQJC44100)and the National Postdoctoral Program for Innovative Talents,China(No.BX20190232).

