Effect of fuel type on the performance of an aircraft fuel tank oxygen-consuming inerting system
Xiaotian PENG, Shiyu FENG, Chaoyue LI, Chen CHEN, Weihua LIU
Key Laboratory of Aircraft Environment Control and Life Support of MIIT, College of Aerospace Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China
KEYWORDS Aviation fuel;Catalytic reactions;Hollow fiber membrane;Inert gas;Mathematical model
Abstract The properties of aviation fuel have a great influence on the performance of oxygenconsuming inerting systems. Based on the establishment of the catalytic inerting process, the flow relationship of each gas component flowing through the catalytic reactor was derived. The mathematical model of the gas concentration in the gas phase of the fuel tank was established based on the mass conservation equation, and the fuel tank model was verified by performing experiments.The results showed that the fuel type exerts a considerably higher influence on the performance of the oxygen-consuming inerting system compared to the corresponding influence on the hollow fiber membrane system, and the relative magnitude of the inerting rates of the four fuel types is RP5>RP3>RP6>JP8. In addition, a higher catalytic efficiency or fuel load rate corresponds to a higher rate of decrease of the oxygen concentration in the gas phase, and the inerting time is inversely proportional to the suction flow rate of the fan.When different fuels are used,the amount of cooling gas and water released from the inerting system are different.Therefore,the influence of fuel type on the system performance should be extensively considered in the future.
1. Introduction
Aircraft safety has always been a matter of considerable concern.The upper space of a fuel tank is filled with a combustible oil and gas mixture, which is a potential safety hazard, and effective measures must be taken to reduce the probability of explosions.Studies have shown that the most effective method is to control the flame transfer and change the ignition limit of the aircraft fuel tank.1Federal Aviation Administration(FAA)considers that the fuel tank of military and civil aircraft is in the inert state when the oxygen concentration in the gas phase space is less than 9% and 12%, respectively.2
Since the 1950s, the safety of aircraft fuel tanks has been ensured by carrying a storage tank filled with liquid nitrogen/-gas nitrogen/Halon 1301. This method has been used in many aircraft,such as the DC-9,F-16,and F-20 aircraft.3,4However,the inert gas carried is limited, which makes it difficult to achieve full inert protection. Therefore, in the late 1970s, the Onboard Inert Gas Generation System (OBIGGS), which produced inert gas via airborne equipment, was promptly developed. Studies have shown that this approach is a relatively better method of carrying inert gas.Although technically difficult,the logistical support requirements are extremely low,and a limit value below the supported fuel combustion during flight can always be achieved5,6.
Nomenclature
η efficiency
˙n molar flow rate
Prreaction exothermic
Hufuel calorific value
p pressure
h enthalpy
ptenvironmental pressure
pssaturated vapor pressure
T temperature
Tddew point temperature
d moisture content
Cpspecific heat
V volume
R gas constants
Subscript
cat catalytic
i inlet
o outlet
F fuel
O oxygen
N nitrogen
C carbon dioxide
H water
cooler cooler
g gas
q liquid
U ullage
A air
total total
With the development of technology, the Hollow Fiber Membrane-based Onboard Inert Gas Generation System(HFM-OBIGGS)has become the most economical and practical technology for aircraft fuel tank fire and explosion suppression,7-10and has been widely used in new military aircraft,such as the F-22, F-35, Boeing 737, Boeing 747 and Airbus A320.11-14However, there are still many problems with the HFM-OBIGGS technology, for example, the low efficiency of the separation membrane leads to a large compensation loss of the aircraft, and the high pressure on the inlet of the separation membrane leads to difficulties in application (such as helicopters), fine membrane filaments and osmotic apertures.The ozone in the gas source can lead to a serious deterioration of the membrane performance,and when the nitrogen rich gas is filled into the fuel tank,the fuel vapor may leak and pollute the environment.15,16
In 2004,when Phyre developed a new deaeration system for the Air Force to remove dissolved oxygen from fuel, an economical, efficient and environmentally friendly oxygenconsuming tank inerting system that does not require engine bleed air was developed. The system has no moving parts,except a low-pressure pump;therefore,it has a low power consumption,compact structure and light weight.This system can solve the problem of the HFM-OBIGGS requiring engine bleed air, and it also does not emit exhaust gases as the fiber membrane system does. Thus, this system is called the Green airborne Inert Gas Generation System (GOBIGGS).17,18
The GOBIGGS was technically validated at the FAA Atlantic City Technology Center in May 2007.19The short range FL-350 flight envelope was selected, with a total flight time of approximately 105 min. The inerted fuel tank was a standard central wing tank with a total tank volume of 0.48 m3and loaded with 64 L of JP-8 fuel.The results showed that the GOBIGGS prototype had an excellent performance.Approximately 7 min after system initiation, the oxygen concentration in the gas phase of the fuel tank was inerted to less than 12%. After 20 min of operation, the oxygen concentration in the gas phase space was reduced to 0%. If the separation membrane is used, it is impossible to achieve such a low oxygen concentration, and the separation efficiency is extremely low. At the stage of subduction, the oxygen concentration increases slightly. However, the maximum concentration is less than 4%, which can readily satisfy the requirements of oxygen concentration control in inerting.In 2011,at the China Lake Naval Weapons Testing Center, the GOBIGGS prototype was tested on all the flight processes including taxi, takeoff,climb,cruise,descent and landing on the UH-60 helicopter and the A-3 attack aircraft. The results showed that the compensation loss was lesser than that of the OBIGGS.20,21In addition, Shao et al. inerted the fuel tank with N2and CO2,and the results showed that the effects of the two gases were quite different.22At present, this technology is still in the experimental stage.Once the technology is mature,GOBIGGS may become a better alternative than OBIGGS,comparable to the HFM-OBIGGS.23,24
Different from HFM-OBIGGS,fuel type has a huge impact on GOBIGGS.25This is due to the different fuel vapor pressures of different fuels(as can be seen from Table 126),and fuel vapor pressure determines the amount of substances involved in the reaction, which in turn affects the amount of oxygen consumed and the amount of carbon dioxide generated.However, the influence of the fuel type on the performance of the GOBIGGS has been neglected in current research. But with the development of air traffic,aircraft often travel to and from opposite ends of the world, and it is inevitable to use different types of aviation fuels. Therefore, it is particularly important to consider the influence of the difference in the physical and chemical properties of different fuels on the inerting system.The influence of the fuel type is a result of primarily the following aspects: (A) different components and calorific values of different fuels lead to a different reaction intensity and heat release;27(B)different fuel densities lead to a different amount of gas dissolved in the fuel;28(C) different components of fuel lead to different flash points and ignition points, which affect the flammability limit;29,30(D)the vapor pressures of different fuels are different, and thus, the partial pressures of gases in the gas phase space and the concentration of reactants are alsodifferent.31In addition,the GOBIGGS requires a cooling medium to take the heat of reaction away, and the problem of water removal before the tank must be considered.
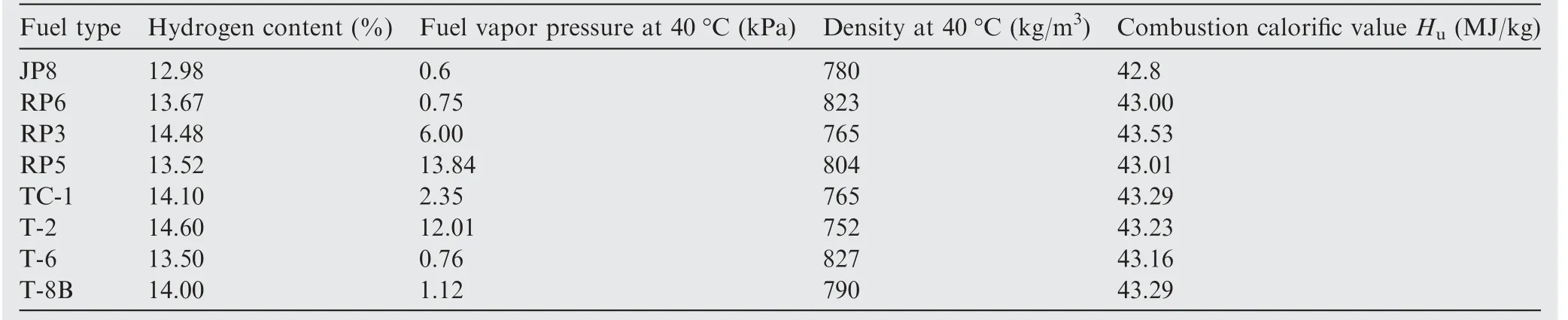
Table 1 Physicochemical properties of fuel types.26
Considering these aspects,a catalytic inerting system flow is designed in this study.Based on some reasonable assumptions,a mathematical model of the catalytic inerting is established.Four fuel types are selected to solve the model. The effects of the oil loading rate, fan suction flow rate and catalytic efficiency on the inerting process are analyzed,and the amount of cooling gas required and precipitation water of the GOBIGGS are calculated to provide a basis for the design and improvement of future systems.
2. Principle and assumption of inerting system
The flow chart of the oxygen consumption inerting system designed in this study and the flow relationship of each part are shown in Fig.1.The basic principle is that the mixture containing fuel vapor, oxygen, nitrogen, carbon dioxide and a small amount of gaseous water vapor is extracted from the gas phase of the tank. The mixed gases are heated to ignition temperature in an electric heater and later reacted in a reactor to form Oxygen-Depleted Air (ODA) consisting mainly of nitrogen and carbon dioxide. The mixed inert gas is cooled by rammed air or fuel in the cooler, dehydrated and injected back into the upper gas space of the tank, thereby achieving the purpose of reducing the oxygen concentration and inerting the fuel tank.
The following basic assumptions are made in this study:
(1) Fuels are composed of complex hydrocarbons;however,this paper holds that the molecular formula of the fuels can be expressed as CaHbwithout considering trace elements such as oxygen, sulfur and nitrogen in the fuel molecules.
(2) Regardless of the heat transfer between the tank and the external environment,and between the gas and the fuel,the temperature in the tank is constant at 40°C. It is considered that the gas temperature in the catalytic reactor is constant at 200°C,the heat generated by the reaction is dispersed by the cooling air, and the mixed inert gas at the outlet of the cooler is cooled to 40°C.
(3) The influence of flow resistance is not considered.
(4) The reaction is complete and produces only CO2and H2O.
(5) The heat transfer between the air and fuel is not considered.
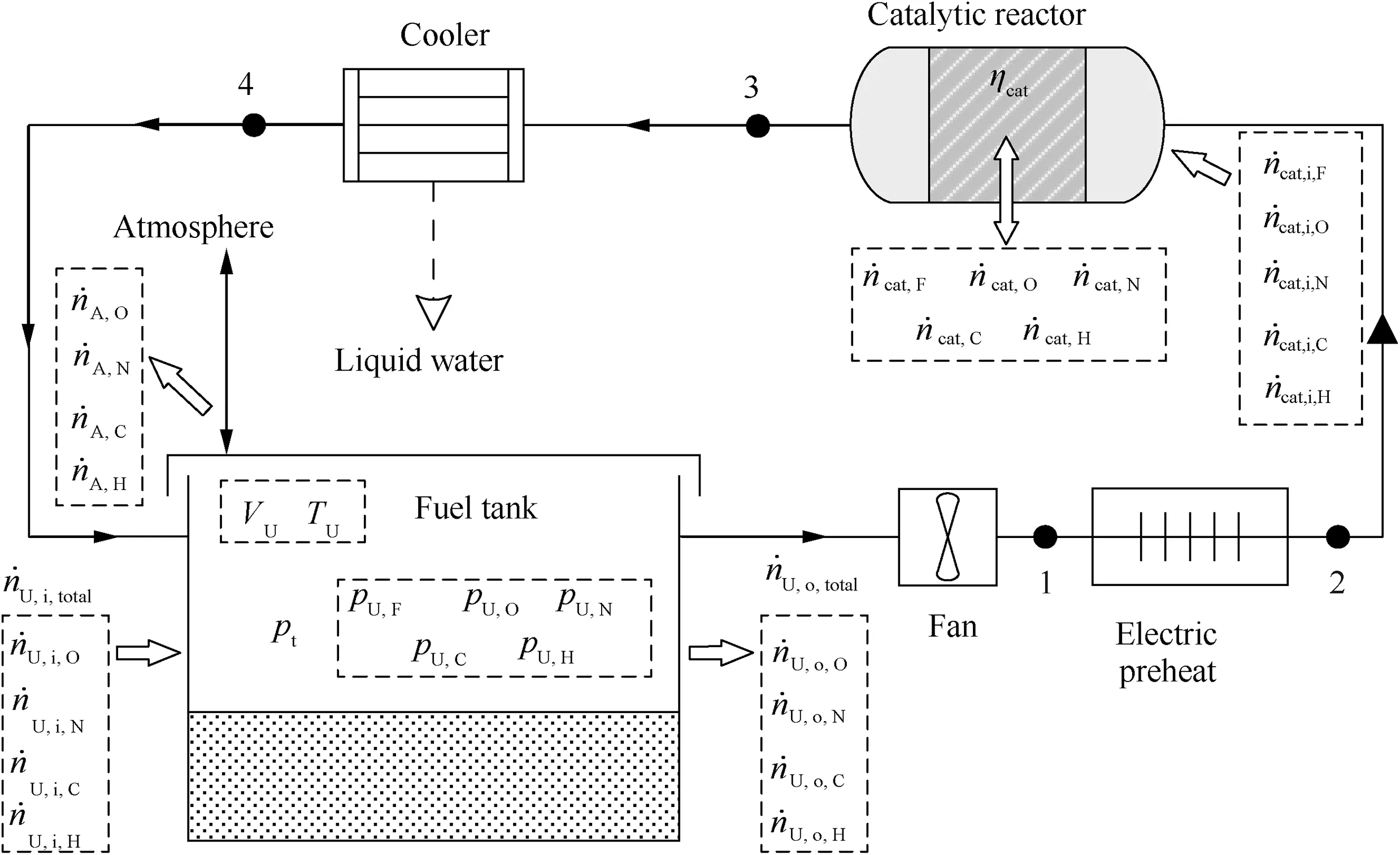
Fig. 1 Flow chart of catalytic inerting system.
(6) The inerting process is performed on the ground. The total pressure of the tank is consistent with the ambient pressure.The partial pressure of the fuel vapor is considered as the saturated vapor pressure, and the saturated vapor pressure is calculated as the Reed vapor pressure,ignoring the difference between the real vapor pressure and Reed vapor pressure at low gas-liquid ratio.
3. Mathematical model
3.1. Catalytic reactor
In a catalytic reactor, the reaction process satisfies the following relationship:
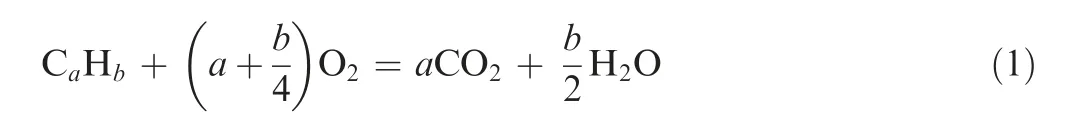
However, even if the amount of oxygen is considered sufficient, not all the fuel in the catalytic reactor can be converted to carbon dioxide and water; thus, the efficiency of the catalytic reactor, ηcatis defined as

(1) If the oxygen entering the catalytic reactor is sufficient to react with the fuel vapor, that is,

the molar flow of the fuel vapor consumed in the catalytic reactor is

(2) If the oxygen entering the catalytic reactor is insufficient to react with the fuel vapor, that is,

then, the oxygen consumed in the catalytic reactor satisfies

The relationship between the fuel vapor and amount of oxygen consumed in the catalytic reactor, and the molar flow relationship between the generated carbon dioxide and water vapor are satisfied:

The reaction exothermic Pris:

The required cooling air flow in the reactor ˙qrcan be calculated by the following formula:
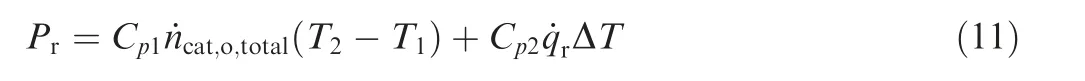
where ˙ncat,i,Fand ˙ncat,i,Odenote the molar flow rates of the fuel vapor and oxygen entering the catalytic reactor, respectively(mol/s); ˙ncat,Fand ˙ncat,Odenote the molar flow rates of the fuel vapor and oxygen consumed in the reactor, respectively(mol/s); ˙ncat,C, ˙ncat,Hdenote the molar flow rates of the carbon dioxide and water vapor generated in the catalytic reactor(mol/s); ˙ncat,o,totaldenotes the total molar flow rates of the gas at reactor outlet;Cp1,Cp2denote specific volume of the reaction gas and the cooling air (J·mol-1·°C-1); T1, T2denote the temperature at the inlet and outlet of the reactor(°C);ΔT denotes the temperature difference of cooling air (°C).
3.2. Cooler
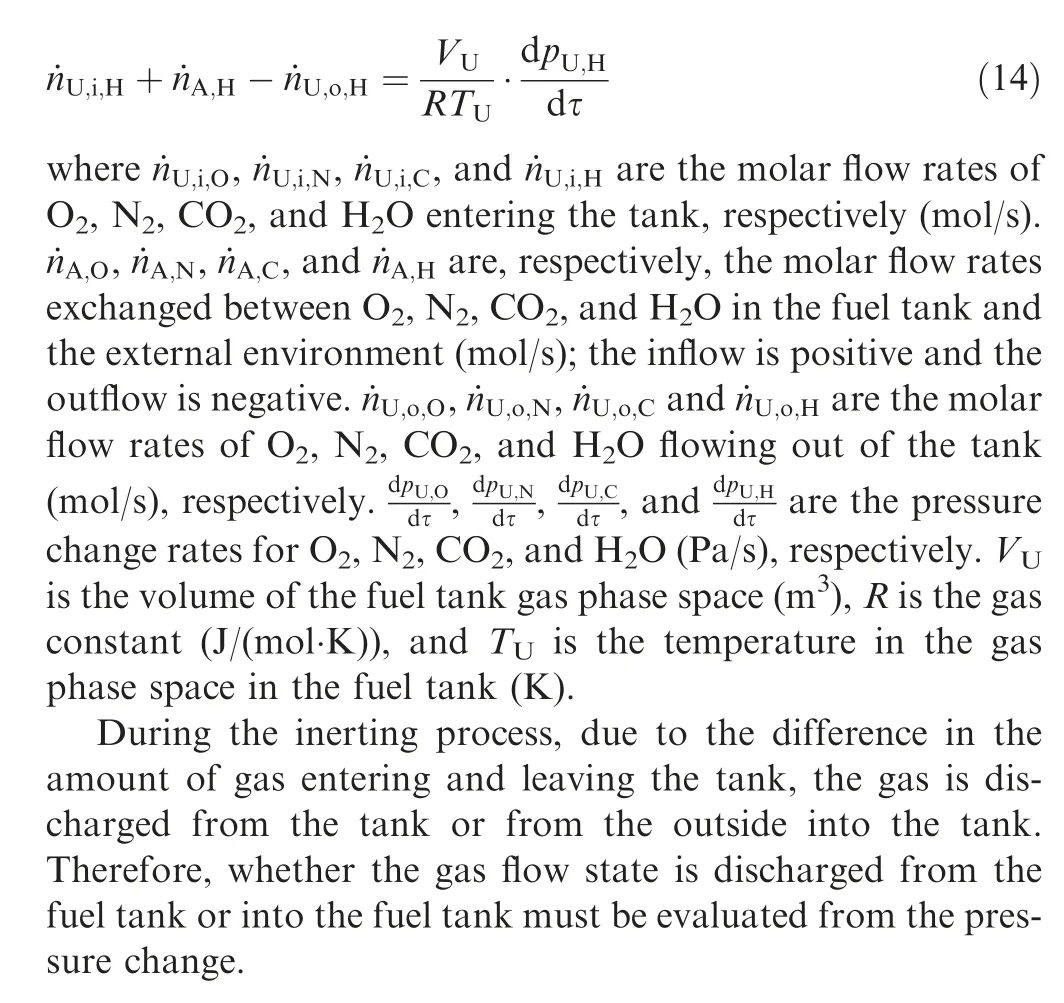
The schematic psychrometric chart of the wet air in the process of air heating,reaction and cooling in the oxygen consumption inerting system is shown in Fig. 2. The state point is the same as that in Fig. 1. The dew point temperature (Td1or Td2) can be determined considering the air state point 3 after the reactor. The outlet gas temperature of the cooler is the same as the ambient temperature T (unit: K), and the outlet air state of the cooler can also be determined.
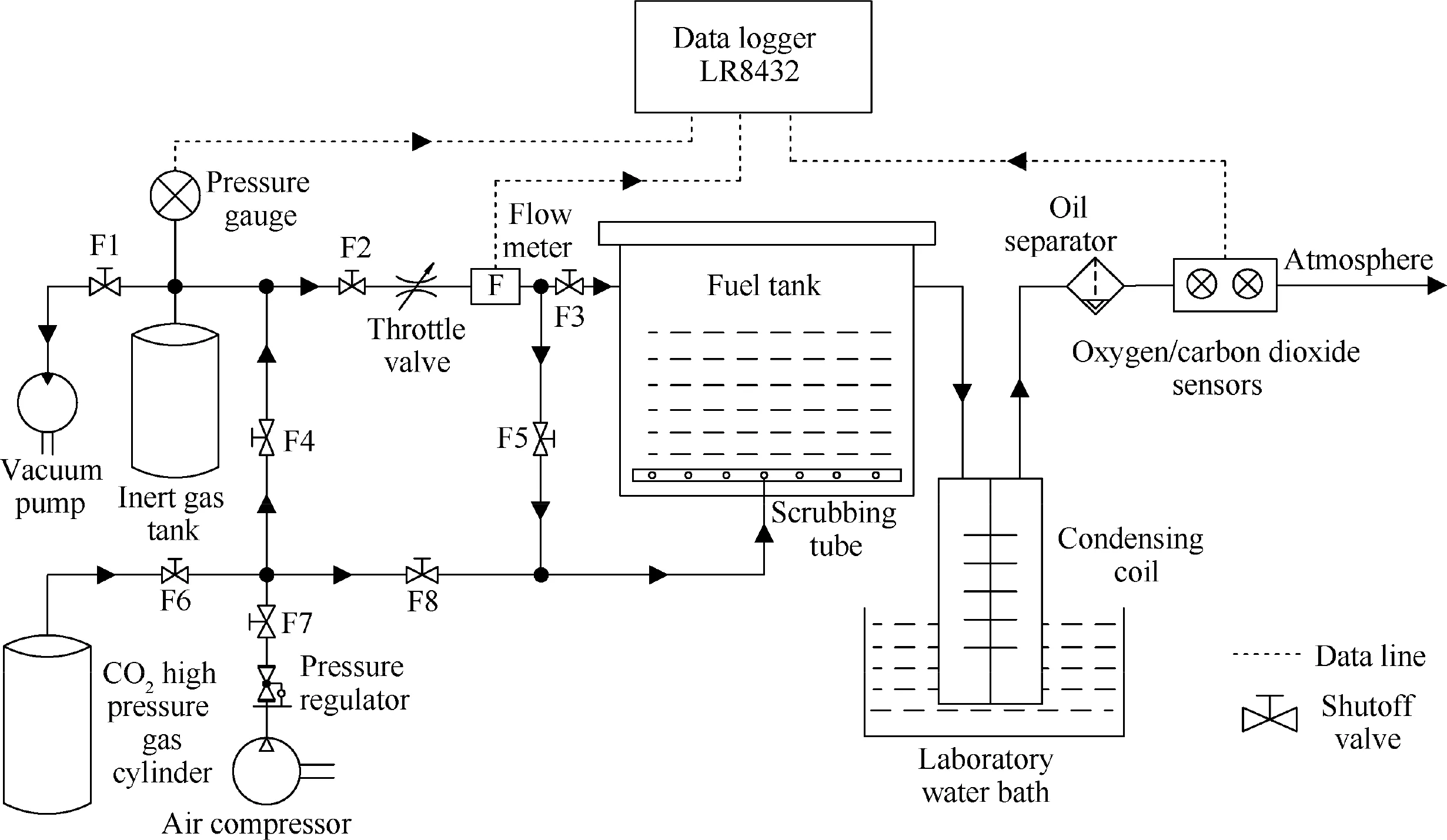
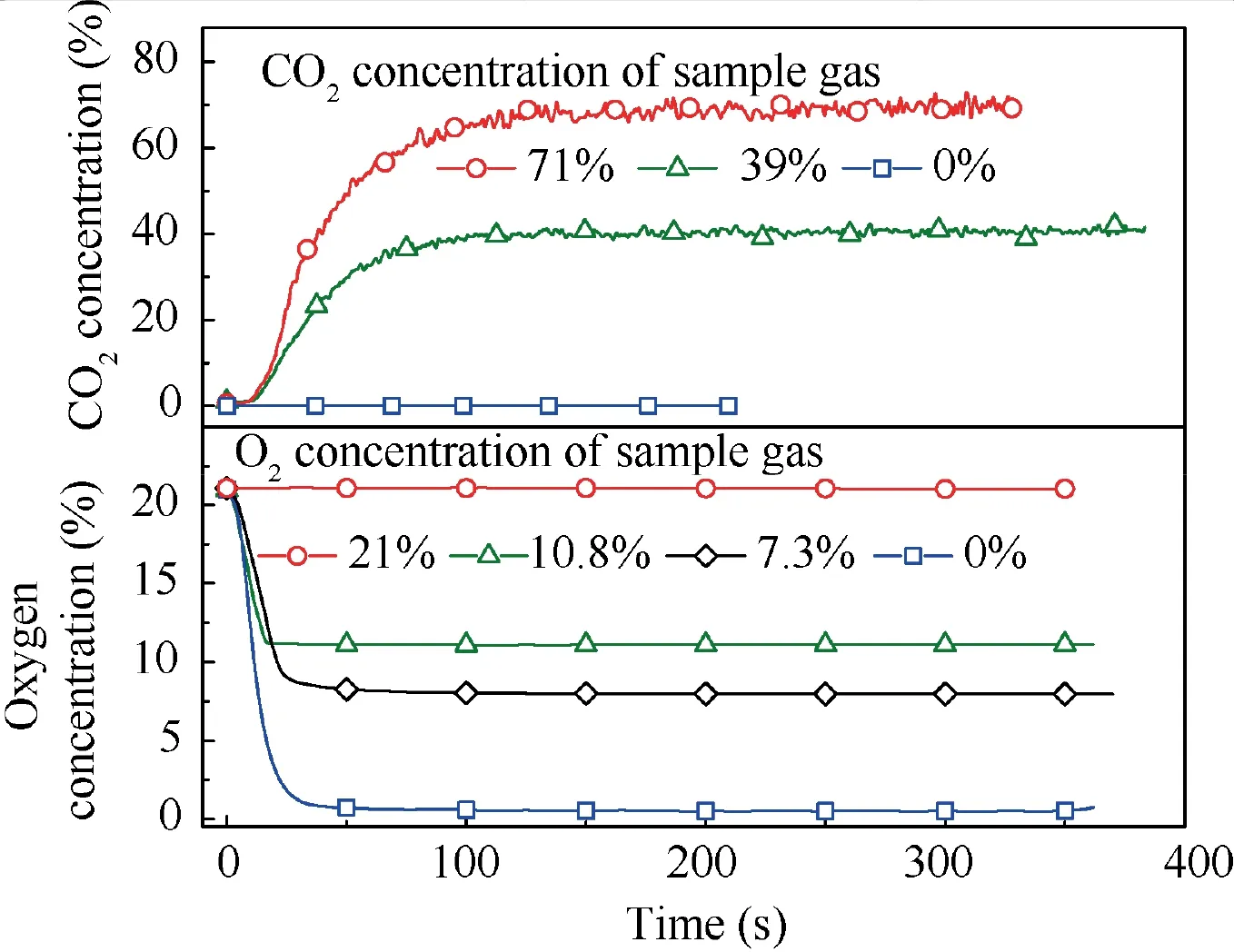
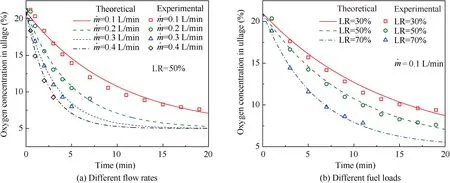
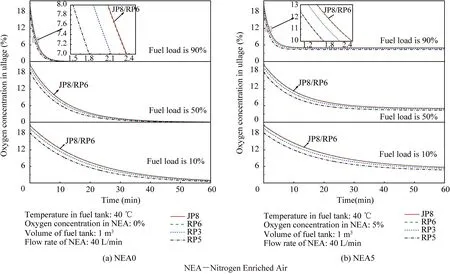
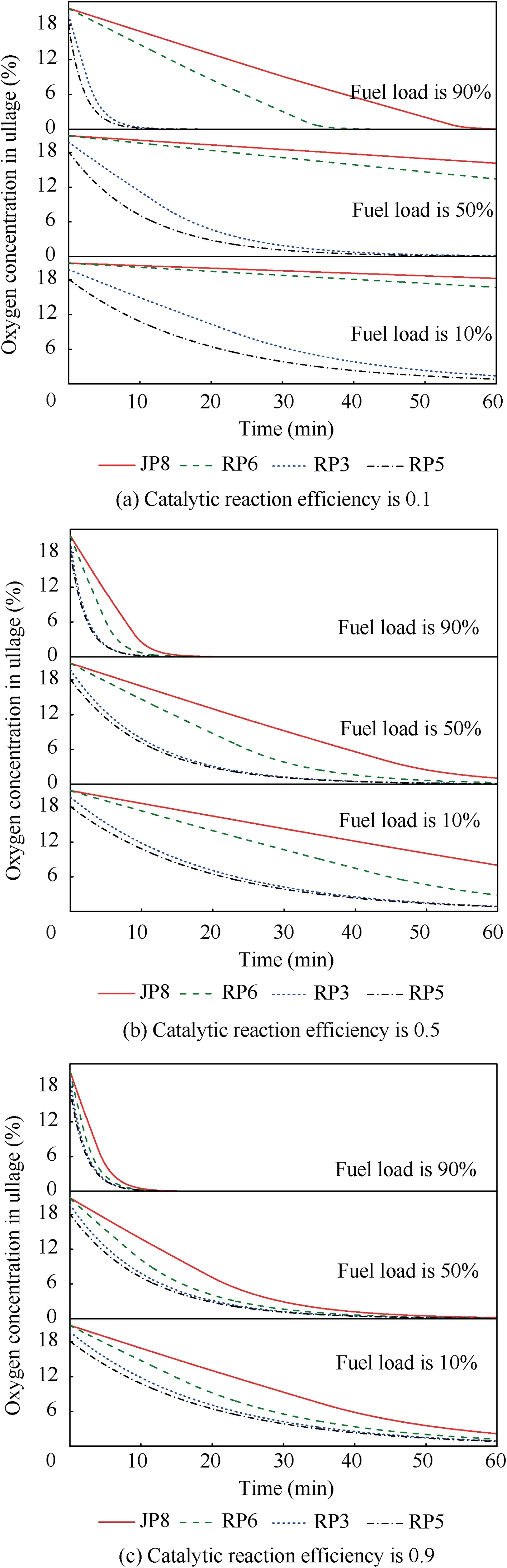
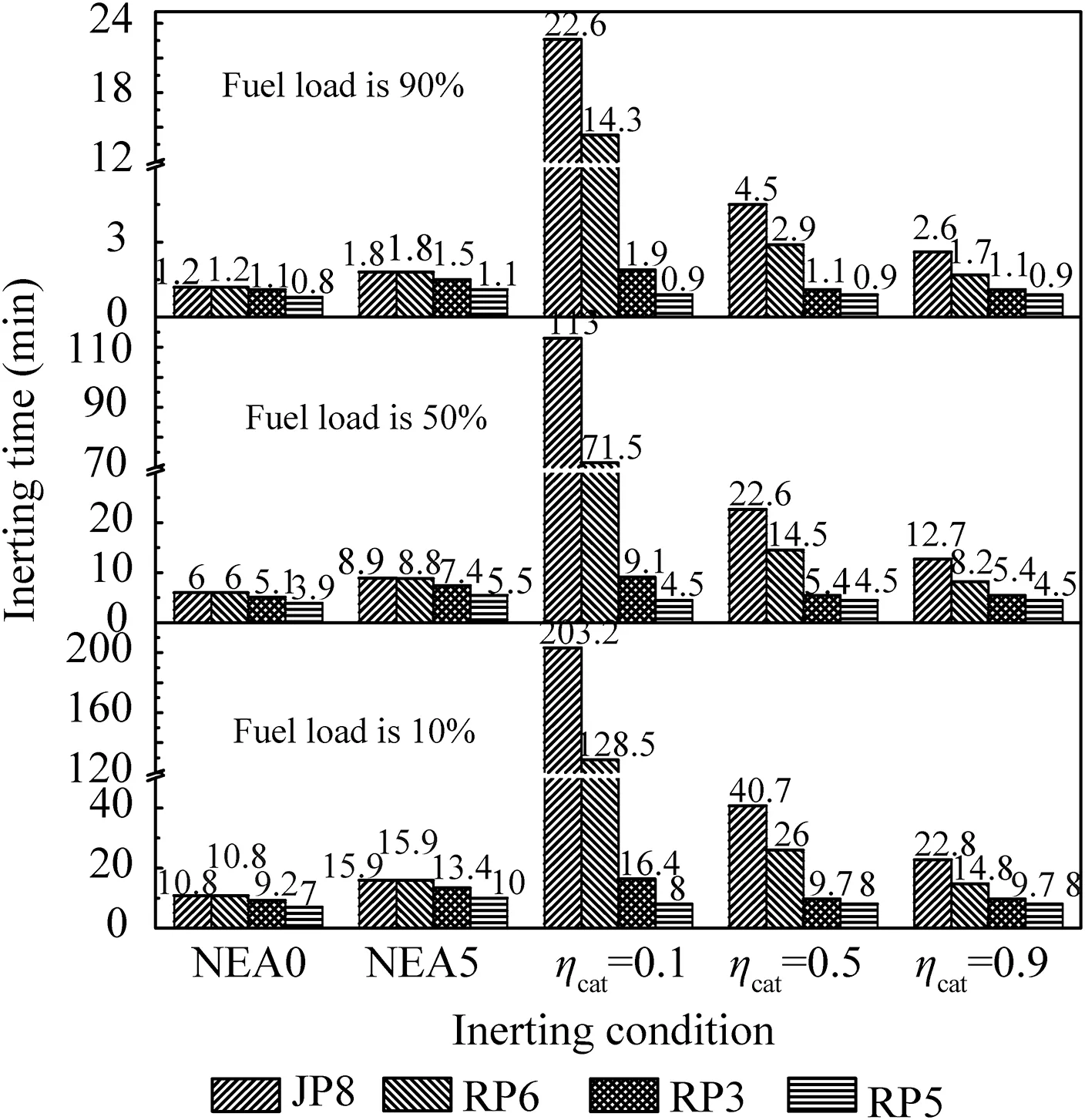
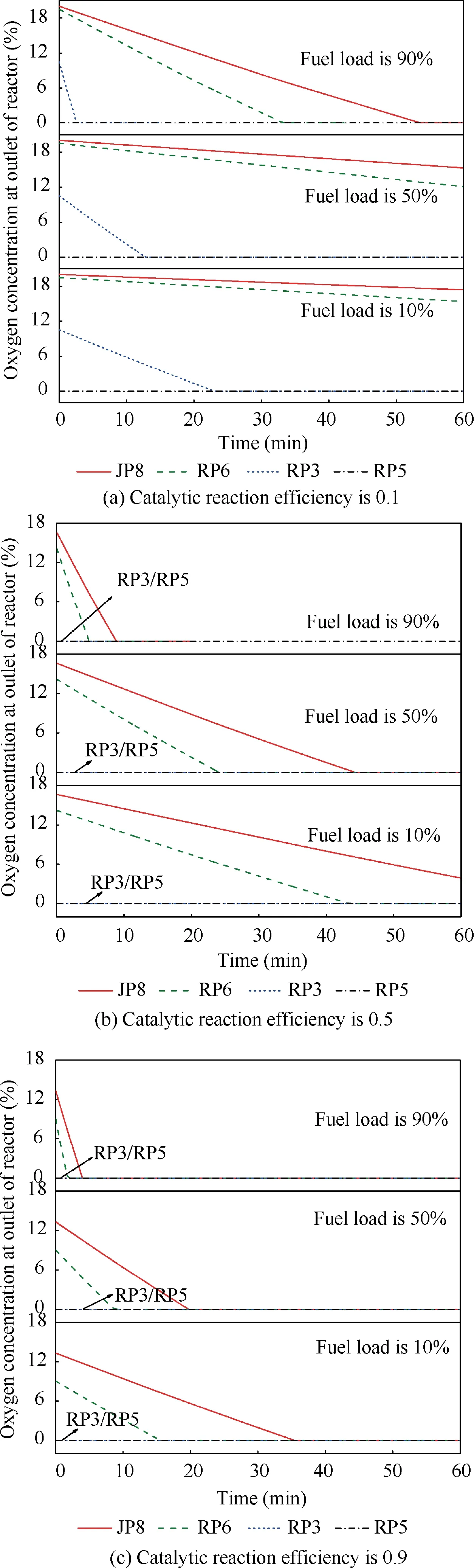
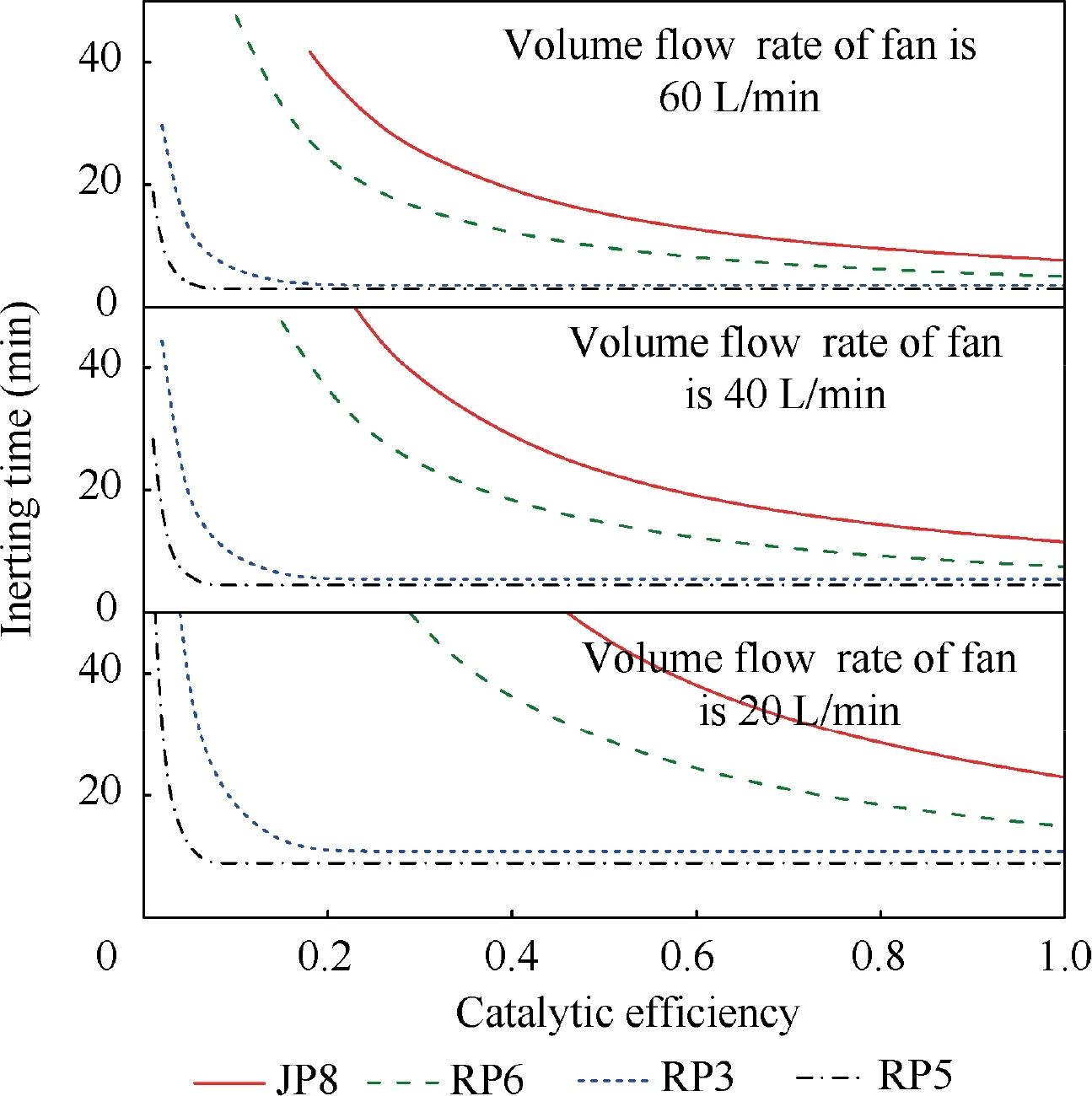
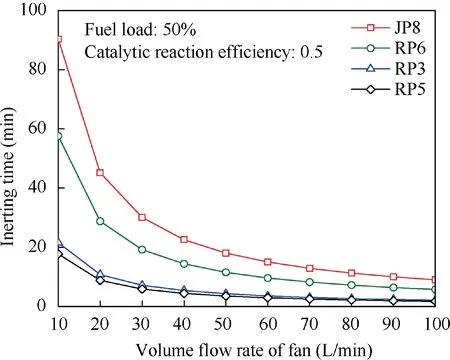
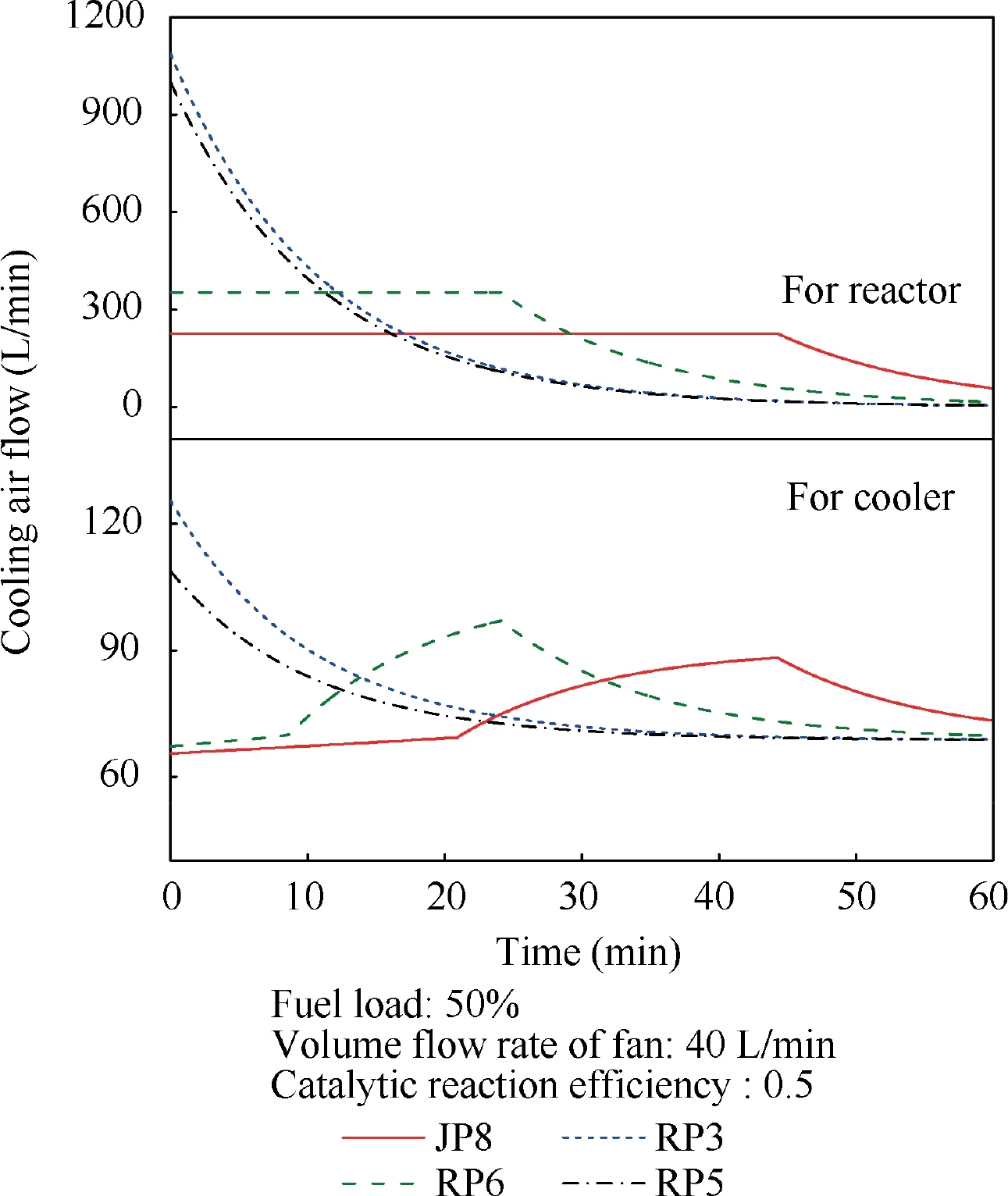
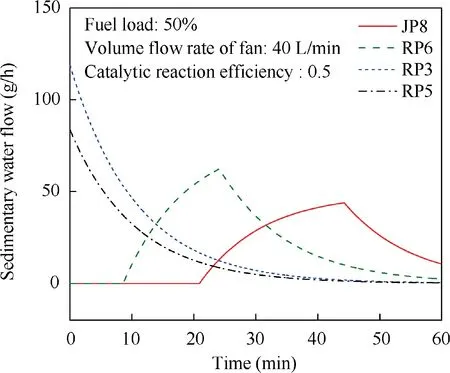
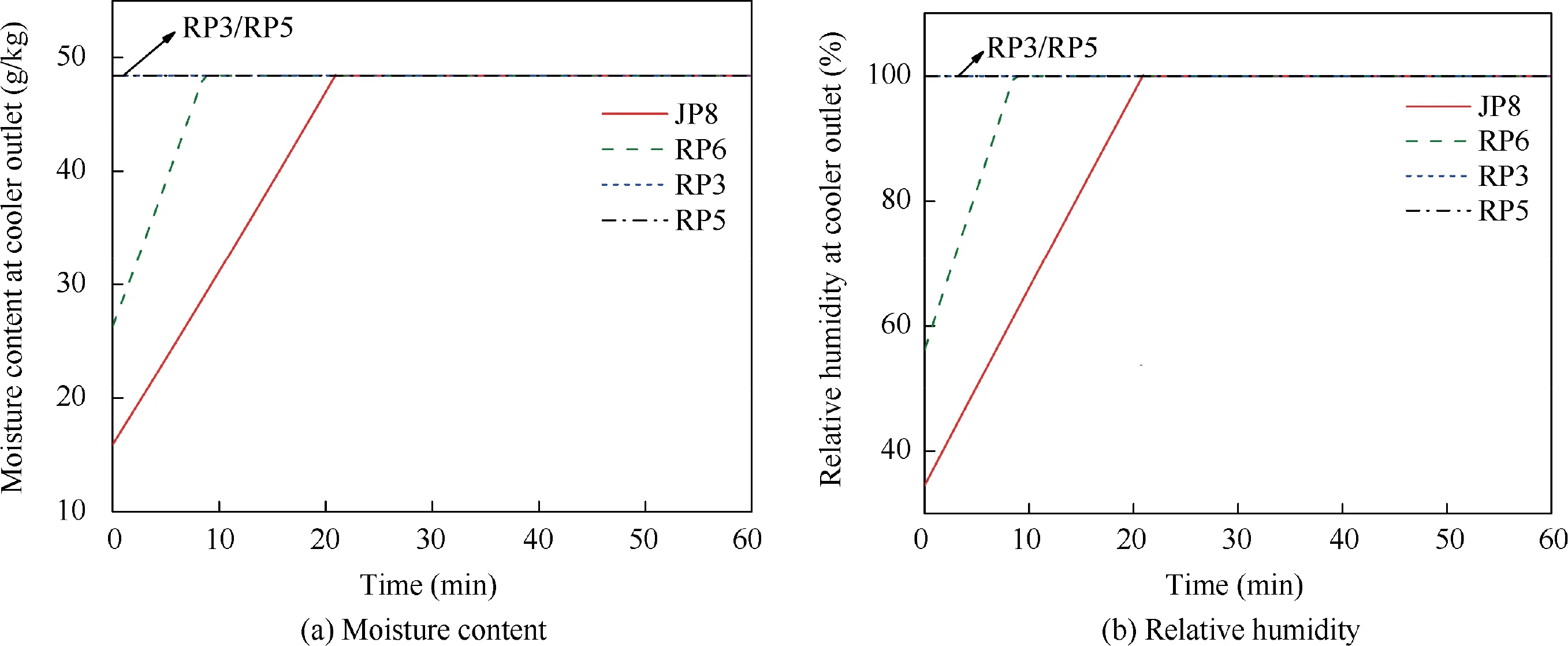
When T>Td1, as shown in Fig. 2(a), no water precipitation occurs in the cooler. When T The required cooling air flow in the cooler ˙qcand water amount released from the cooler can be calculated by the following formula: where ˙ncooler,i,total, ˙ncooler,o,totaldenote the molar flow rate of the gas at inlet and outlet of cooler (mol/s); ˙ncooler,i,h, ˙ncooler,o,h,˙nhdenote the molar flow rate of inlet and outlet of cooler and separated water vapor;hcooler,i,hcooler,odenote the enthalpy value of gas at inlet and outlet of cooler(J/kg);Mairdenotes the relative molecular mass of air (kg/mol). The fuel gas phase space is used as the control body to establish a mass conservation equation for oxygen, nitrogen,carbon dioxide, water vapor and other components.Because of the ground performance study of the inerting system, the dissolved and escaped gas in the fuel can be neglected as follows: Fig. 2 Schematic psychrometric chart of catalytic inerting system. (1) When the gas flowing into the tank is sufficient to pressurize the tank, the following relationship exists: Subsequently, the gas in the fuel tank is discharged to the external environment in a proportional manner, and the amount of exhaust gas satisfies the following relationship: (2) When the gas flowing into the fuel tank is insufficient to pressurize the fuel tank,the following relationship exists: Subsequently, the external gas flows into the tank, and the inflow gas flow is satisfied: where xA,Oand xA,Nare the volume fraction of oxygen and nitrogen in the air, respectively. At the same time,the sum of the partial gas pressure in the tank is the same as the pressure of the external environment,pt,which satisfies Considering that the development of catalysts in the reactor is not yet mature, the performance of the reactor is determined by defining the catalytic efficiency. Therefore, it is difficult to construct a complete system closed loop experimental bench. In view of this aspect, the fuel tank model of the system core is verified according to the existing test conditions.A fuel tank ullage washing apparatus was constructed, and its flowchart is shown in Fig. 3. The experimental system is shown in Fig. 4. A rectangular fuel tank with dimensions of 250 mm×50 mm×180 mm (L×W×D) is employed; the jet fuel is RP-3, and the ambient temperature is 30°C. The experimental instruments include a high pressure CO2/N2cylinder, an inert gas tank, a pressure transducer (HSTL-800), a vacuum pump (FY-1H-N), an air compressor, a pressure regulator (IR2000-02), O2and CO2concentration sensors (MAX250B, COZIR-W), a water bath (DC-8030), a condensing coil, and an oil separator. The parameters and accuracy of the instruments utilized in the experiment are listed in Table 2. Each experiment consists of three steps: the preparation of the ODA, ullage washing, and fuel scrubbing using air. Fig. 3 Schematic diagram of experimental apparatus. Fig. 4 Experimental system. Table 2 Experimental equipment and parameters. (1) First, the inert gas tank is evacuated with a vacuum pump and filled with a specific proportion of air and CO2using the air compressor and CO2high pressure gas cylinder. (2) After the pressure reading in the inert gas tank stabilizes,the F2 and F3 shutoff valves are opened, and the flow rate is controlled to a specific value by adjusting the throttle. Subsequently, the ODA is directly introduced into the ullage to vent the ullage air out of the fuel tank;the outflow goes through the condenser and oil separator, and subsequently flows into the O2/CO2measurement device. (3) After reading the data, the bottom of the fuel tank is connected to the air compressor, and the air is used to scrub the fuel to remove the inert gas;this process is followed by the preparation for the next experiment. The O2and CO2sensors are calibrated using sample gases before the sensors are actually used. The CO2concentrations are 0%, 39% and 71%, whereas the O2concentrations are 0%, 7.3%, 10.8% and 21%. The calibration results are plotted, as shown in Fig. 5. The maximum deviation is within 5%for the CO2sensor and 0.7%for the O2sensor,indicating that the accuracy of the sensors is acceptable. Fig. 5 Calibration of O2/CO2 sensor. Using the RP3 fuel with ODA(5%O2,19%N2,76%CO2),experimental and theoretical calculations were carried out under different fan flow rates ( ˙m) and fuel loading rates(LR). The results of the comparison of the oxygen concentration in the ullage are shown in Fig. 6. It can be seen that the experimental and calculation results are mostly in agreement.The average relative error between theoretical and experimental results is 3.2%. It is thus considered that the model calculation results are highly reliable and can be used for further research calculations. To compare the influence of fuel types on the inertia modes of the HFM-OBIGGS and GOBIGGS, based on the HFMOBIGGS model in the literature,32a helicopter fuel tank with a volume of 1 m3was selected.From the first four types of aviation fuel listed in Table 1,pure nitrogen gas NEA0 and NEA5 with an oxygen concentration of 5% were used to wash the fuel tank. The variation of oxygen concentration in the gas phase is shown in Fig. 7. Considering the presence of fuel vapor, the initial oxygen concentration in the upper part of the tank is slightly less than 21%, and the inerting rate of NEA0 is higher than that of NEA5. A larger fuel loading rate corresponds to a smaller volume of gas phase space and higher rate of decrease of the oxygen concentration. In addition, there exist some differences in the time when the four fuels reach the safe oxygen concentration of 12%.Among these fuels, JP8 and RP6 take the maximum time,while the RP5 fuel takes the shortest time, because for the HFM-OBIGGS, the fuel vapor pressure of different fuels is the main factor affecting the inerting effect if the solubility of gases are ignored, and a larger fuel vapor pressure corresponds to a smaller oxygen content in the gas phase space and higher rate of displacement of the fuel tank.It can be concluded that the fuel type affects the performance of the HFMOBIGGS to a certain extent, but the effect is not significant. Based on the previous mathematical model,the GOBIGGS is solved.First,the relationship between the oxygen concentration in the ullage and the time under different oil loading rates is presented, as shown in Fig. 8. Similar to in the HFMOBIGGS, the RP5 takes the shortest time, and a higher fuel vapor pressure corresponds to a shorter inerting time. Fig. 6 Comparison of theoretical calculation and experimental results of tank washing. Fig. 7 Effect of fuel types on HFM-OBIGGS inerting system. To more clearly compare the difference in the performance of the two systems corresponding to different fuels, the statistical inerting time of 12% was considered, as shown in Fig. 9.It can be seen that in the GOBIGGS, the inerting rate of different fuels varies considerably. For example, when the catalytic efficiency is 0.5 and the loading rate is 50%, the time difference between the JP8 and RP5 inerting to 12% is 18.2 min;however,for the HFM-OBIGGS,the corresponding difference is only 3.3 min. In addition, for the GOBIGGS, a smaller fuel loading rate corresponds to slower inerting. For example, when the catalytic efficiency is 0.5, the inerting time for the JP8 fuel at the three fuel loading rates is 40.7, 22.6, and 4.5 min. A smaller fuel rate corresponds to a larger difference in the inerting time required for different fuels. Taking RP5 and JP8 as examples, the inerting times at the three oil loading rates are 32.7, 18.2, and 3.6 min. In addition to the difference in the volume of the gas phase occupied by the fuel vapor, the most important reason is that the reaction intensity is directly determined by the vapor pressure of the fuel when different fuels are used. When the fuel load rate is small, the volume of the gas phase space increases, and the amount of oxygen needed to be replaced by the inert gas increases; consequently, the inerting rate decreases,which in turn affects the oxygen concentration participating in the reaction, as can be noted from the variation of the oxygen concentration at the outlet of the catalytic reactor, shown in Fig. 10. For the JP8 and RP6 fuels, a smaller fuel loading rate means that more oxygen is available in the catalytic reactor. Because the RP3 and RP5 fuels have a higher fuel vapor pressure, when the catalytic efficiency is 0.5, the fuel vapor is excessive, and no oxygen is present at the outlet of the reactor. More importantly, the catalytic efficiency has a significant impact on the inertia performance.A higher catalytic efficiency corresponds to lesser oxygen content at the outlet of the reactor,and the oxygen concentration at the upper part of the tank decreases at a higher rate. Fig. 11 shows the relationship between the inerting time and the catalytic efficiency required for the inerting of the fuel tank to 12% for the four aviation fuels at different fan flow rates. It can be seen that for JP8 and RP6, a greater catalytic efficiency corresponds to lesser inerting time; however, for the RP3 and RP5 fuels, when the catalytic efficiency is higher than a certain value, the amount of oxygen involved in the reaction is insufficient,and the inerting time no longer changes with the catalytic efficiency. The relationship between the inerting time and the catalytic efficiency of different fuel types is considerably different, due to the different fuel vapor pressures. When the catalytic efficiency is constant, the relationship between the inerting time and the fan flow rate is as shown in Fig. 12. A larger flow rate of the fan corresponds to lesser inerting time required, and the inerting time of the four fuel types is inversely proportional to the flow rate of the fan.That is,when the flow rate of the fan is n times the original flow rate,the inerting time is 1/n the original time. Unlike the HFM-OBIGGS, the GOBIGGS needs to provide additional cooling air to remove the reaction heat and to cool and precipitate the water vapor after the reaction.Assuming that the temperature difference between the inlet and outlet of the cooling air is 100°C, the amount of cooling air needed in the reactor and cooler and the amount of water released from cooler are calculated, as shown in Figs. 13 and 14, respectively. Fig. 8 Effect of fuel types on GOBIGGS inerting system(volume flow rate of fan: 40 L/min). Fig.9 Time required to inert to 12%under different conditions. Fig. 13 indicates that for the RP3 and RP5 fuels, oxygen is always insufficient in the reactor, and the reaction intensity decreases with the decrease in the oxygen concentration in the tank. Because RP3 has a lower vapor pressure than that of RP5, the amount of oxygen that can participate in the reaction is relatively large, and thus the amount of cooling air required is higher than that for RP5. For JP8 and RP6, the oxygen is sufficient at the beginning, the reaction intensity is unchanged, and the amount of cooling air required for the RP6 fuel is higher than that required for JP8. Later, the amount of oxygen becomes insufficient,and the amount of cooling gas in the reactor also decreases gradually. Combined with Fig. 14, it can be noted that the variation trends of the cooling air and precipitated water in the cooler is the same, and the cooling gas and precipitated water required for the RP3 and RP5 fuels decrease gradually.In contrast,for the JP8 and RP6 fuels,the dew point temperature of the cooler outlet gas is higher than the ambient temperature for a certain period of time,and no water precipitation occurs.As the process progresses, liquid water begins to appear, and the amount of cooling air required increases rapidly until the fuel vapor is excess. Subsequently, the reaction begins to weaken, and the amount of cooling gas and water released from the cooler begins to decrease gradually. It can also be seen from Fig. 13 that the amount of cooling air required in the reactor is considerably greater than the amount of cooling air required in the cooler. Fig. 15 shows the humidity and relative humidity of the air at the inlet of the tank. For the JP8 and RP6 fuels,with a low fuel vapor pressure, no water precipitation occurs at the beginning. The humidity and relative humidity increase gradually. After the dew point temperature increases, the outlet of the cooler is always saturated, the relative humidity is 100%, and the humidity does not change. For the RP3 and RP5 fuels, the gas entering the tank is saturated at the place of reaction due to the occurrence of constant water precipitation. Fig. 10 Effect of fuel type on oxygen concentration at catalytic reactor outlet (volume flow rate of fan: 40 L/min). Fig. 11 Relationship between inerting time and catalytic efficiency under different fan flow rates (fuel load: 50%). Fig. 12 Relationship between inerting time and fan flow rate. Fig. 13 Amount of cooling air required in cooler and reactor. Fig. 14 Amount of water released from cooler. Fig. 15 Gas state parameters at cooler outlet (fuel load: 50%; volume flow rate of fan: 40 L/min; catalytic reaction efficiency: 0.5). The fuels JP8,RP6,RP3 and RP5 were selected as the research objects. The effects of key parameters such as the oil loading rate, catalytic efficiency and fan flow rate on the inerting efficiency were analyzed and compared with those of the hollow fiber membrane system, and the following conclusions were derived: (1) The fuel vapor pressure not only affects the gas phase oxygen integral fraction of the tank,but also determines the proportion of the fuel vapor and oxygen entering the catalytic reactor. Therefore, the research shows that the influence of the fuel type on the performance of the GOBIGGS is considerably greater than the corresponding influence on the HFM-OBIGGS performance. (2) When excessive oxygen is present in the reactor,a higher fuel vapor pressure corresponds to a higher inerting rate.The relative inerting rate of the four fuels in the GOBIGGS is RP5>RP3>RP6>JP8. In addition,a higher fuel loading rate corresponds to a larger inerting rate. (3) A higher gas flow rate drawn from the tank means that less time is required to reach the safe oxygen concentration of 12%, and the inerting time of the four fuels is inversely proportional to the flow rate of the fan. In addition, a higher catalytic efficiency corresponds to a shorter inerting time. (4) For the RP3 and RP5 fuels with a higher fuel vapor pressure, liquid water is precipitated at the beginning of the reaction. For JP8 and RP6, sufficient oxygen is present, and no water precipitation occurs at the reaction site. With the progression of the inerting process,the liquid water precipitation and reaction intensity decrease successively. However, the mathematical model established in this study is relatively limited. For example, the performance of the catalytic reactor is characterized only by the constant reactor efficiency; in particular, the temperatures before and after the reactor are set to a fixed value, which is different from the actual situation. Therefore, the mathematical description of the catalytic process in the reactor and the performance of the heat transfer system should be further considered in future work. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgement This work was supported by National Natural Science Foundation of China Civil Aviation Joint Fund (No.U1933121),Postgraduate Research & Practice Innovation Program of Jiangsu Province (No.KYCX19_0198). The Fundamental Research Funds for the Central Universities and Priority Academic Program Development of Jiangsu Higher Education Institutions.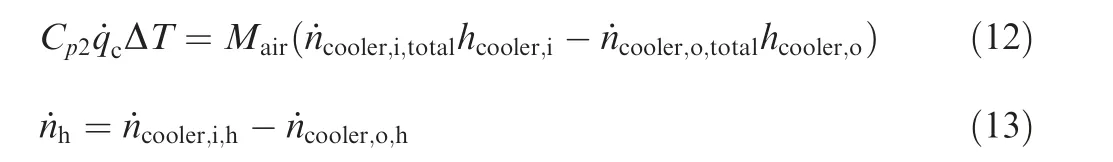
3.3. Tank
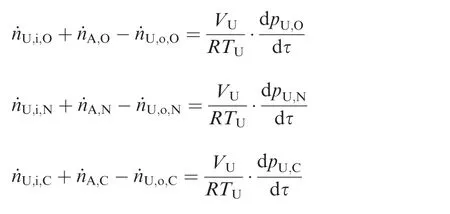
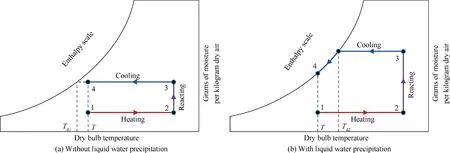
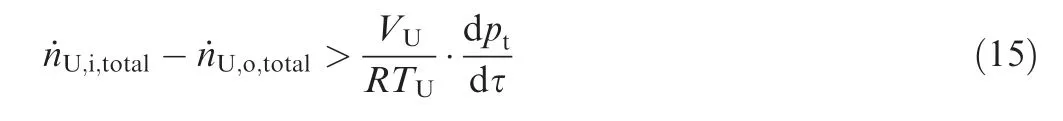

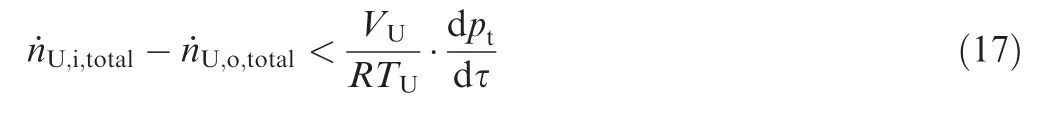


4. Model validation
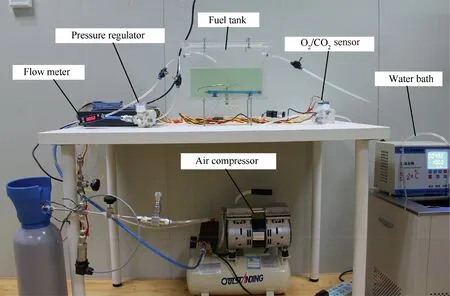

5. Results and discussion
6. Conclusions
 CHINESE JOURNAL OF AERONAUTICS2021年3期
CHINESE JOURNAL OF AERONAUTICS2021年3期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- Criteria for hypersonic airbreathing propulsion and its experimental verification
- Effect of scavenge port angles on flow distribution and performance of swirl-loop scavenging in 2-stroke aircraft diesel engine
- Ship detection and classification from optical remote sensing images: A survey
- Variational method based robust adaptive control for a guided spinning rocket
- Inertial parameter estimation and control of non-cooperative target with unilateral contact constraint
- Theoretical modeling of vectoring dual synthetic jet based on regression analysis
