Monitoring lime and cement improvement using spectral induced polarization and bender element techniques
Bte Bte, Junnn Co, Chi Zhng, N Ho, Song Wng
a Institute of Geotechnical Engineering, College of Civil Engineering and Architecture, Zhejiang University, Hangzhou, 310058, China
b Department of Civil and Environmental Engineering, Colorado School of Mines, Golden, Colombia, 80401, USA
c Department of Geology, University of Kansas, Lawrence, KS, 66045, USA
d Department of Civil Engineering and Construction Management, Georgia Southern University, Statesboro, GA, 30458, USA
Keywords: Particle size and distribution Spectral induced polarization (SIP)Bender element (BE)Cement hydration Complex conductivity Shear wave velocity Heterogeneity
ABSTRACT Spectral induced polarization (SIP) and bender element (BE) techniques show a high sensitivity to particle size,particle distribution and content of generated hydration products,which essentially govern the efficiency of ground improvement.In this context,both SIP and BE were integrated on a column setup to monitor the processes of lime and cement stabilization. A 5 mmol/L Na2CO3 solution was injected into the sand-lime mixture to produce CaCO3 precipitation,while deionized water was injected into the sandcement mixture to induce the hydration of cement.The average diameters of the precipitated particles or clusters were calculated from the relaxation time, which was a significant parameter of SIP signals, via the Schwarz equation. Two pairs of BE were used to demonstrate the heterogeneity of the product precipitation, which was probably caused by the location of the inflow and outflow on the SIP-BE column. SIP and BE showed the capability of nondestructively monitoring the spatiotemporal chemical evolution processes, which could be helpful for engineering applications.
1. Introduction
Lime and cement are the most commonly used chemicals, for ground improvements with the objectives of increasing the strength, bearing capacity, and resistance of soft soils, to decrease permeability and settlement, and to improve stability of the longterm performance of foundations (Croft, 1967; Bell, 1988, 1996;Krizek et al.,1992;Nelson and Miller,1992;Little,1995;Taha et al.,2002; Guthrie et al., 2007; Elandaloussi et al., 2019; James, 2020;Wang et al., 2020). Extensive studies include chemical reaction mechanisms of lime and cement in soils, physico-chemical factors affecting the strength of stabilized materials, and engineering concerns such as design process, construction techniques, and quality control (Diamond and Kinter, 1965; Ola, 1977; Sherwood,1993; Bell,1996; Katz et al., 2001; Kavak and Akyarlı, 2007; Tang et al., 2007; Tingle et al., 2007; Zheng et al., 2020). However, heterogeneities,both spatially and temporally,are still a major concern in the practice of using these materials. For the initial quality assessment after soil stabilization,previous studies focused on two in situ nondestructive methods:downhole P-wave velocity logging and spectral analysis of a surface wave (SASW) testing method(Madhyannapu et al., 2010). The SASW method is based on the dispersive characteristics of seismic surface waves of the Rayleigh type traveling in the vertically heterogeneous medium to obtain average shear wave velocity profiles in the measured soil areas(Nazarian and Stokoe, 1984). Testing results from these studies showed that both methods mentioned above could be good indicators of the strength and stiffness properties of the treated soils.However,particle size and distribution of the produced precipitates after treatment were also reported to have significant influences on the efficiency of the ground improvement and mechanical properties of geomaterials(Cao et al.,2019).In addition,the distribution pattern of the produced particles, i.e. suspended in the pore fluid,surface coating or contact cementation,also plays a significant role(Fernandez and Santamarina,2001;Cao et al.,2019).Until now,this particle-level information is still beyond reach with existing in-situ testing methods.Therefore,an efficient monitoring system that can measure the particle size and distribution, solid content, strength,stiffness and heterogeneous properties of soils after the ground improvement is highly desired.
The spectral induced polarization(SIP)technique has emerged as a nondestructive geophysical method for the monitoring of particle size and the distribution of a porous medium (Slater and Lesmes,2002; Atekwana and Slater, 2009; Zhang et al., 2012). It has been applied in many areas including process monitoring of abiotic induced carbonate precipitation(Wu et al.,2010),zero valence iron oxidation(Wu et al.,2005;Slater and Binley,2006),mortar,cement and concrete property evaluations (Kaouane, 2016; Khajehnouri et al., 2019, 2020), mineral precipitation in porous media (Zhang et al., 2012), and biofilm formation (Atekwana and Slater, 2009;Albrecht et al.,2011).A brief explanation and simple working principle of SIP are described as follows. Under alternating electrical fields,for a negatively charged particle surface,a locally alternating electrical field with phase lag could be introduced in the electrical diffuse layer due to the direction alignment between the electrical field and the negative charges of the particles (Sposito and Prost,1982; Bate and Burns, 2014; Chu et al., 2019). Since the inherent frequency of the local electrical field is completely out of phase with the frequency of the external alternating electrical field,a polarization (or relaxation) is induced (Hayt and Buck, 2005). SIP can be expressed with the complex conductivity(σ*)of a saturated porous medium.At low frequencies(<1000 Hz),σ*can be written as

where ω is the angular frequency; σelis the direct current (DC)conductivity of the porous medium caused by electrolytic conduction;are the frequency-dependent real and imaginary components of the surface complex conductivityrespectively;and i is the imaginary unit.The DC conductivity σelis governed by the DC conductivity of the pore fluid, porosity, pore size distribution and connectivity of the electrolyte filled pore space(Archie,1942).Surface complex conductivity(σ*surf)relates to the mineralogy of the medium, particle size and electrical conductivity of the pore fluid (Breede et al., 2011). Alternatively, the Cole-Cole model is also used to describe the complex conductivity(σ*) of geomaterials (Cole and Cole, 1941; Pelton et al.,1978; Wu et al., 2010):

where σ0is the DC conductivity; m is the chargeability and represents the polarization magnitude,and m = 1-σ0/σ∞,in which σ∞is the conductivity at high frequency; c is the shape parameter,ranging from 0.2 to 0.8 for unconsolidated soils(Zhang et al.,2012);and τ is the mean relaxation time. τ and the magnitude of the SIP responses, which is revealed by the magnitude of imaginary conductivity, are the two significant properties for analysis. τ represents the average particle size (Schwarz, 1962), while the magnitude of imaginary conductivity represents the contents of the precipitation.τ can be expressed by

where fpis the frequency (Hz) when the peak value of σ′′is observed, d is the average particle diameter (m), and Dsis the diffusion coefficient (m2/s).
Alternatively, the bender element (BE) technique, which is a mature and effective nondestructive technique in measuring the shear wave velocity of geomaterials, is also sensitive to the presence of particles during lime and cement modification (Lee and Santamarina, 2005; Ferreira et al., 2011; Kang and Bate, 2016;Moon and Ku, 2016; Zhu et al., 2018; Bate et al., 2019). If the two aforementioned innovative techniques are combined in one testing system, the real time responses of the stabilization process with both lime and cement can be fully monitored and examined. In addition, many studies have been conducted on the hydration of cement paste previously (Carette and Staquet, 2016; Zhu et al.,2018; Ma and Kawashima, 2019). However, it was found that some of the methods were not quite reliable, especially on estimation of the initial and final settings (Ma and Kawashima, 2019).Therefore,SIP-BE,as a combined technique,holds high potentials to more accurately monitor the hydration process and better measure the hydration parameters of cement.
The objective of this study is to examine the feasibility of the SIP technique in monitoring the processes of lime and cement stabilization and unveil the fundamental mechanisms of soil improvement in terms of electrical and physicochemical properties of geomaterials. The following tasks are performed:(1)conduct lime and cement stabilization tests on coarse grained soils;(2)monitor the changing trends of complex conductivities and the shear wave velocities of geomaterials by SIP electrodes and BE; (3) verify and validate the reliability of the SIP and BE testing system; and (4)elucidate the fundamental mechanisms of lime and cement stabilization of soil based on SIP and BE testing results.
2. Materials and experimental methods
2.1. Materials
Ottawa 50-70 sand(U.S.Silica Holdings Inc.,Chicago,IL),type I/II Portland cement and standard hydrated lime (Ca(OH)2, Mississippi Lime Company) were used for this study. The diameter of 50%finer(D50),maximum(emax)and minimum(emin)void ratios of Ottawa sand are 0.26 mm, 0.87 and 0.55, respectively.
Ottawa sands were immersed in 70% alcohol for 24 h and then washed with deionized water three times followed by oven-drying(100°C) for at least 24 h before use. A plastic tube(90 mm×60 mm×0.64 mm,height×diameter×thickness)was placed in the center of the SIP-BE column (300 mm × 100 mm,height×diameter)to separate the sand and mixtures of sand-lime and sand-cement.Both the plastic tube and the inner surface of the SIP-BE column were flushed with 70%alcohol and then rinsed with deionized water three times before testing.
2.2. Sand-lime mixture preparation
The soil-lime sample was prepared as follows. First, 3-cm thick pure sand at the bottom was compacted into the column at a relative density(Dr)of 93.3%.Second,the plastic tube of 9 cm in height was placed in the center of the column. Hydrated lime (46.4 g) and Ottawa sand(434.7 g)at a lime/sand ratio of 1:3(by volume)were homogenized and compacted into the plastic tube(porosity=32.8%for sand). Third, the rest of the spaces between the column and plastic tube was filled with compacted pure sand(Dr=93.3%).Then,the plastic tube was quickly removed from the column.Pure Ottawa sand with Dr=93.3%was further compacted into the column until the total sample height was 15.1 cm(Fig.1).The total mass of Ottawa sand was 2000 g.A 100 kPa vertical stress was applied on the top of the sample via a load frame (Master loader HM-3000, Humboldt MFG. Co., Norridge, IL). After the vertical load was stabilized,deionized water was introduced into the sample for 4 h from the bottom of the column through a peristaltic pump to saturate the sample.Finally,a solution of 5 mmol/L Na2CO3(Fisher Scientific,CAS No.497-19-8)(electrical conductivity=1.129 mS/cm)was pumped into the sample to produce CaCO3. The flow rate was always maintained at 10 mL/min using the pump. During the CaCO3generation process, a continuous flow of Na2CO3solution was maintained in the first 6 h. Then, a sequence of fluid pumping (about 1 h) + reacting (0.5 h) + testing (0.5-1 h) was followed to ensure sufficient reaction time.
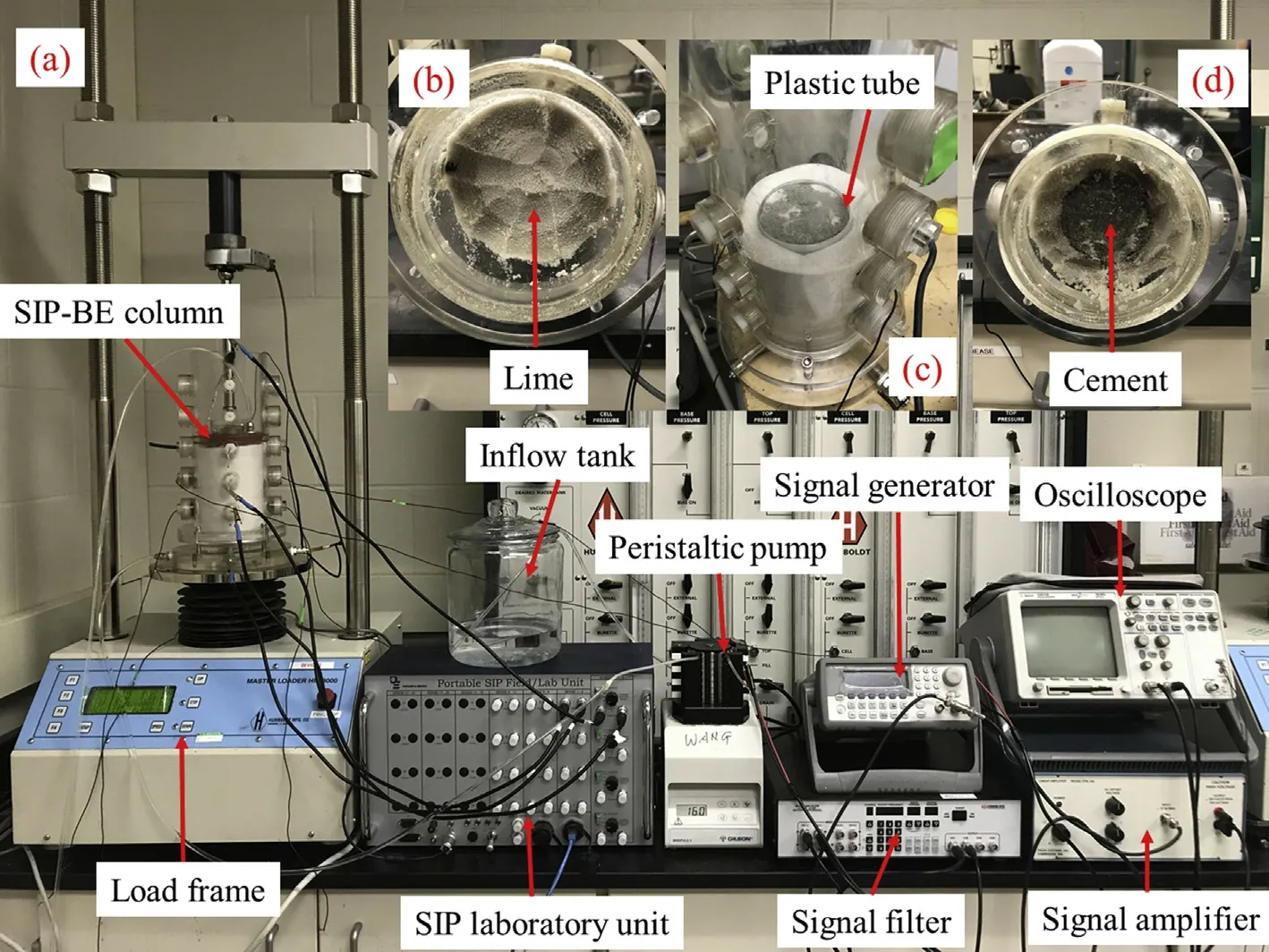
Fig.1. (a) Experimental setup; (b) Sand-lime mixture; (c) Sand-cement mixture with a plastic tube; and (d) Sand-cement mixture after treatment.
2.3. Sand-cement mixture preparation
The sand-cement sample preparation procedures were very similar to those for sand-lime mixture except for the following differences. Portland cement (132.1 g) and Ottawa sand (351.2 g)were mixed homogeneously at a cement sand ratio of 1:2 (by volume) first and then compacted into the plastic tube. The initial water cement ratio was controlled at 0.2. The total sample height was 15 cm and the total mass of Ottawa sand was 1860 g. The relative density(Dr)of the Ottawa sand was 85%.A vertical stress of 5 kPa was then applied.After the load-induced settlement(judged by the Taylor’s square root time method(Terzaghi et al.,1996))was stable, deionized water was introduced into the sample from the bottom of the column via a peristaltic pump at a flow rate of 10 mL/min.Two pore volumes of deionized water were first pumped into the sample. After approximately 18 h and 47 h, another pore volume of water was introduced.
The experimental setup is shown in Fig.1. Both SIP signals and shear wave velocity(Vs)were measured with a self-developed SIPBE column(Fig.1).The size of the acrylic column was 0.3 m×0.1 m(length × inner diameter). Pre-drilled holes aligned with even 0.05 m spacing along the side of the column for SIP electrodes and BEs (Fig. 1). Solutions were introduced to the sample from the bottom and discharged from the top (Fig.1a). Room temperature was maintained at around 23°C throughout the test.
3. Results and discussion
The sand-lime mixture and sand-cement mixture are discussed as follows. SIP and Vsresults are mingled in the discussion with a focus on the evolution of the microscopic properties (size and content of the particles) and their effects on macroscopic properties, i.e. Vs.

Fig. 2. Evolution curves of real conductivity (at 10 Hz), effluent conductivity and pH value for a sand-lime mixture.
3.1. Sand-lime mixture
The time evolution curves of real conductivity(σ′)at 10 Hz and the DC conductivity of effluent (σf) showed similar trends (Fig. 2),which demonstrated that electrolyte was the major contributor to the real conductivity;while the surface conductivity of solids in the soil matrixis secondary.The 10 Hz was selected here as the representative real conductivity of the whole frequency spectrum, because the major relaxation occurred below 10 Hz.Therefore,the real conductivity suffered very limited influences by the relaxation. The formation factor (F) is a parameter quantifying the decrease in conductivity of the rock due to the presence of insulating mineral grains (Archie, 1942; Zhang et al., 2012). The equation is F = σf/σ′,where the average F value of the soil column in this study is 1.77.The effluent pH value fluctuated slightly around 11.4 (Fig. 2). The effluent conductivity decreased from 0.49 S/m to 0.12 S/m in the first 10 h and then remained at 0.12 S/m afterward.The initial decrement of the outflow conductivity could be attributed to the loss of hydrated lime that was flushed out. This was evidenced by the observed white powder in the outflow tube.
The shear wave velocity(Vs)evolution curves of four pairs of BEs(BE 1-2, 3-4,1-4 and 2-3), i.e. Vs,12, Vs,34, Vs,14and Vs,23, are presented in Fig. 3. The following observations can be made:
(1) Four stages in the curves can be identified, i.e. the initial increase (Stage I: 0-6 h), temporary stability (Stage II: 6-12 h), a secondary increase (Stage III: 12-25 h), and an eventual plateau (Stage IV: after 25 h). The plateau of Vscurves after 25 h suggested the cessation of the production of calcium carbonate via a chemical reaction:

(2) The hierarchy of Vs(Vs,14> Vs,34> Vs,12≈Vs,23) reveals the heterogeneity of the produced calcium carbonate in the lime hydration reaction (Eq. (4)).
Typical imaginary conductivity curves measured at different testing times are also shown in Fig. 3. The above four stages identified by the Vsresults were also in accordance with the changing trends of imaginary conductivities (σ′′). During the first 6 h, both the major peak of the σ′′curve (σ′′peak) and the corresponding frequency of the major peak(fp)decreased with elapsed time(Fig.3a).Decrement of fprevealed the increment of average particle size based on the Schwarz equation (Eq. (3)), while the decrement of σ′′peakexhibited a decrement in particle quantities or in the net surface charges of the particles(Wu et al.,2010;Zhang et al.,2012).Given the high initial contents(25%by volume),lime particles were believed to be the reason for the initial high magnitude of the imaginary conductivity. With the increment of time and the hydration process, lime was either consumed to generate larger, yet less electrically charged CaCO3precipitates,or simply flushed out as mentioned previously.This resulted in a lower σ′′magnitude(from 0.0017 S/m to 0.0002 S/m),and a lower relaxation frequency(from 199.5 Hz to 0.4 Hz). The particle size calculated from the Schwarz equation (Eq. (3)) increased from 0.0026 mm to 0.067 mm: the former was comparable to the individual size of the lime powder,while the latter was typical of the aggregate size of CaCO3from lime hydration (Moorehead, 1986; Wu et al., 2010; Cizer et al., 2012).Above results suggest that SIP holds high potential to capture the detailed information of the size and distribution of the particles,which are unavailable through other means of measurements.
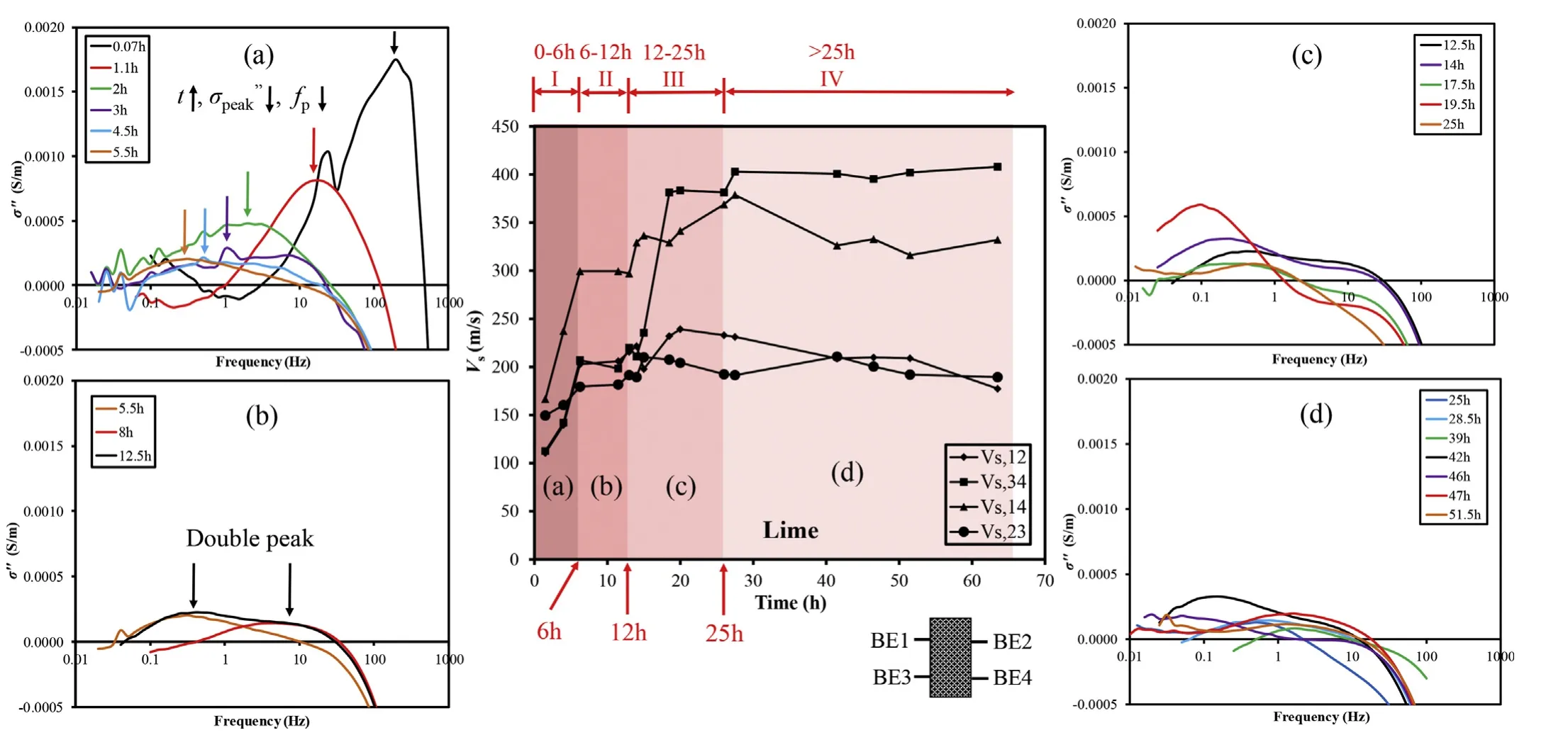
Fig. 3. Evolutions of the imaginary conductivity and shear wave velocity curves of the soil-lime mixture.
From 6 h to 12 h,nearly constant σ′′peaks of around 0.0002 S/m were observed,while fpshifted from 0.3 Hz to 6 Hz and eventually became dual peaks(0.3 Hz and 6 Hz)(Fig.3b).This suggested both a steady particle size and quantity in the sand-lime matrix,indicating a dormant period.Coincidently,Vsvalues remained nearly constant(Fig. 3). From 12 h to 25 h, two major σ′′peakvalues ranging from 0.002 S/m to 0.006 S/m were measured, and the corresponding fpvalues were located in the range of 0.1-1 Hz and 6-12 Hz,respectively (Fig. 3c). The major average precipitated particle size changed from 0.0378 mm to 0.119 mm(Eq.(3)),which suggested an increase of aggregate size.After 25 h,the measured ranges of major fpand the magnitudes of σ′′decreased moderately, indicating that both major average particle sizes and quantities of precipitation increased only mildly (Fig. 3d). Meanwhile, Vsalso remained constant (Fig. 3).
The average particle sizes calculated by Eq.(3)were tabulated in Table 1.The diffusion coefficient in Eq.(3)was adopted according to the chemical speciation in the pore fluid: Before 25 h, most of the pore fluids of the soil sample was dominated by the Ca(OH)2solution; afterward, the injected Na2CO3solution became the dominant fluid due to the depletion of Ca(OH)2.Therefore,the diffusion coefficients before and after 25 h were 1.12 × 10-9m2/s for 0.02 mol/L Ca(OH)2solution (Sugiyama et al., 2003) and 1.15 × 10-9m2/s for 0.005 mol/L Na2CO3solution (Leaist and Noulty,1985), respectively. After the first 6 h, an average particle size of around 0.04 mm was consistently presented, which was close to the average size (0.02 mm) of the calcium carbonate generated with CaCl2and Na2CO3solutions in a glass bead column(Wu et al., 2010).
It was determined that for a similar CaCO3particle size, the association pattern of precipitates originating from different CaCO3generation methods could result in significantly different stiffnesses. A study by Cao et al. (2019) measured Vsand σ′′of Ottawa 50-70 sand mixed with eggshell powders,which was composed of 99%CaCO3.The average particle diameter of the eggshell powder at 100 kPa vertical stress, estimated by the Schwarz equation, was primarily 0.026 mm with some larger than 0.412 mm,which were similar with that in this study(0.03-0.21 mm).The content of the CaCO3powder used in Cao et al. (2019) was 33.1%, which was significantly larger than the calculated total content of CaCO3precipitates generated in this study (1.2%). However, the measured shear wave velocities ranged from 92 m/s to 189.2 m/s under the vertical stress from 5 kPa to 100 kPa, which was significantly less than the Vsvalues(ranging from 189 m/s to 408 m/s)under 100 kPa vertical loading in this study. It was postulated that eggshell powder primarily filled the voids in the sand matrix, unlike the postulated cementation association at the sand particle contacts in this study. In addition, vertical stress could increase the contact of the eggshell powder with sand particles.
The increments of the shear wave velocities (ΔVs) at the CaCO3content of 1.2% for five different studies with similar association patterns,i.e.cementation at the particle contacts(Weil et al.,2012;Martinez et al., 2013; DeJong et al., 2014; Cao et al., 2019), are compared in Fig. 4. Except for this study using physico-chemical reactions and the Cao et al. (2019)’s study using the physical mixing of CaCO3powder, the other three adopted the microbial induced carbonate precipitation (MICP) method. It was observed that Vsvalues measured in this study were in the same range of the MICP studies.This suggested that the effectiveness of soil treatment by physico-chemical reaction of this study was as effective as that of the MICP method, and both were more efficient (in terms of Vsincrement)in soil improvement than simple physical mixing of the CaCO3particles, i.e. eggshell powder in Cao et al. (2019).

Table 1 Calculated average diameters of the precipitated particles.
3.2. Sand-cement mixture
The evolution curve of the real conductivity (σ′) of the sandcement mixture at 25 Hz is presented in Fig. 5. The 25 Hz was selected as the representative real conductivity of the whole frequency spectrum due to the same reason with sand-lime mixture,i.e. the major relaxation of sand-cement mixture occurred below 25 Hz. Thus, the real conductivity suffered very limited influences by the relaxation. It was observed that:
(1) During the 1st injection of water,σ′first increased and then decreased. At approximately 6 h, the maximum σ′value of 0.19 S/m was achieved.
(2) In both the 2nd and 3rd injections,σ′increased again.
(3) At around 184 h,σ′became stable at approximately 0.14 S/m.
The possible reason of the above changing trends of σ′could be elaborated as follows. Portland cement has four primary phases(components of clinker), i.e. tricalcium silicate (C3S, 50%-70%),dicalcium silicate (C2S, 10%-25%), tricalcium aluminate (C3A,around 10%), and tetracalcium aluminoferrite (C4AF, up to 15%).Gypsum is often added to the cement as the predominant source of sulfate to moderate the initial flash set, i.e. an early loss of workability of the cement (Borgerson, 2007; Kosmatka and Wilson,2011). Directly after water introduction, gypsum, C3S and C3A in the cement quickly dissolved in water,which resulted in a dramatic increase of the ion concentration(Skalny and Young,1980;Moukwa et al.,1991).The major ions in this period were Ca2+,OH-and SO42-(Tamás,1982;Diamond,1983;McCarter and Curran,1984;Moukwa et al.,1991).Therefore,the real conductivity,which depends mostly on the pore fluid conductivity, increased. The ion concentration in the pore fluid would reach supersaturation (Williamson, 1968;Moukwa et al.,1991).Beyond the supersaturation state,some of the ions in the pore fluid would precipitate.For example,after reaching a peak value of 0.194 S/m at around 6 h, the real conductivity decreased with time during the first water injection(Fig.5).During the 2nd and 3rd injections of deionized water,the real conductivity decreased and then increased again. This was primarily caused by the initial dilution of the pore fluid and then by continuous cement hydration. At elapsed time, unreacted cement hydrated and ions were released. At 184 h after initial injection, the cement in the column was completely hydrated and the real conductivity stabilized (Fig. 5).
The evolution curves of the shear wave velocities and imaginary conductivities (σ′′) from the SIP technique for the sand-cement mixture are presented in Figs. 6 and 7, respectively. In order to minimize the influences of Maxwell-Wagner polarization and dielectric effects at high frequencies, as well as the changes of the pore fluid, phase at 10 Hz was selected as the representative one shown in Fig.6.The shear wave velocity evolution curves could be divided into four regions, which seemingly coincide with the four stages of the hydration process of cement (Fig. 6); while σ′′responses during those four stages were also distinctively different and elaborated below:
(1) Stage 1 (0-2.4 h): Vsincreased from 220 m/s to 550 m/s for BE 1-2 and 3-4 and to approximately 800 m/s for BE 1-4 and 2-3(Fig.6).σ′′peakincreased from 0.01 S/m to 0.038 S/m,and the major fpranged from 15.8 Hz to 31.6 Hz(Fig.7a).The latter correspond to an average particle size range of 0.91-1.28 μm with the diffusion coefficient of the cement paste solution of 20.39×10-12m2/s adopted based on the result of Yang and Cho(2003).The above initial trends of both Vsand σ′′were attributed to the precipitation of ettringite crystals,which mildly increased the stiffness and SIP responses(Fig.7a).With the first injection of water,the gypsum(C SH2)in the cement immediately dissolved and generated a sulfaterich solution for the pore fluid. In the first few minutes, C3A hydrated with water and became the aluminaterich gels (Moukwa et al., 1991; Kosmatka and Wilson,2011). These gels could react with sulfate ions in the pore fluid to produce the small needle-like crystals of ettringiteattached on the surface of the clinker. The chemical reaction isEttringite crystals could bridge the sand particles and increase both contact areas and the coordination number (i.e.number of contacts per particle)significantly(Fig.7a,inset).Therefore, Vsoverall increased. With an electrical diffuse layer accumulation around the ettringite crystals, the generation of ettringite crystals led to strong σ′′responses(Fig. 7a), as revealed by the highmagnitude (up to 0.038 S/m).From Eq.(3),the calculated average particle size ranged from 0.91 μm to 1.28 μm,which agreed with the size range of ettringite, i.e. 0.5-2 μm long and <0.2 μm wide as provided in Gao et al. (2002) and the scanning electron microscope (SEM) image given by Artioli and Bullard(2013).

Fig. 4. A comparison of the shear wave velocity increments for different studies at CaCO3 content of 1.2% (A, B, C and D are the locations of the bender elements).

Fig.5. Changing trend of real conductivity at 25 Hz in the cement treated soil sample.

Fig. 6. Evolution of shear wave velocity curves and phase curve at 10 Hz for the soilcement sample.
(2) Stage 2(2.4-6 h):Vswas slightly increased for all four pairs of the BE (Fig. 6). The magnitudes of σ′′decreased with elapsed time,and fpincreased to ≥100 Hz(Fig.7b). Because of the limited quantities of gypsum in cement, the concentrations of sulfate ions in the pore fluid dramatically decreased due to the complete consumption of gypsum(Fig. 5). The ettringite crystals became unstable under this environmental condition and began converting to another solid phase called monosulfoaluminate(Kosmatka and Wilson, 2011). The chemical reaction isThe specific surface area of the monosulfoaluminate (5.7 m2/g) was reported to be smaller than that of ettringite (9.8 m2/g) (Baur et al., 2004),and the surface charge densities of the monosulfoaluminate were also found to be lower than those of ettringite(Elakneswaran,2009;Ge and Wang,2017).These two factors could be the main reasons for the continuous σ′′decrement.Another possible reason might be the slight hydration of the C3S phases of cement paste to calcium-silicate-hydrate (CS-H)gel,which rendered the sand-cement mixture more of a plastic semifluid body that shielded the electrical signals,i.e.the ion mobility decreased because of the plasticity of the sand-cement mixture.The cement was likely in the dormant period, which indicated that few hydration products would be generated during this stage(Moukwa et al.,1991).Limited changes of Vssuggested that the contact areas of the sand particles might also have minor changes due to the morphology changes of ettringite to the monosulfoaluminate phases.An SEM image of monosulfoaluminate crystals given by Phenrat et al. (2005) was exhibited in Fig. 7b. Previous study showed that the average particle size of monosulfoaluminate crystals was ≤0.5 μm (Gao et al., 2002),which was smaller than that of ettringite crystals (0.91-1.28 μm). Meanwhile, all fpvalues measured at this stage were ≥100 Hz (Fig. 7b), which suggested a particle size of≤0.51 μm according to the Schwarz equation (Eq. (3)). The agreement between the measured monosulfoaluminate crystal size from SIP and the calculated particle size from SEM (Phenrat et al., 2005) validated the usefulness of the Schwarz equation and suggested that monosulfoaluminate crystals were the major products during this stage.
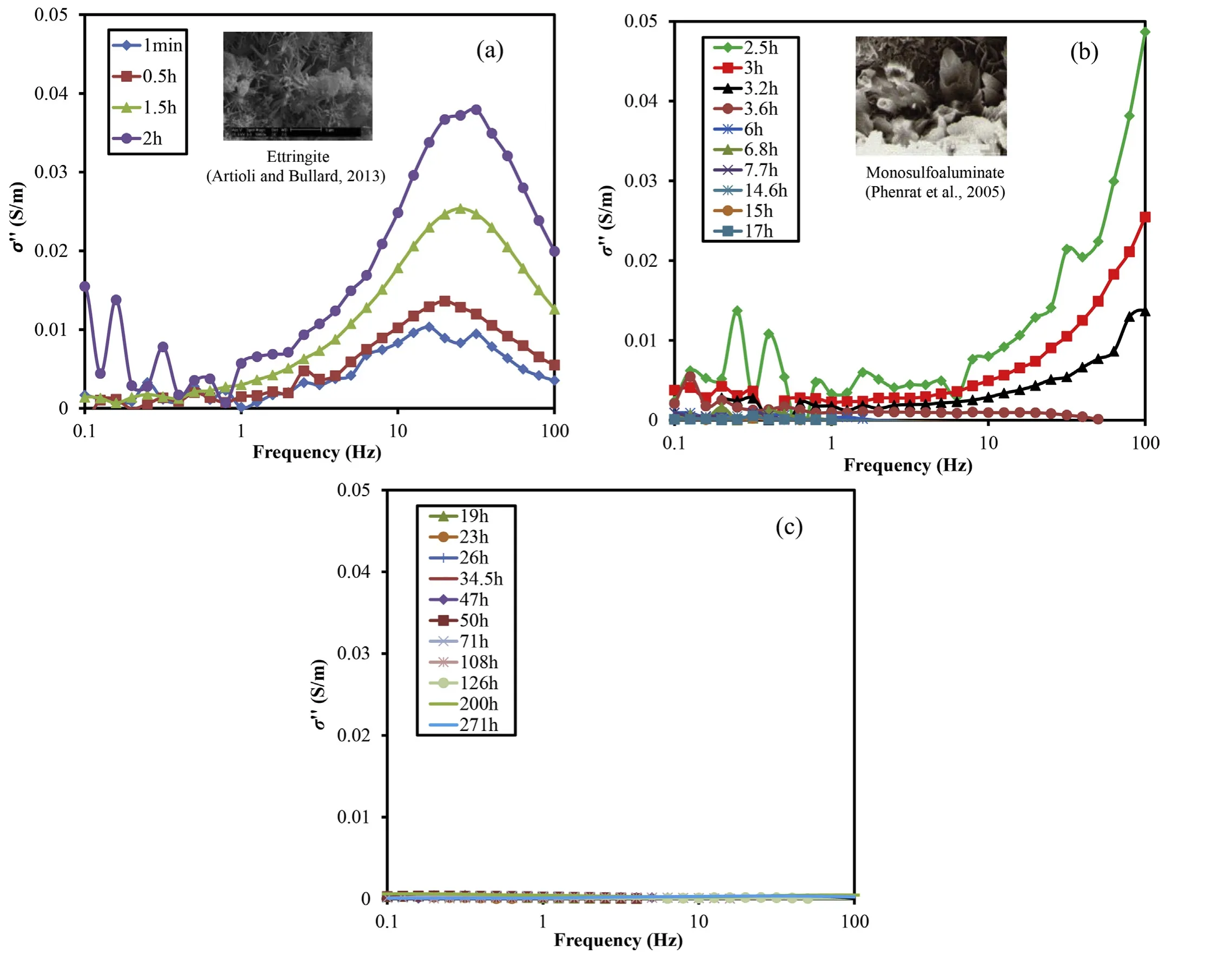
Fig. 7. Evolutions of the imaginary conductivity curves for the soil-cement sample.
(3) Stage 3 (6-35 h): Vsincreased mildly (Fig. 6). The magnitudes of σ′′almost equaled zero. No fpvalues were detected(Fig.7b and c).The solid products nucleated and precipitated from the gradual hydration of C3A,C4AF and C3S phases that occupied greater volumes than the original cementitious materials and consequently some of the space between the cement grains was filled.Eventually,the hydration products connected adjacent grains and formed a continuous solid network (Kosmatka and Wilson, 2011). The initial set of cement was probably achieved during this stage (Soliman et al., 2015). Since the cement was still plastic and had little rigidity before the initial set of cement, Vsincreased gently,which agreed with a prior study conducted on the Vsmeasurement in mortar (Zhu et al., 2018).
(4) Stage 4 (>35 h): Vsincreased significantly, especially for BE 1-4 and 2-3(from 900 m/s to 1500 m/s). Still almost no σ′′and fpvalues were detected during this stage. The further hydration of C3S and C2S to C-S-H gel(the primary source of strength) and CH (calcium hydroxide) phases significantly increased the strength and stiffness of the sand-cement mixture. The cement paste transformed from a semifluid to a solid state. Hence, Vsincreased dramatically. After around 184 h, the hydration reactions of C3S and C2S were nearly completed. Therefore, it coincides with no significant changes in the phase of the complex conductivity and Vs(Fig. 6), and in fp(Fig. 7).

Fig. 8. A schematic sketch of the sand-cement mixture with probable and different degrees of hydration.
It was noticed that Vsvalues measured from BE 1-4 and 2-3 were significantly larger than those from BE 1-2 and 3-4 (Fig. 6),which revealed the heterogeneous properties of the sand-cement mixture. The Vsvalues for BE 1-4 and 2-3 were very similar over the entire period, while for BE 1-2 and 3-4, they were similar before 42 h approximately.Afterwards,the Vsfor BE 3-4 increased significantly.The possible reason for this was depicted in Fig.8.BE 3 and 4 were located near the inflow port where the water cement ratio was slightly higher than the originally designed water cement ratio(0.2).Generally,the cement paste with a higher water cement ratio needed a longer time to hydrate and could eventually achieve a higher strength and stiffness.However,BE 1 and 2 were near the outflow area. There could be a region near BE 1 and 2 where the hydration process of the cement was not adequately reacted due to the potential preferential flow (Cao et al., 2019; Ye et al., 2019).Consequently, the strength and Vsvalues of that region were relatively low. The middle part of the sample was potentially fully hydrated and precipitated faster than the region with higher water cement ratio, whereas larger Vsvalues were also measured.
4. Conclusions
Lime and cement stabilization experiments on Ottawa 50-70 sands were conducted in this study. SIP and BE techniques were integrated to efficiently and nondestructively monitor the stabilization processes. During the stabilization, complex conductivities were detected by SIP and shear wave velocities were measured using BE. At different chemical reaction stages, the produced particle sizes were analyzed according to the values of peak frequency for imaginary conductivity and the Schwarz equation.Based on the testing results, the following conclusions can be made:
(1) Real conductivities measured from the SIP technique could be a good indicator for the cessation of the chemical reactions and stability of the testing system. Electrolytic DC conductivity was observed as a major component of the real conductivity in this study.
(2) Changing trends of imaginary conductivity or phase and shear wave velocities coincided with each other for both lime and cement treatments. This revealed that unique chemical reactions occurred in specific time periods of different improvement methods.The SIP-BE testing system held high potential to effectively mimic and monitor the entire lime and cement treatment processes.
(3) The agreement of the measured particle sizes from SIP in this study and the calculated particle sizes from previous studies for specific particles produced under similar testing conditions validated the usefulness of the Schwarz equation and the reliability of the SIP technique.
(4) In lime treatment, most of the CaCO3crystals probably precipitated at the contact areas of Ottawa sands, which suggested that the soil treatment using the physico-chemical reaction of this study is as effective as that by MICP method.
(5) Profiles of the shear wave velocity measured from BE revealed the heterogeneous properties of soil-lime and soilcement mixtures after the treatment. This heterogeneous property was probably caused by the location of the inflow and outflow on the SIP-BE column of this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research is sponsored by the Basic Science Center Program for Multiphase Evolution in Hypergravity of the National Natural Science Foundation of China(Grant No.51988101) and Ministry of Science and Technology of China (Grant No. 2019YFC1805002).Financial support from the Overseas Expertise Introduction Center for Discipline Innovation(Grant No.B18047)is also acknowledged.Insightful and constructive comments from the anonymous reviewers are sincerely appreciated, which helped improve the quality of this paper immensely.
 Journal of Rock Mechanics and Geotechnical Engineering2021年1期
Journal of Rock Mechanics and Geotechnical Engineering2021年1期
- Journal of Rock Mechanics and Geotechnical Engineering的其它文章
- Improved prediction of slope stability using a hybrid stacking ensemble method based on finite element analysis and field data
- An integrated laboratory experiment of realistic diagenesis, perforation and sand production using a large artificial sandstone specimen
- Predicting uniaxial compressive strength of serpentinites through physical, dynamic and mechanical properties using neural networks
- Axial response and material efficiency of tapered helical piles
- Rock brittleness indices and their applications to different fields of rock engineering: A review
- Application of artificial intelligence to rock mechanics: An overview
