Association between retinopathy, nephropathy, and periodontitis in type 2 diabetic patients: a Meta-analysis
Hui-Qun Wu, Xiao Wei, Jin-Yi Yao, Jian-Yan Qi, Hui-Min Xie, Ai-Min Sang, Kui Jiang
1Department of Medical Informatics, Medical School of Nantong University, Nantong 226001, Jiangsu Province, China
2Department of Ophthalmology, Aきliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China
Abstract
INTRODUCTION
D iabetes mellitus (DM) is a kind of chronic disorder that could not be felt with serious symptoms at its early stage. DM was affecting over 217 million people worldwide in the last decade, and its prevalence is still increasing, and it is estimated that the prevalence of diabetes for all age-groups worldwide was estimated to be 4.4% by the year 2030[1]. The progress of DM-related microvascular dysfunctions are associated with cerebral and peripheral microvascular complications[2-3]. It’s recognized that vascular complications of diabetes are the most serious manifestations of diabetes, of which retinopathy (DR) and diabetic nephropathy (DN) are two major contributors to end-stage blindness and renal disease, respectively[4].
Periodontitis, another chronic inflammatory disease has been reported to aあect almost 50% of the general population globally[5]. Those individuals with chronic periodontitis could develop periodontal pockets, however, they might be unaware of their clinical periodontal status due to being asymptomatic and painless during the most process of its course[6]. The periodontitis was discovered to be associated with a variety of systemic diseases and conditions, which was in need of attention[7-10]. Similarly, such association was also echoed in DM[11-12]. Previous clinical investigations showed that the risk of periodontitis is greater in DM patients, and the disease course of diabetes might result in more severe tooth loss[13]. Moreover, the periodontitis has a significant impact on diabetes control, incidence and complications[14-15]. Recently, the risk of peripheral arterial disease was high in patients with periodontitis has recently been discosed[16]and the severity of periodontitis was found to be significantly associated with the number of microvascular complications too[17]. Several clinical investigations revealed that the periodontitis had certain association with DR in those DM patients[18]. And the similar findings were indicated in those DM patients with DN[19]. However, such association reported in each individual publication has not been verified in large population. Despite several summarized analyses have been conducted to prove the relationship between diabetes and periodontitis[20-22], similar summarized studies have not yet been performed to confirm the association between periodontitis and microvascular complications in type 2 diabetic (T2D) patients. Therefore, we conducted this Meta analysis to summarize the results of previous studies and investigate such potential association between periodontitis and microvascular complications in T2D patients in order to provide more reliable evidence for correlated clinical researches.
MATERIALS AND METHODS
Search StrategySeveral electronic databases were available for our comprehensive search including China National Knowledge Infrastructure (CNKI), Chinese VIP Information (VIP), Wanfang, Web of Science, ScienceDirect and PubMed and were queried for relevant citations (updated to Mar 2019). By virtue of the following search terms: “periodont*”, “alveolar bone loss”, “anodontia” , “edentulous” and “toothless”, “diabet*”, “diabetic retinopathy”, “fundus”, “DR”, “proliferative diabetic retinopathy”, “PDR”, “non-proliferative diabetic retinopathy”, “NPDR”, “microaneurysm*”, “neovascularization”, “cotton-wool spots”, “hard exudate*”, “soft exudate*”, “h*emorrahge”, “bleed*”, “degeneration” “blind*”, “diabetic nephropathy”, “DN”, “albuminuria”, “dialysis”, “serum creatinine”, “urea nitrogen”. Some of above terms were combined to generate a subset of citations that address the purpose of our investigation. The reference lists of relevant articles were later under prudent scrutiny in quest of potential qualified studies beyond the electronic searches.
Inclusion and Exclusion CriteriaThe trials were included if they have satisfied the following criteria: 1) According to the updated CDC-AAP case definition for monitoring periodontitis in 2012, the number of periodontitis categories was expanded from 3 to 4,i.e., no, light, moderate, and severe periodontitis[23]. 2) Trials that included patients with DM which was comply with the standard of WHO (1985) or American Diabetes Association (ADA, 1997)[24]. 3) Diagnosis of DR was determined by the Guideline for DR Clinical Diagnosis and Treatment which was developed by the Eye Institute of Chinese Academic of Ophthalmology in 2014 and other guidelines[25-27]. 4) DN was diagnosed on the presence of proteinuria >0.5 g/24h, a stage referred to as overt nephropathy, clinical nephropathy, proteinuria, or macroalbuminuria[28]. 5) Adequate information about the occurrence of periodontitis was available. 6) The language was written in English or Chinese. The exclusion criteria were as follows: 1) The subjects of study were patients accompanied with other diseases aあecting teeth, eye or kidney organs. 2) Insuきcient data about DR, DN, and periodontitis. 3) The literature reporting language used failed to be English and Chinese.
Quality Assessment and Data ExtractionBy virtue of the Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in Meta-analyses and RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark), the quality of the included studies was evaluated thoroughly. Using the “star system”, the study was judged from three broad perspectives: the choice of the study group; the comparability of each group; and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively[29]. On the basis of the NOS, each study will be scored as high quality for studies receiving at least 8 stars; medium quality for those awarded seven stars, or low quality if studies had fewer than seven stars[30-31]. Nothing is more crucial for a good quality case-control study than the adequate definition and representativeness of the case, the definition and selection of the controls, acceptable comparability of cases and controls on the basis of the design or analysis, low no-response rate, ascertainment of exposure, together with the same method of ascertainment for cases and controls given the pretty established scale. Three reviewers (Qi JY, Wei X, Yao JY) independently extracted data with a piloted extraction form, and reviewed all data prudentlyviaidentifying all studies by the other author (Wu HQ). The following items was extracted the from the reports: year of publication, inclusion or exclusion criteria, sample size, ages, sex, the number of T2D patients with microvascular complications, the number of patients with periodontitis, and other co-variables such as smoking, total cholesterol, hypertension, metabolic syndrome events etc. We screened abstracts of articles and obtain full articles of studies that met all predetermined.All the duplicated studies were excluded. Two reviewers (Qi JY, Wei X) estimated the quality of the included studies. If there were divergences, they would reach a consensus after a discussion with a third reviewer (Wu HQ).
Statistical AnalysisIn pursuit of improving the accuracy of these tests, subgroup analyses were used to identify the testrelated or other factors responsible for heterogeneity. Utilizing RevMan (version: 5.3) to perform Meta analysis, odds ratio (OR) and its 95% confidence interval (CI) were calculated for statistical analysis. In addition, heterogeneity was assessed using a Chi-square test and quantified by inconsistency indexI2. Generally, inter-group heterogeneity was evaluated byI2and heterogeneity. While the heterogeneity value was less than 0.1, pooled OR was estimated by either using a random eあect model or using a fixed eあect model. A two-sidedPvalue which is less than 0.05 denotes statistical significance. What’s more, funnel plots were utilized for investigating publication and other biases in this Meta analysis.
RESULTS
The literature search yielded 2086 references from PubMed, Web of Science, the CNKI, VIP, Wanfang databases, eight articles[17-19,32-36]out of which were qualified for this inclusion finally according to the flowchart. Figure 1 presented the study selection process.
Summary Characteristics of Included StudiesAll included studies met inclusion and exclusion criteria. Eight studies from different regions including USA, Japan, Korea, Iran and China were finally included for this Meta analysis. In all 3987 subjects, there were 1207 T2D patients accompanying with microvascular complications and 1734 patients with periodontitis as well. The age of the included population ranged from 30 to 69y. Each study recruited both men and women in the case and control groups. However, only Ricardoet al[33]included the same number of male and female subjects. Four of articles were only concerned with the association between periodontitis and DR, and another three, Hanet al[18], Sharmaet al[32], Ricardoet al[33]referred to association between periodontitis and DN. While Nittaet al[17]recruited subjects with overall microvascular complications of diabetes. The current WHO body mass index (BMI) cut-off points of 23, 27.5, 32.5, and 37.5 kg/m2were refered[37]. In the literatures we included in the study, the BMI of the people was over 22, indicating that some patients were overweight to different extent. Diabetes was defined as HbA1c≥6.5% or fasting plasma glucose (FPG) ≥7.0 mmol/L. Prediabetes was classified as HbA1c between 5.7% and 6.4%[38]. In our study, the values of HbA1c were more than 7%, showing that patients blood glucose level were not well controlled. In addition, studies have shown that baseline blood glucose HbA1c control has no significant effect on longitudinal changes in diabetic complications. A worsening or persistent failure of glycemic control is associated with an increased risk of increased complications of diabetes[13]. In these eight articles, Ricardoet al[33]studied 51.6% hypertensive patients. What’s more, Sharmaet al[32]involved nearly four fifths patient who drunk a lot in their study. The detailed description of the characteristics of included studies could be observed in Table 1.
Figure 1 Flow chart of study selection.

Figure 2 The methodological quality of the included studies.
Quality of Included StudiesThe methodological quality of the included studies was graphically demonstrated in Figure 2. The overall quality of the included studies was variable. The comparability of cases and controls on the basis of the design or analysis had introduced high bias in some studies whereas the no-response rate was fairly low in all studies. In most studies, the definition and selection of the controls were vaguely revealed, and the ascertainment of exposure, together with the same method of ascertainment for cases and controls were opaque in some studies as well.
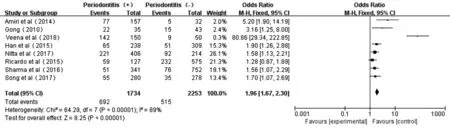
Figure 3 Forest plot comparing DR/DN with non-DR/DN in diabetic patients with periodontitis and non-periodontitis.
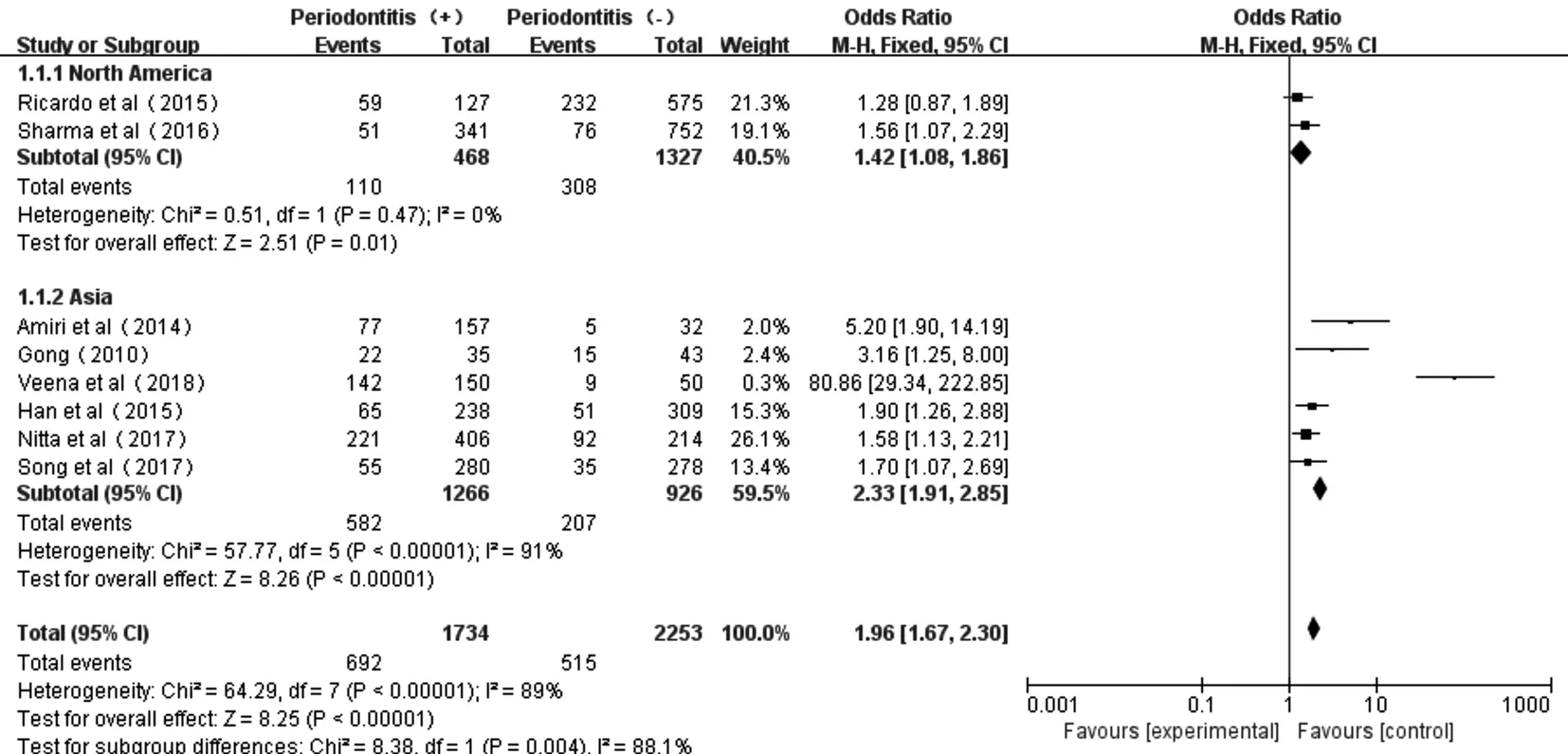
Figure 4 Forest plot comparing DR/DN with non-DR/DN in subgroup Asian and North American diabetic populations.
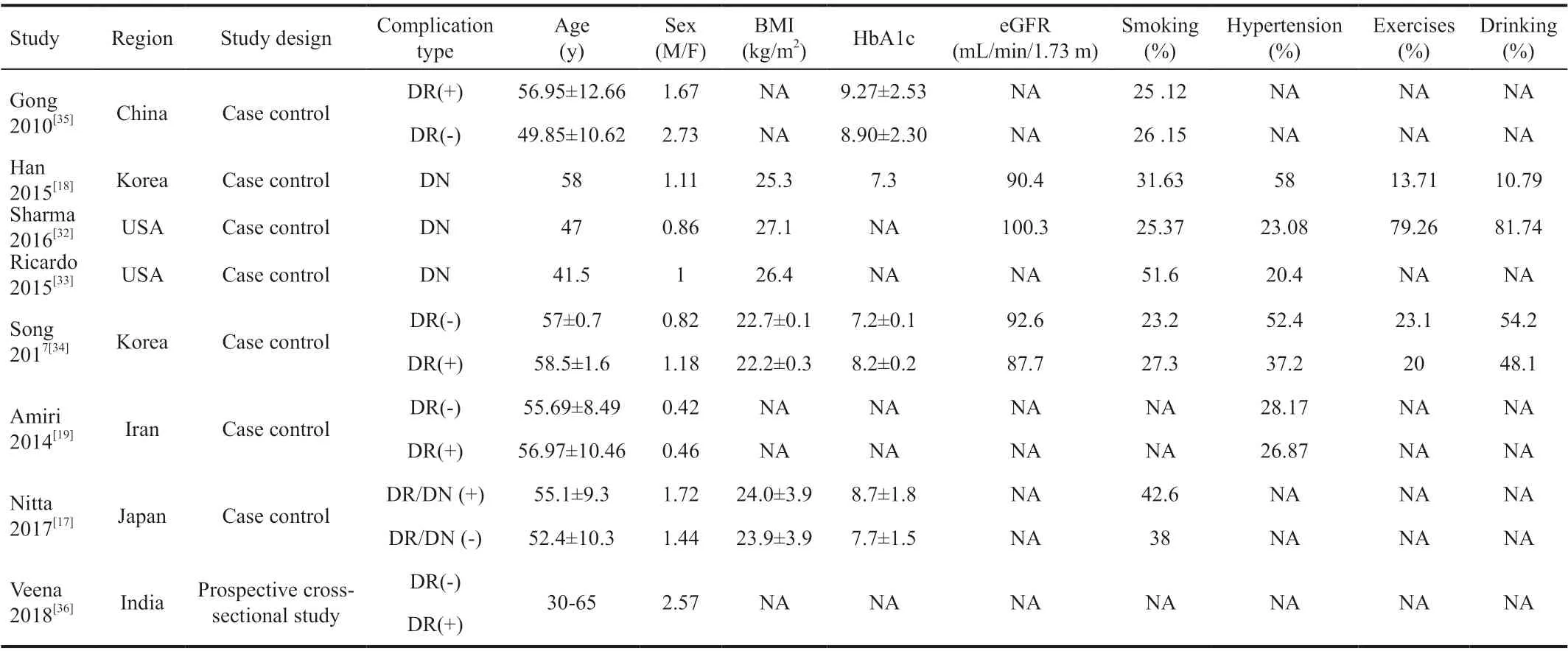
Table 1 Characteristics of the included studies
Association Between Periodontitis and DR or DN in T2D PatientsThe Meta forest plot presented little heterogeneity of the eight studies[17-19,32-36](P<0.00001,I2=89%); and the total eあect size OR andZvalue were 1.96 (95%CI: 1.67-2.30) and 8.25 (P<0.00001), respectively, indicating that periodontitis was associated with overall microvascular complications (Figure 3).
Moreover, the subgroup investigations among the studies in Asian and North American populations showed acceptable heterogeneity too (North American:P=0.47,I2=0; Asian:P<0.00001,I2=91%). And the subtotal effect size OR in North American (1.42, 95%CI: 1.08-1.86) and Asian populations (2.33, 95%CI: 1.91-2.85) all confirmed the existed association between periodontitis and diabetic microvascular complications. However, the strength of such association between periodontitis and DR or DN T2D patients was more obvious in Asians than that in North Americans (Figure 4).
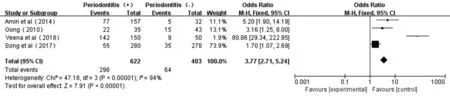
Figure 5 Forest plot comparing DR with non-DR in diabetic patients with periodontitis and non-periodontitis.
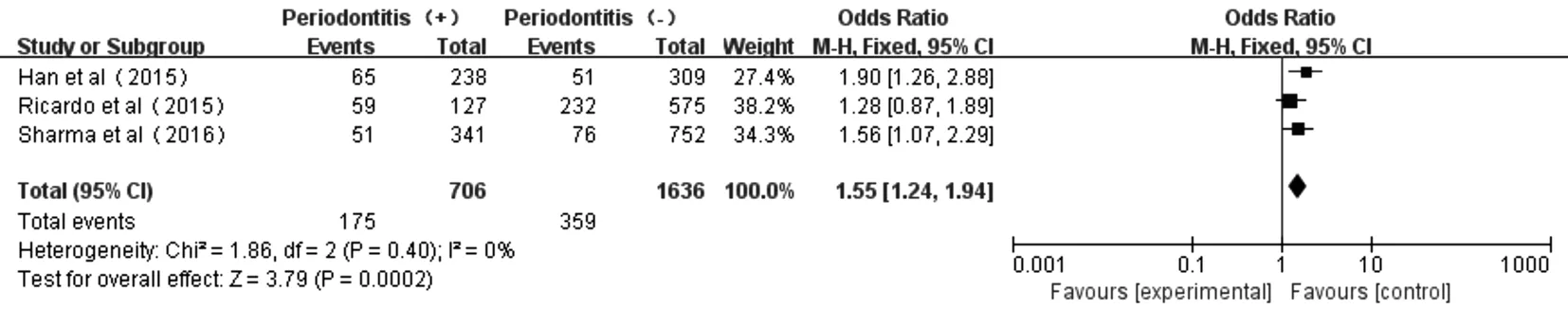
Figure 6 Forest plot comparing DN with non-DN in diabetic patients with periodontitis and non-periodontitis.
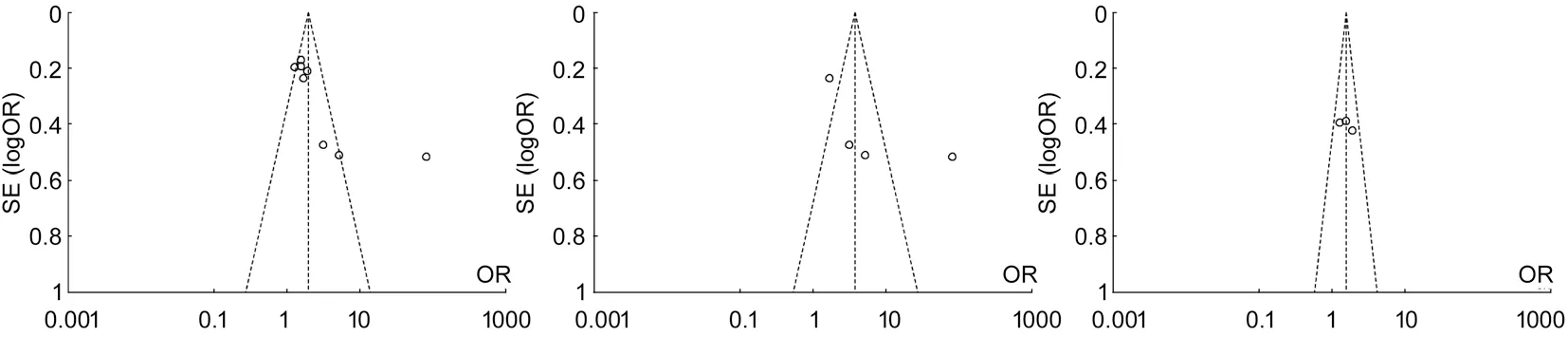
Figure 7 Funnel plots between diabetic patients with periodontitis and non-periodontitis in diあerent comparions A: Comparing DR/DN with non-DR/DN; B: Comparing DR with non-DR; C: Comparing DN with non-DN.
Association Between Periodontitis and DRAs shown from the forest plot of association between periodontitis and DR (Figure 5), there was a certain degree of heterogeneity (P<0.00001,I2=94%) in these four studies[19,34-36]. The total eあect size OR in this study was 3.77 (95%CI: 2.71-5.24), and theZvalue was 7.91 (P<0.00001), indicating the association between periodontitis and DR (Figure 5).
Association Between Periodontitis and DNThe forest plot of association between periodontitis and DN was shown in Figure 6. Three studies[18,32-33]displayed no heterogeneity in patients with periodontitis and non-periodontitis (P=0.40,I2=0). The total eあect size OR in this study was 1.55 (95%CI: 1.24-1.94) and theZvalue was 3.79 (P=0.0002), indicating that periodontitis was associated to DN.
Publication BiasFunnel plots of each group of Meta analysis were shown, and those funnel plots without outlines were due to the use of the random eあect model (Figure 7).
DISCUSSION
Diabetes has been validated as a significant risk factor for periodontitis, which risk is estimated to increase approximately three times in diabetic individuals compared with those nondiabetic individuals[38]. The possible reason is that diabetes could lead to inflammation in periodontal tissues. For instance, compared with those non-diabetic persons with periodontitis, levels of prostaglandin E2 (PGE2) and interleukin (IL)-1β in gingival crevicular fluid (GCF) were higher in diabetic patients with periodontitis[39]. It’s found that DM patients with HbA1c>8% had a considerably higher GCF IL-1β level, and both HbA1c and glucose were considered as independent predictors of an elevated GCF IL-1β level[40]. The possible reason might be due to that hyperglycaemia was a cause of the activation of pathways that increase inflammation, oxidative stress and apotosis[41].
It is generally believed that the initial factor of periodontitis is a large number of bacteria in dental plaque, and periodontitis was recognized as an oral disease that could contribute to lowgrade chronic inflammation, thus producing proinflammatory cytokines and promoting systemic inflammation[42].In those patients with periodontitis, some inflammatory cytokine such as serum levels of IL-6 and C-reactive protein (CRP) were increasing, and the elevated levels of such systemic inflammatory biomarkers were believed to be connected with DM[43]. Tumor necrosis factor (TNF)-α and IL-6 are the main inducers of acute-phase proteins, including CRP, and both have been shown potentially contributing to insulin resistance[44-45]. Moreover, the serum levels of IL-6 and CRP could be used to predict future occurrence of DM[46], suggesting the possible mechanisms underlying the association between periodontities and DM in our findings. Interestingly, Casanovaet al[42]reviewed the relevant articles and summarized the two way relationship between periodontitis and DM. Chronic periodontitis may enhance insulin resistance and increase the risk of multiple long-term complications of diabetes[47]. That is, the systemic inflammation associated with periodontitis might enhance the diabetic state and the resulted hyperglycemia further increase the risk of microvascular complications. The two of such microvascular complications, DR and DN, were investigated in our study. It confirmed that association between retinopathy, nephropathy, and periodontitis, and that the outcome of periodontal destruction is significantly more frequent and severe in subjects with DR. Improved control of hyperglycemia has been proven to reduce the risk of microvascular complications[48]. Therefore, simultaneous periodontal treatments should be implemented to improve glycemic control and assuage periodontitis in diabetes patients with periodontitis. In this way, it might also inhibit the development and progression of diabetic microvascular complications such as DR and DN.
As is prevalently perceived by all, Meta analysis is a comprehensive statistical method that has been under ever-growing utilization for combining and integrating data from a multitude of independent studies. However, the results of Meta analysis depend on the quality of primary researches included for further analysis, having implications on the summarized results. Even though a definite association between periodontitis status and the occurrence of diabetic microvascular complications has been established, this study cannot identify a cause-and-eあect relationship given its case-control design. Furthermore, the limited populations of the studies and indefinite DM duration might limit the generalization of our conclusions.
Despite the above-mentioned weaknesses, this Meta analysis approves the association between the DR, DN and periodontitis in T2D patients. Moreover, it is in need of large-sample, wellconducted prospective clinical investigations to assure that association.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Key R&D Program of China (No.2018YFC1314900; No.2018YFC1314902); Nantong “226 Project” and Excellent Key Teachers in the “Qing Lan Project” of Jiangsu Colleges and Universities [No.(2018)III-436].
Conflicts of Interest: Wu HQ,None;Wei X,None;Yao JY,None;Qi JY,None;Xie HM,None;Sang AM,None;Jiang K,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
