Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
Yu-Jie Cen, De-Bo You, Wei Wang, Yun Feng
1Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China
2Beijing Key Laboratory of Restoration of Damaged Ocular Nerve, Peking University Third Hospital, Beijing 100191, China
Abstract
INTRODUCTION
Corneal blindness is the second leading blinding eye disease in the world (only rank after cataract), aあecting 10 million people and causing huge social burden[1]. The current main treatment option is allogeneic corneal transplantation, including full-thickness (penetrating keratoplasty, PK) or partial (lamellar keratoplasty, LK or endothelial keratoplasty, EK) corneal transplantation. Cornea is a relatively immune-privileged area because of the absence of blood vessels and has the highest success rate in human organ transplantation[2]. Postoperative neovascularization may highly increase rejection rate thus led to transplant failure. Among all kinds of corneal transplantation, LK, which only substitute epithelium and stroma part of cornea, has the lowest rejection rate[3]. However, aging of society population and the prolongation of life expectancy decrease the resource of corneal donor grafts[4-5]. The growing incidence of contagious diseases (e.g.AIDS, syphilis, hepatitis,etc.) and the steady increasing popularity of corneal refractive surgery have further exacerbated this shortfall for corneas under such situation are unacceptable for transplantation[6]. All these lead to a sharp shortage of donor corneas in eye bank. Therefore, ophthalmologists have been seeking substitutes for cadaveric corneal grafts.
With the development of cross-disciplinary studies of cell biology, molecular biology, materials science and bioengineering, constructing tissue engineered corneasin vitrois a topic attracted great research interest. Acellular corneal matrix (ACM) has showed similar physiological and biochemical function to normal corneal tissue, in the meantime possessed good histocompatibility[7-8]. Xenogenic decellularized corneal matrix (XDCM) as a heterograft has already been applied to clinic to substitute human cornea in LK[9]. In our early experiments, we confirmed that XDCM grafts can maintain transparency 1mo postoperatively and permit the growth-in of stroma cells and nerve fibers[10]. But the disadvantages of XDCM postoperative neovascularization at latter stage remain unsolved thus restrict its further clinically application.
Mesenchymal stem cells (MSCs) are self-renewing, multipotent stem cells[11], which have the potential of differentiating into bone, cartilage, lung, skin and other tissues[12-13], and are often used as seed cells for tissue engineering because of this multilineage potential[14]. MSCs could be isolated from neonatal birth-associated tissue (including placenta, umbilical cord, cord blood,etc.) or adult tissues (including bone marrow, peripheral blood, adipose tissue,etc.). Cell surface expression include positive expression of CD90, CD73, CD105 and negative detection of CD34, CD45, HLA-DR, CD11b[15]. Meanwhile, MSCs and their derivatives play key roles in T cell suppression and regulatory T cells (Tregs) activation, which have made them be involved in studies of allogeneic grafting in order to reduce post-transplant immune rejection[16-19]. In corneal chemical injury model, MSCs also showed antiinflammatory and anti-angiogenic capabilities[20]. Previously study from our team showed that MSCs, under ACM microenvironment, presented beneficial factors for corneal recovery[21], indicated that MSCs combined with XDCM may improve corneal post-transplant prognosis.
In present study, we hypothesize that MSCs could reduce neovascularization after XDCM-LK and constructed an engineered corneal graft using rabbit bone marrow MSCs as seed cells and canine XDCM as scaffold. The aim was to construct a competent corneal lamellar substitute in order to alleviate the shortage of human corneal donor.
MATERIALS AND METHODS
Ethical ApprovalAdult New Zealand white rabbits and research dogs were obtained from the Peking University Animal Science Research Center. Animal protocols were in accordance with ARVO (Association for Research in Vision and Ophthalmology) Statement for the Use of Animals in Ophthalmic and Vision Research. All animal experiments were approved by Medical Science Research Ethics Committee of Peking University Third Hospital, China (No.S2019341).
Isolation and Culture of Rabbit Mesenchymal Stem
CellsRabbit femur and tibia were isolated and cut under sterile conditions. Flushed bone marrow cavity five times with Dulbecco’s minimal essential medium (DMEM; Life Technologies, Grand Island, NY, USA). The cell suspension was slowly moved into Percoll gradient media (Sigma-Aldrich, USA) and centrifuge (CENTRI5810, Beckman Coulter, USA) at 2000 rpm for 20min. The whitish cellular layer rich in mononuclear cells was collected in a medium constituted by DMEM, fetal bovine serum (FBS, 10%), penicillin (1%) and streptomycin (1%). After centrifuging at 1500 rpm for 5min, the cell pellet was collected and re-suspended in 5 mL DMEM then incubated at 37℃ under condition with 5% CO2balanced with air. Fresh medium was replenished every two days, and cell morphological changes were observed daily under inverted microscope (TE2000-S, Nikon, Japan).
Rabbit Mesenchymal Stem Cells Identification
Flow cytometryRabbit MSCs (106cells) were re-suspended in 100 μL phosphate buあered saline (PBS) and incubated with primary antibodies (1 μg) for 30min on ice. After spinning and multiple washes with buffer, the cell samples were incubated with fluorescein isothiocyanate (FITC) goat antimouse secondary antibodies (Santa Cruz, USA) for 30min on ice. After rinsing with PBS two times for 5min, they were resuspended in 500 μL 1% paraformaldehyde PBS solution, and set aside for measurement. Analyses were carried out by flow cytometry and its supporting software to detect CD34, CD45, and CD90. The cell population was taken from the histogram box according to control group [forward scatter (FSC) and side scatter (SSC)] to detect the fluorescence expression rate of the sample group.
In vitro diあerentiation of mesenchymal stem cellsOsteogenic and adipogenic differentiation was induced as reported method[21]to verify the multipotent characteristics of isolated cells. Rabbit MSCs were seeded at a density of 1×104cells/mL in 6-well plate and cultured in two kinds of diあerentiation media.Osteogenesis differentiation medium constituted by DMEM supplemented with 0.1 μmol/L dexamethasone (Sigma, UK), 50 μg/mL L-ascorbic acid (Sigma, UK), 10 mmol/L β-glycerophosphate (Sigma, UK), and 10% FBS. The medium was replenished twice a week during 21-day observation period. To visualize osteogenic diあerentiation, cells underwent von Kossa staining.
Adipogenic diあerentiation was induced by DMEM supplemented with 1 μmol/L dexamethasone, 0.5 mmol/L IBMX (Sigma, UK), 10 μg/mL insulin (Sigma, UK), 100 μg/mL indomethacin (Sigma, UK), and 10% FBS. The induction medium was changed every 3 to 4d, and cells were cultured for at least 14d. To show the presence of adipocyte-like cells, oil-red O staining was performed.
Xenogenic Decellularized Corneal Matrix Preparation
Dogs were sacrificed with an overdose of sodium pentobarbital (100 mg/kg). After enucleation, the whole eye globes were sterilized with 5% tobramycin in PBS for 5min. Then the corneas were harvested along the limbus and immediately fixed in neutral buffered 2% formaldehyde for 2h. After washed with distilled water, the corneas were decellularized in 1.5 mol/L NaOH at 60℃ and washed with distilled water extensively for 30min. The resultant decellularized corneal matrix tissues were incubated in 0.5% aspartic acid to eliminate the residual fixative, washed and individually packed for sterilization. This method has been used in our previous study[21].
Mesenchymal Stem Cells Culture on Xenogenic
Decellularized Corneal MatrixThe grown second generation of MSCs were seeded onto XDCM and cultured in Petri dishes with DMEM (Life Technologies, Grand Island, NY, USA) medium to construct the new graft. Morphology was observed with inverted microscope and under scanning electron microscopy (SEM) for 30d.
Scanning Electron MicroscopyTissue-engineered rabbit MSC on canine XDCM grafts (XDCM-MSC grafts) were fixed in 2.5% glutaraldehyde solution for 2h at 4℃ and washed 3 times for 10min with distilled water. The samples were postfixed with aqueous solution of 1% osmium tetraoxide for 1h, washed with distilled water, dehydrated and critical point dried. Samples were mounted and coated with gold-palladium sputtering and viewed under SEM (FEI Quanta FEG 600, Oregon, USA).
Lamellar Keratoplasty on RabbitsLK was performed on 10 rabbits, which were randomly placed into 2 groups and accepted two diあerent kinds of corneal grafts: 1) XDCM group (n=5): xenogenic decellularized corneal scaあolds; 2) XDCMMSCs groups (n=4): tissue-engineered corneal grafts made up with XDCM and MSCs, with MSCs adhesion layer on the surface side (MSCs were cultured onto XDCM for 14d).
All surgical procedures followed the principle of sterility. All grafts were 4 mm in diameter and were sutured onto the host rabbit cornea by 10-0 nylon sutures at 12 points along graft edge. All postoperative experimental rabbits were not given antibiotics, steroidal anti-inflammatory or anti-metabolic drugs.
Post-transplant General Ocular Observation and EvaluationThe corneal changes were observed under slit lamp microscopein vivo, with fluorescein staining to assess epithelial status. Corneal transparency, extent of neovascularization and epithelium defect were evaluated and scored at 1, 7, 30, and 90d post-implantation. All grading and measuring works were done three times by three researchers respectively and take average as the final scores or measurements.
Neovascularization was graded according to previous reported scoring system[22]: A score of 1 was given for each quadrant aあected; a score of 1 for superficial vessels (anterior one-third of corneal thickness) and 2 for deep vessels (posterior twothirds of corneal thickness); and a score of 1 for peripheral vessels (outer one-third), 2 for mid-peripheral vessels (middle third) and 3 for central vessels (central third). When one quadrant had more than one vessel, the highest vessel score was accounted for that quadrant. This grading gave a maximum score of 6 for each quadrant and 24 for the whole cornea.
The degree of corneal opacification was scored[23], with 1 for mild stromal opacity; 2 for moderate stromal opacity; 3 for severe corneal opacity with visible iris; and 4 for opaque cornea with iris not visible.
Corneal epithelial recovery status was evaluated by fluorescein staining, green fluorescein part represent epithelium defection. Corneal epithelial defect percentage (%) =(area of epithelial defect/area of whole cornea)×100%. Area was measured by Image J software (National Institutes of Health, USA) on each rabbit eye external images.
Post-transplantin vivoConfocal MicroscopyAt 3mo after transplantation, post-operative rabbit eyes were examinedin vivousingHeidelberg Retina Tomograph-II (HRT-II) in combination with Rostock Cornea Module, in central and peripheral regions[10]. After local anesthetization (0.4% oxybuprocaine hydrochloride eyedrops), rabbit eyes were subjected to confocal scan from corneal epithelium to corneal endothelium, in which carbomer gel (Visidic; Dr. Mann Pharma, Berlin, Germany) was used as coupling medium. At least three images were collected anterior (epithelium layers), inside and posterior (rabbit corneal stroma) of the transplanted grafts.
Statistics AnalysisStatistical analysis was performed by SPSS 20.0 of Windows software (version 20.0, SPSS, Inc., USA). The Shapiro-Wilk test verified that all corneal scores were non-normally distributed, and then non-parametric test was performed. Data are presented as the medians±interquartile range withP<0.05 considered as statistically significant.
RESULTS
Rabbit Mesenchymal Stem Cells Isolation and CultureThe rabbit MSCs were successful isolated and showed adherent growth with fusiform or oval shape 24h after plated. At 3rd day, cytoplasmic prominences were observed in some of cells. At 7th day, cell number increased rapidly, with most of MSCs showed spindle shape with abundant cytoplasm, and gradually fused to a film (Figure 1A). Two weeks after seeding, the number of fused adherent cells was up to 80%, and then the cells were cultured for passage. MSCs of passage 2-3 were collected for later use.
Identification of Rabbit Mesenchymal Stem CellsFlow cytometry showed that the surface marker expression of rabbit MSCs we isolated were CD90 positive (Figure 1B) and CD34, CD45 negative (Figure 1C).
Rabbit MSCs we collected were multipotent and could diあerentiated into adipocytes or osteoblasts when cultured in corresponding induction medium. With osteoblast induction medium, MSCs generally turned into flat and wide shape (Figure 1D), and the formation of calcium nodules in the cytoplasm were detected by von Kossa staining (Figure 1E), which showed the differentiation potential to osteoblasts. Cultured with adipogenic induction media, multiple lipid droplet could be observed in cytoplasm (Figure 1F). Oil-red O staining set apart the lipid droplet in red (Figure 1G), which indicated the MSC induction of adipogenesis.
Construction and Morphology of Bioengineered MSC
XDCM GraftRabbit bone marrow MSCs were seeded on XDCM. MSCs, in the beginning, were round, oval or small short spindle, not in uniform size, and the nucleus could not be clearly identified. Several hours later, most of MSCs changed from the round to short spindle shape, with some protuberances (Figure 2A). By the 3rdday, short spindle cells increased, and the protuberances were more obvious. At the 7thday, the number of cells significantly increased, and cells were observed attaching to XDCM in colony form, becoming more hypertrophic. At the 14thday after seeding, MSC proliferated to greater cell number, and cells covered about 70% of XDCM. At this time, the cell growth was vigorous, most of them showing a long spindle appearance and gradually fused into a film (Figure 2B). As early as one day after seeding on XDCM, SEM showed that MSCs adhered to the surface of XDCM. These MSCs were in good growing status with hypertrophic cell bodies, rough surface, protrusions and some villi-like structures (Figure 2C, 2C1). Three days after seeding, MSCs showed an expansive growth pattern on XDCM, and gradually evolved into spindle shape (Figure 2D). Cell surfaces were smoother than before but with obvious microvillus structure (Figure 2D1). On 14thday, SEM image showed that MSC density was significantly increased (Figure 2F), and the spindle-shaped cells extended toward both poles with small fluff on the cell surface. Pseudopodia between MSCs were connected to each other and gradually merged into film and small fluあ was visible on the cell surface (Figure 2F1).
Post lamellar keratoplasty observationAll nine rabbits survived and were observed for three months after LK, without eye infections and acute immune rejection. One rabbit from XDCM-MSC group died 2wk after surgery because of acute gastrointestinal infection and the data of this rabbit was excluded.For XDCM group (n=5), from one day to one week after the surgery, the corneal grafts were transparent (Figure 3E-3F2). One month after LK, the cornea grafts showed mild edema, corneal neovascularization started with a few blood vessels gradually grew inward transplanted grafts from the corneal limbus (Figure 3G-3G2), the epithelium remain partial defected (Figure 3G2). Three months after the surgery, the epithelium were fully recovered (Figure 3H2), obvious neovascularization were seen in the grafts thus reduced corneal transparency, most of vessel started from the suture site and grow into XDCM grafts (Figure 3H-3H2).

Figure 2 XDCM-MSCs morphology under inverted microscope and SEM A-B: Morphology of MSCs at 1st and 7th day after seeding onto XDCM observed by inverted microscope (×10). Scale bar: 100 μm. C-F: Morphology of MSCs 1, 3, 7, and 14d after seeding onto XDCM observed by SEM (×500). C1-F1: Morphology details with greater magnification respectively.

Figure 3 Post-operative observation by slit lamp A-H: Images with diあuse light source; A1-H1: Images with slit light source; A2-H2: Images with fluorescein staining. Fluorescent green area represented corneal epithelial defect.
As for XDCM-MSC group (n=4), all the grafts were edematous one day after the operation (Figure 3A-3A2) and gradually alleviated after one week (Figure 3B-3B2). Two out of four rabbits have epithelium fully recovered at one month (Figure 3C2). Three months after LK, corneas were much more transparent, 75% of rabbit corneas did not manifest neovascularization, and only one rabbit had vessels grown into the implant periphery (Figure 3D-3D2).From one day to one week post-operatively, corneal neovascularization scores showed no significant differences between two groups. While three months after surgery, neovascularization score of XDCM group was significantly higher than XDCM-MSC group, withP<0.05 (Figure 4A).

Figure 4 Corneal surface evaluation between XDCM and XDCM-MSC groups A: Corneal neovascularization scores; B: Corneal opacity scores; C: Percentage of corneal epithelial defect (%). aP<0.05; bP<0.01.
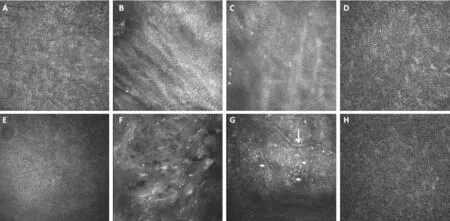
Figure 5 In vivo confocal microscopy images anterior, inside and posterior of grafts A-D: XDCM-MSC group. A: Epithelium; B: Superficial part inside transplanted grafts; C: Deep part inside transplanted grafts; D: Rabbit corneal stroma; E-H: XDCM group. E: Epithelium; F: Superficial part inside transplanted grafts; G: Deep part inside transplanted grafts; H: Rabbit corneal stroma. Short arrows: Nerves inside grafts. Long arrows: Neovessels inside grafts.
At one day and one week after operation, opacity score of XDCM-MSC group was significantly higher than XDCM group, withP<0.01. And no significant diあerences were shown between two groups at one month and three month postoperatively (Figure 4B).
Post-Transplant in vivo Confocal Microscopy ImagingNerve fibers were observed in 2 rabbits of XDCM group (Figure 5G) 3mo after surgery, which had fewer branches than normal corneal stroma nerves. However, no regenerated nerve fibers were found in the eyes of the XDCM-MSC group.
Confocal examination also showed that in XDCM group collagen fibers of the grafts formed a three-dimensional network structure with a number of cells grew in, collagen tissues were arranged in a quiet ordered way but still less ordered compared with normal cornea tissues (Figure 5F). The collagen tissue structure of XDCM-MSC group was basically the same as that of XDCM group, but much more cell-absent area (Figure 5B, 5C) could be observed in the grafts at the time point of 3mo after operation. Morphology of corneal epithelial cells in the two groups had not significantly different from those in normal eyes (Figure 5A, 5E). Rabbit corneal stroma cells posterior to the grafts were dimly visible (Figure 5D, 5H) and endothelium layer could not be observed.
DISCUSSION
It has been confirmed that MSCs could express all of the genes of ectoderm, endoderm and mesoderm, providing the theoretical foundation of MSC multiple differentiation potential[24-25]. Artificial bone, muscle, blood vessels, skin, and nerves derived from MSCs are under clinical trials. Meanwhile, MSCs have been used in co-transplantation therapy to improve transplant success rate[26-27]. In the field of ophthalmology, MSCs have been induced to differentiate into retinal cells and corneal epithelial cells in some studies[28-30]for direct cell replacement therapy, and also have been used to suppress immune rejection after corneal and retinal transplantation[17,31-33]. Ohet al[20]and Maet al[34]all demonstrated the anti-inflammatory and anti-angiogenic abilities of MSC in chemical burned ocular surface model. MSC could upregulate thrombospondin-1 (TSP-1) to inhibit neovascularization by disrupting vascular endothelial growth factor receptor-2 (VEGFR-2) and CD47 signaling[35-36]. Brayet al[37]also exhibited the immunosuppressive properties of corneal limbus-MSCs and provided a possible allogeneic strategy, but withoutin vivoexperimental verification.
We chose to use bone marrow MSCs to construct the substitute corneal graft for their simplicity of gaining[11]and relative strong cell passage ability, though fetal MSCs have greater propagation capacity in general[38]. In the current study, a high purity of MSCs was obtained by Percoll separation and cell adherent methodin vitro. The cells showed adherent growth pattern at 1 to 2h after inoculation, and adherence was basically completed after 4h. The passaged cells were consistent, with fusiform appearance, and arranged in parallel or concentric manner. Two methods are employed to identify MSCs: surface membrane marker detecting and inverse extrapolation identification[39-40]. Flow cytometry analysis revealed that most cells isolated in our study were CD90 positive and CD34, CD45 negative, which correspond with MSC surface biomarker characteristics[15]. The multipotential differentiation ability proved by osteogenic and adipogenic induction further confirmed their identity.
The immunogenicity of ACM was low, which only account for 1.62% of all cell-mediated immunity[41]. ACM could provide a suitable microenvironment for cells to grow onto, Yoerueket al[42]had successfully cultured human corneal cells in decellularized corneas. Xuet al[43]used XDCM as a scaffold for cornea reconstruction. ACM alone as corneal lamellar transplantation substitute has been plagued by postoperative neovascularization and subsequent immune rejection[44]. Our previous study reported that MSCs may present beneficial factors for corneal recovery under the ACM microenvironment[21]. Based on these works, we decided to culture MSCs onto XDCM to form the bio-engineered graft. We observed that MSCs showed adherent growth pattern on XDCM. By day 14, LK surgery time point of this study, the number of cells significantly increased and covered up to about 70% XDCM surface, cells were in spindle-shape and wellarranged, small fluあ and pseudopodia could be observed which indicated good viability.
Forin vivoexperiment, we lamellar transplanted our bioengineered corneal grafts, which constructed by rabbit MSCs cultured on canine XDCM for 14d, on to rabbit corneal stroma implant bed and found that the incidence of angiogenesis three months after transplantation of XDCM-MSC group was significantly lower than that in the XDCM group. The edema of XDCM-MSC grafts in the first week was because that these grafts did not go through dehydration before transplantation. XDCM-MSC graft edema gradually alleviated after surgery and by the time of 3mo XDCM-MSC grafts showed a more transparent appearance than XDCM grafts under slit lamp
observation. The average opacity score of XDCM-MSC group was lower than that of XDCM group, though there were no statistical significance between two groups, which may due to the limited sample number. At one month the corneal epithelium defection percentage of XDCM-MSC group significantly lower than that of XDCM group, showed that the epithelium recovery courses of XDCM-MSC group were faster. These results suggested that MSCs could help resist neovascularization and promote ocular surface repair after heterograft transplantation, therefore may enlarge the source range of corneal substitute.
The reason for the relative larger area of cell-absent zone in XDCM-MSC group is unknown. We thought that stromal cells from peripheral rabbit implant bed grow gradually into the graft tissue and in turn started stromal cell reconstruction. In common state, cornea stromal cells are dormant, and can be activated into fibroblast cells under stimulations such as trauma, surgery, inflammation, some cornea stromal cells can further transform into myofibroblasts and perform migration[45]. Besides, no regenerated nerve fibers were observed in XDCMMSC group 3mo after the operation, though several studies showed a nerve-stimulate abilities of MSCs[46-47]. We speculated that this was because of the corneal avascular anatomic characteristics. Since MSCs could inhibit neovascularization and inflammatory response to keep the transparency of corneal graft, at the same time, this inhibitory eあect may reduce innergraft vasogenic chemokines that drive corneal stroma cell migration and nerve growth factor that induce innervation[46]. Less stromal cells and less neovascularization cause the lack of nutrition metabolism in grafts may also attribute to the delay of nerve regeneration. Further experimental studies are needed to confirm these hypotheses. Besides the temporary noninnervation status of the graft would not reduce the success rate of transplantation[7].
To the best of our knowledge, this is the first article to cotransplant bone marrow MSC with XDCM hence prevent postoperative neovascularization. However, some limitations do exist: 1) The sample size was small, increased sample volume is needed in the future to further confirm the result; 2) This study did not labeled and tracked MSCs, so the MSC existence duration on ocular surface is unclear. Whether MSCs only had immune regulation eあect or had mesenchymal epithelial transition (MET) procedure need further investigate. Still, our research preliminarily revealed the high development potential of MSC in the field of cornea transplantation. Further studies of comparing anti-neovascularization abilities of MSCs co-transplantation and postoperative steroids are needed in the future. And co-transplantation of MSCs is promising to be applied to clinic to reduce the use of steroids.
In conclusion, we constructed a novel corneal bio-engineered graft, which used MSCs as seed and XDCM as carrier. The graft could successfully reduce post-operatively neovascularization and remain good clarity after three months, which indicate that cotransplantation of MSCs could be used to help increase corneal transplantation successful rate and enlarge the source range of corneal substitute in order to overcome the shortage of cadaveric cornea tissue.
ACKNOWLEDGEMENTS
Authors’ contributions:Data curation: Cen YJ, You DB; Formal analysis: Cen YJ, You DB; Funding acquisition: Feng Y; Investigation: Cen YJ, You DB; Methodology: Feng Y, Wang W; Software: Cen YJ, You DB; Supervision: Wang W; Writing-original draft: Cen YJ; Writing-review & editing: Cen YJ, You DB.
Foundations:Supported by National Natural Science Foundation of China (No.81700799); Clinical Medicine Plus X-Young Scholar Project, Peking University.
Conflicts of Interest: Cen YJ,None;You DB,None;Wang
W,None;Feng Y,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
- RNA interference targeting NOX4 protects visual function in an experimental model of retinal detachment by alleviating blood-retinal barrier damage
