Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
Yao-Wen Song, Jun-Yu Chen, Xu Li, Li Wang, Zhi-Qiang Pan
1Shanxi Eye Hospital, Taiyuan 030002, Shanxi Province, China
2Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthalmology and Visual Science Key Laboratory, Beijing 100730, China
Abstract
INTRODUCTION
The corneal endothelium is a monolayer of polygonal cells functioning as an ion exchanger, thus acting as a barrier to maintain corneal transparency. Corneal endothelial dysfunction induces stromal edema, which gradually develops to bullous keratopathy. Endothelial keratoplasty (EK) is currently an effective treatment for corneal endothelial dysfunction[1]. Compared to penetrating keratoplasty, EK decreases postoperative immunological rejection and significantly prolongs the graft survival time[2]. However, the limitations of EK are the complicated procedure and donor shortage. Hence, methods for culturing corneal endothelial cells (CECs)in vitroand further injection of the cultured cells into the anterior chamber directly along with Rho-associated kinase (ROCK) inhibitors are emerging[3-4]. Kinoshitaet al[5]cultured human CECs from 7 donor corneas and injected the cultured cell suspensions with Y-27632 into the anterior chambers of patients diagnosed with bullous keratopathy. After 2y of follow-up, all patients maintained corneal transparency with no significant complications. Although this result was encouraging, Kinoshitaet al[5]described that human CECs cultured from single donor cornea can be provided for only two patients, as only the subcultured cells in the second to third passages were suitable for clinical use. This was further confirmed by Bartakovaet al[6]who showed that human CECs passaged four times enter a mesenchymal state, gradually losing their barrier function.
Because of the donor shortage, feasible alternatives for human corneal tissues are needed. The porcine cornea resembles the human cornea with respect to its tissue structure and biomechanical properties[7]. Pigs are abundant and easily accessible[8]. The apoptotic and necrotic percentages of porcine CECs were significantly lower than those of humans[9]. Further, the xenoantigens in porcine CECs were significantly weaker than those of porcine corneal epithelial and stromal cells[10]. Among all porcine strains, the Wuzhishan miniature porcine corneas exhibit very close characteristics as those in humans[11]. Previously, Liuet al[12]showed that Xeno-Descement’s stripping automated EK from Wuzhishan pig to rhesus monkey was eあective. Our results showed that five of seven monkeys gained corneal transparency within 30d postoperation. Among them, 4 porcine corneal endothelial grafts survived for more than 180d. Based on our previous study, we explored the influence of the ROCK inhibitor Y-27632 on an inbred Wuzhishan porcine CEC suspension and its potential for corneal endothelial dysfunction treatment. We investigated the eあects of Y-27632 on cultured porcine CEC proliferation and explored whether this system can be used to treat corneal endothelium dysfunction.
MATERIALS AND METHODS
Ethical ApprovalAll procedures for the housing and treatment of animals in our experiments strictly complied with the ARVO Statement for the use of animals in Ophthalmic and Visual Research. This study was also approved by the Animal Ethics Committee of Capital Medical University, China (No.AEEI-2016-092).
Primary CulturesFour porcine corneas from an inbred line of Wuzhishan miniature pigs (WZSP; aged 10-12mo, wild-type, 26thgeneration) were the sources of primary cultured cells. The pigs were kindly gifted by Grand Life Science and Technology Company (Beijing, China). Porcine globes were thoroughly washed with 5% povidone-iodine solution and soaked in sterile phosphate-buffered saline (PBS; Gibco, Grand Island, NY, USA) containing 10000 U/mL tobramycin for 30min. Porcine corneas with scleral rims were excised in a 10-cm culture dish (Falcon, Corning, Inc., Corning, NY, USA) and rinsed with sterile PBS containing 1% antibiotic (5000 units/mL of penicillin and 5000 μg/mL of streptomycin, Gibco). The corneas were placed, endothelial side up, in a sterile 10-cm culture dish, treated with 0.25% trypsin (Gibco), and incubated for 30min at 37℃. The cells were then scraped from the corneal endothelium, centrifuged at 1000 rpm for 5min, and resuspended in RPMI 1640 medium (Catalogue number C11875500BT, Gibco) with 1% antibiotic and 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). Porcine corneal endothelial cells (PCECs) were seeded into a 6-well culture plate (Corning) and then incubated at 37℃/5% CO2until reaching confluence. The confluent cells were trypsinized and counted using a cell counting chamber and passaged further at ratios of 1:2 to 4.
Immunofluorescence AnalysisPrimary cultured PCECs were grown to 80% confluence and transferred to a glass slide in a 6-well culture plate. After attaching to the glass slide, the PCECs were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), and blocked with 10% FBS. PCECs were incubated at 4℃ overnight with 1:400-dilution rabbit monoclonal antineuron-specific enolase (NSE) antibody (Zeye Biological Technologies Co., Ltd., Shanghai, China) and then incubated at room temperature with a 1:400-dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (Zeye). The samples were treated with antifade mounting medium containing DAPI (Solarbio Science & Technology Co. Ltd., Beijing, China). Images were captured using a fluorescence microscope (BX41, Olympus, Tokyo, Japan).
MTT AssayY-27632 was used to evaluate PCECs proliferation at different working concentrations (0, 10, 30, 100, and 200 μmol/L)[13-15]. The experiment was performed in quadruplicate. Cultured PCECs in passage two were plated in a 96-well plate (Corning) at a density of 5×104cells/well. After the cells had adhered, PCECs were treated with diあerent concentrations of Y-27632 (Selleck Chemicals, Houston, TX, USA) for 48h. Next, 20 μL of MTT was added to each well and incubated for 4h. To record the optical density (OD) at 490 nm, 200 μL dimethyl sulfoxide (Sigma) was added to each well and the plate was gently shaken for 10min.
5-Ethynyl-2’-deoxyuridine-labeling AssayWhen passage two PCECs reached 80% confluence, they were treated with diあerent concentrations of Y-27632 for 48h. Next, the PCECs were treated with 10 μmol/L 5-ethynyl-2’-deoxyuridine (EdU; Abcam, Cambridge, UK) for 3h. The PCECs were fixed using 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, and 0.5 mL Click-iT reaction cocktail (Abcam) was added to each well. After gentle mixing, the plates were incubated for 30min in the dark. The samples were counterstained with DAPI for 10min, and images were captured with a fluorescence microscope.
In Vivo StudiesSeven New Zealand rabbits (4 males and 3 females, aged 80-120d, weighing 2.0-2.5 kg) were purchased from the LongAn Laboratory Animal Breeding Center (Beijing, China). Five rabbits (R1-R5) were injected with the PCEC suspension and Y-27632 into their anterior chambers and the other two rabbits (R6-R7) were used as controls. The experimental procedure was performed as follows: rabbits were anesthetized with 2.5% sodium pentobarbital auricular vein injection at a dose of 0.1 mL/kg, and the left eye of each rabbit was operated. A small 2-mm incision was created in the limbus of the rabbit’s eye and viscoelastic was injected into the anterior chamber to protect the lens. All ranges of the corneal endothelium from the substrate center were removed using a reverse hook. The limbus incision was sutured with a 10-0 suture before removing the viscoelastic from the anterior chamber. All rabbits were fed normally for 1wk. After that, slit lamp was used to determine whether the rabbit corneas maintained their edema and opacity. A 0.2-mL cell suspension containing 5×105PCECs and 100 μmol/L Y-27632 was injected into the anterior chamber of R1-R5. After the surgery, the injection group was injected with 3.5 mg betamethasone (Schering-Plough, Kenilworth, NJ, USA) subconjunctivally and were placed face down for 3h to promote the attachment of PCECs to the posterior substrate. During the observation time, levofloxacin eye drops (Santen, Osaka, Japan) and tobramycindexamethasone eye ointments (Alcon, Puurs, Belgium) were applied three times per week for all rabbits until the end of the observation period.
All rabbits were sacrificed at 14d post-surgery. Rabbit globes were enucleated, and corneal sections were prepared. To perform trypan blue-alizarin red staining, 0.25% trypan blue solution (Servicebio, Wuhan, China) 0.2% alizarin red solution (Servicebio) were slowly dripped onto the rabbit corneal endothelium surface in turn for 90s. The morphology of PCECs was captured and observed by light microscopy (Leica).
Hematoxylin-eosin (HE) staining was performed to observe the morphological changes of rabbit corneal tissues. The 5-μm corneal sections were placed into xylene, 100% ethanol, and 75% alcohol successively and then stained with hematoxylin solution for 5min. The sections were stained with eosin for 5min after gradient dehydrating with 85% and 95% alcohol. The sections were dehydrated again with ethanol and xylene until they became transparent, after which they were mounted with neutral gum. Light microscopy was conducted to observe the tissues and acquisition of images for analysis.
In addition, immunofluorescence analysis was performed to detect pump and tight junction protein expression in the rabbit corneal endothelium. After deparaffinization with xylene and ethanol, the tissue sections were transferred to citric acid antigen repair buffer (pH 8.0) in the microwave for antigen retrieval. The tissue sections were then blocked with 3% bovine serum albumin (BSA) for 30min. Anti-Na+/K+-ATPase or antizonula occludens-1 (ZO-1) primary antibodies (Invitrogen, Carlsbad, CA, USA) were used. After overnight incubation at 4℃, a 1:500 dilution of FITC-conjugated secondary antibody (Invitrogen) was added, and the samples were incubated for 50min. Cell nuclei were counterstained with DAPI. The tissue sections were mounted with anti-fade mounting medium and captured using a fluorescence microscope (Zeiss).
Statistical AnalysisDunnett multiple-comparisons test was used to analyze the statistical significance among multiple sample sets such as OD values in MTT assay. Values shown on graphs were expressed as the mean±SE. SPSS20.0 software was used for data analysis (SPSS, Inc., Chicago, IL, USA).P<0.05 was considered as statistically significant.
RESULTS
Primary Culture and Cell Identification of PCECsThe trypsinized PCECs adhered to the 6-well culture plate within 24h after isolation from the porcine corneas. Upon visualization using a microscope, they remained as small scattered clusters. A confluent monolayer of oval or hexagonal cells was observed between 14-21d. However, the cells located on the edge of cellular layers showed an elongate and flatten pattern, which slightly diあered from canonical PCECs (Figure 1B). The cell density was approximately 2×106/well. The presence of NSE-positive cells was detected by immunofluorescence analysis (Figure 1).
Y-27632 Increases Proliferation of Subcultured PCECsWe observed an increase in the proliferation of subcultured PCECs with all concentrations of Y-27632 ranging from 10-100 μmol/L by counting the cells using a cell counting chamber. Of all concentrations, the cells treated with 100 μmol/L Y-27632 exhibited the highest proliferation rate. However, when 200 μmol/L Y-27632 was used, it significantly reduced the proliferation of PCECs, and the cells were prone to endothelialmesenchymal-transition (EnMT). The MTT assay results agreed with the microscopy findings. We observed a significant increase in cell viability in the 100 μmol/L Y-27632 group compared to the control. In addition, the EdU-labeling assay in 48h PCEC subcultures showed increased EdU-positive cells upon treatment with Y-27632 compared to untreated control cells (Figure 2). These findings indicate that Y-27632 increases the proliferation of PCECsin vitro.
Y-27632 and PCECs Restored Corneal TransparencySeven corneas from rabbits, R1-R7, showed edema and opaque conditions within 48-72h after surgery. Y-27632 injected along with the PCECs led to gradual improvement in corneal transparency. And some mild edema signs prevailed in the injection group corneas treated with the PCEC suspension and Y-27632 after postoperative 14-day follow-up. In contrast, the R6-R7 rabbit corneas had visible edema and an opaque exterior, although the peripheral opacity area was decreased. This indicates that damaged rabbit CECs rarely achieved selfhealing. Hence, combination treatment with Y-27632 and PCECs resulted in visible improvement in the R1-R5 rabbit’s corneal transparency (Figure 3). Further trypan blue-alizarin red staining showed that PCECs adhered to the posterior substrate had a clear border and attached closely with each other. Most were living cells, but their shape was irregular and polygonal. Moreover, the cell nucleus was not significantly stained. Based on the HE staining results, the rabbit cornea of the injection group showed regular thickness and a dense structure. Attached PCECs in the posterior R1 corneal surface were visible. In the control group, the edema cornea including loosened stroma and damaged endothelium structure was visible in the images. In addition, immunofluorescence analysis revealed positive expression of Na+/K+-ATPase and ZO-1 in the R1 corneal endothelium. The expression of these two markers was very low in the R6 corneal endothelium. This indicates that dense tight junctions are crucial for improving the morphology of injected PCECs for successful grafting (Figure 4).

Figure 1 Culture of PCECs from the inbred line Wuzhishan porcine corneal endothelium A: Post-extraction 2-3d, the primary cells aggregated into to form clusters. B: Confluent cultured cells showed a morphology of paving stones after 14-21d. The cells in the culture flask’s periphery were elongated and flattened because of a lack of contact inhibition. Scale bar: 100 μm. C: Immunofluorescence analysis to identify NSE-positive cells in the monolayer with DAPI (labeled by arrows). Scale bar: 50 μm.

Figure 3 Animal studies in New Zealand rabbits Seven rabbit eyes represented opacity and edema at postoperative 48h. However, PCECs injected into the R1-R5 eyes gradually functioned over time. The R1-R5 corneas partly restored to transparency at postoperative 14d, whereas the central area of R6-R7 cornea still showed visible opacity and edema (R1 and R6 corneas were shown in the figure).
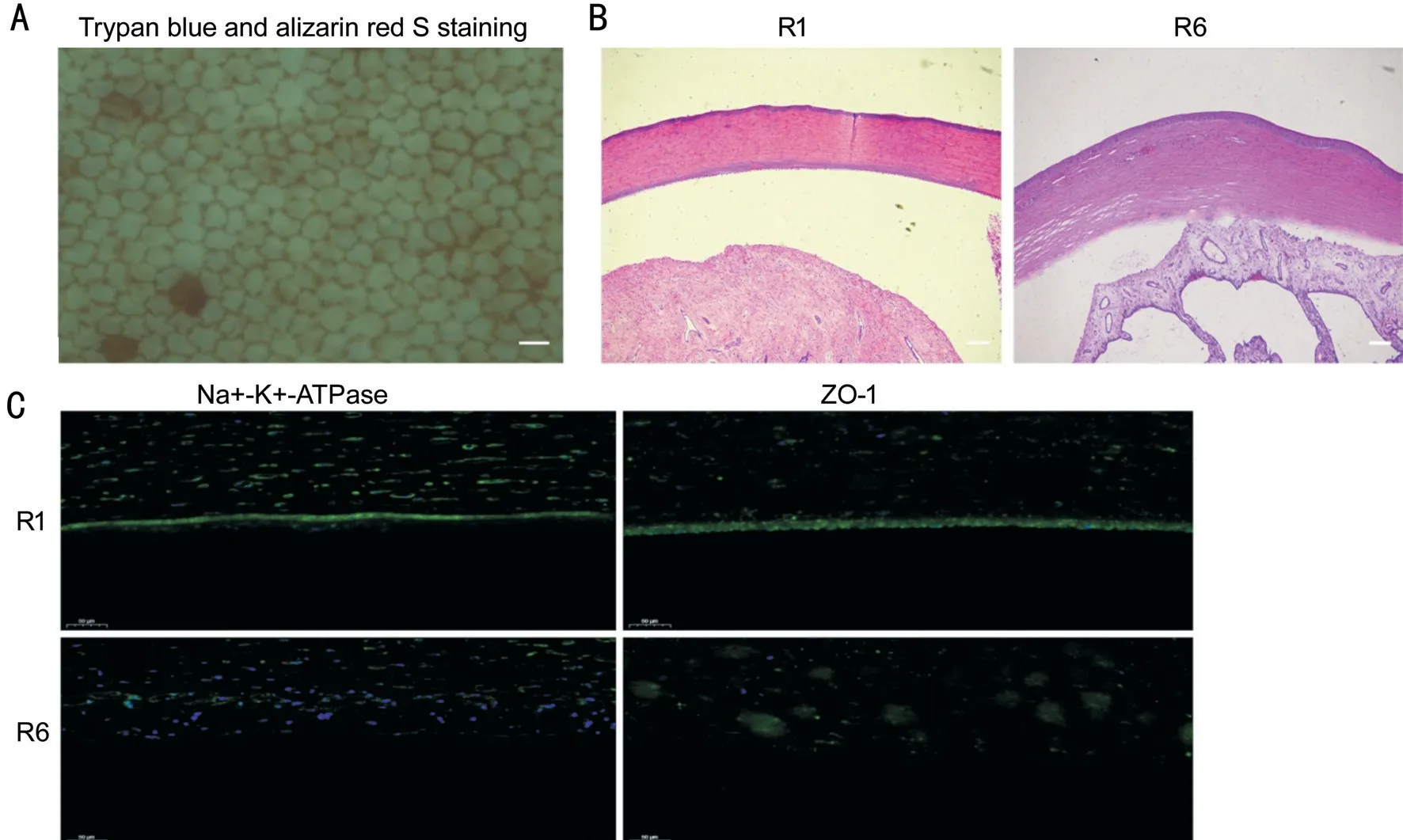
Figure 4 Some staining examinations in rabbit corneal tissues A: Morphology of PCECs attached to rabbit posterior corneal substrate determined by staining with trypan blue-alizarin red. Scale bar: 50 μm. B: HE staining showed that the R1 cornea represented regular thickness and dense structure, whereas edema, thickness, and damaged endothelium structure was observed in the R6 cornea. Scale bar: 200 μm. C: Immunofluorescence staining showed significant positive expression of Na+/K+-ATPase and ZO-1 in the R1 corneal endothelium. However, these two functional markers showed low staining in the R6 corneal endothelium. Scale bar: 50 μm.
DISCUSSION
ROCK inhibitors affected myosin light chain (MLC) phosphorylation to promote contraction of myofilaments, which leads to cell adhesion[16]. Y-27632 is a selective ROCK inhibitor that targets the protein kinases activity of ROCK I and ROCK II, thereby inhibiting the phosphorylation of MLC. This aids in cytoskeleton regulation, stress fiber formation, and cell contraction[17]. Some studies also demonstrated that at specific concentrations, it restrains ROCK activity to promote cell proliferation, migration, and adhesion. Although Y-27632 is still in the phase of basic research and clinical trials, it has been confirmed to eあectively dampen ROCK activity in a few human epithelial and endothelial cells[18]. Importantly, Y-27632 has been implicated in corneal diseases, and given that inbred WZSP corneas are ideal donors for treating endothelial keratoplasty because of their thickness, refractive properties, and similar size as in humans, so in this study, we derived PCECs from several inbred WZSP corneal endotheliums. We designed cellular experiments to observe the role of Y-27632 on PCECs’ proliferation, functional protein expression, adhesion and migration, and its appropriate concentration for enhancing cell populations and functions should be determined explicitly. Then we tested PCECs’ eきcacy with Y-27632 for treating corneal endothelial dysfunction in rabbits.
In addition, other studies indicated that ROCK inhibitors upregulated cell division cycle factor 25A (Cdc25A) by stimulating the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cdc25A positively regulates the degradation of P27Kip1. This indicates that inhibiting ROCK activity can promote cell proliferation[14,19]. Wanget al[15]reported that Y-27632 influenced the mitogen-activated protein kinases family members extracellular signal-regulated kinase and p38, both of which have important roles in cellular processes, such as proliferation and diあerentiation. In this study, we observed that Y-27632 significantly promotes PCEC proliferation and the cultured cells exhibited a normal morphology. We demonstrated that 100 μmol/L was the optimal concentration of Y-27632 for inducing PCEC proliferation. In agreement with our studies, Okumuraet al[3,20]injected a CEC suspension into the anterior chamber with 100 μmol/L Y-27632. Some previous studies indicated that the effect of Y-27632 on cell proliferation was dose-dependent, and in some cases, Y-27632 could suppress cell growth and proliferation[21-22]. In fact, it is confirmed that the positive eあect of Y-27632 on PCEC proliferation declined at Y-27632 concentrations over 100 μmol/L.
We attempted to inject the subcultured PCECs into the anterior chamber of rabbits. Porcine CECs attached to the posterior corneal substrate of rabbits in the presence of 100 μmol/L Y-27632. Considering that rabbit CECs possess the capacity to regeneratein vivo, we scraped the rabbit’s corneal endothelium completely and guaranteed that the corneal edema were still visible 1wk later, and then PCECs were just injected into the rabbits’ anterior chamber. After double staining of the posterior corneal surface, trypan blue penetrated the damaged or dead corneal cell membrane, and then stained the nucleus revealing the cell’s activity. Alizarin red reacted with calcium ions between cells. Staining of the Descement membrane highlighted the border of undamaged CECs. Thus, this double staining indicated that the morphology of cultured PCECs was not same as that of original CECsin vivo. Briefly, these cells were not manifested as hexagons but showed an irregular polygonal pattern. Some cells exhibited stretching but contacted each other densely enough to perform pump and barrier functions. We confirmed that cultured cells secreted functional proteins and adhered to the growth matrixin vitro. Therefore, the CEC density is the most significant factor for evaluating postoperative EK or cell injection efficacy as long as no obvious EnMT changes occur. HE staining revealed the completed corneal endothelium structure in the injection group. Immunofluorescence analysis showed positive expression for Na+/K+-ATPase and ZO-1 in the PCECs. The corneal endothelium in the control rabbit exhibited weak expression of the above two surface proteins, indicating that damaged rabbit CECs rarely possessed regenerate potential to fill the corneal central region. Some rabbit corneas injected with PCECs did not completely recover to transparency, possibly because PCECs induced inflammatory reactions and xenotransplantation rejectionin vivo, despite the topical immunosuppressive regimes and Y-27632 addition. Studies to determine the mechanism underlying this immune response and methods for overcoming this response are needed before these cells can be used in corneal endothelial dysfunction therapy. Further, the small sample size is a disadvantage of our study, which can be overcome in the future to provide more convincing results.
In conclusion, we revealed the role of Y-27632 in improving the proliferation potential of inbred line Wuzhishan miniature PCECs. Our animal study indicates that PCEC injection into the anterior chamber is practical for treating corneal endothelial dysfunction in rabbits. In the future, we will attempt to increase the number of cultured PCECs by incorporating modifications in the collection and surgical procedures. Our findings provide insight into the use of PCECs with the ROCK inhibitor, Y-27632, for eあectively treating corneal endothelial dysfunction.
ACKNOWLEDGEMENTS
The authors thank Beijing Grand Life Science and Technology Company for providing porcine globes.
Foundation:Supported by National Natural Science Foundation of China (No.81470608).
Conflicts of Interest:Song YW,None;Chen JY,None;Li X,None;Wang L,None;Pan ZQ,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
- RNA interference targeting NOX4 protects visual function in an experimental model of retinal detachment by alleviating blood-retinal barrier damage
