RNA interference targeting NOX4 protects visual function in an experimental model of retinal detachment by alleviating blood-retinal barrier damage
Kai Dong, Nan Yang, Jie Ding, Yuan-Ye Yan, Li Lu, Yi-Sai Wang
1Department of Ophthalmology, Anhui Provincial Hospital, Anhui Medical University, Hefei 230001, Anhui Province, China
2Department of Ophthalmology, the Second People’s Hospital of Hefei, Hefei 230001, Anhui Province, China
3Department of Ophthalmology, Wannan Medical College, Wuhu 241001, Anhui Province, China
4Department of Ophthalmology, Eye Center, the First Aきliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China
Abstract
INTRODUCTION
Retinal detachment (RD) is defined as the detachment of the retinal neuroepithelial layer and the pigment epithelium, and RD is a common blindness-causing disease. Although surgery can reduce the damage to the retinal anatomy in most patients, the postoperative visual function of patients is still unsatisfactory. In recent years, a large number of experimental studies have found that the unsatisfactory postoperative recovery of visual function after RD is related to the degeneration of retinal photoreceptor cells[1]. There are many mechanisms of photoreceptor death, including necroptosis, apoptosis and autophagy. Degterevet al[2]found that autophagy can also be activated during necroptosis. Moreover, our group detected the morphological changes of necrotic apoptosis and the high expression of reactive oxygen species (ROS) in photoreceptor cells[3-4].
The integrity of the blood-retinal barrier (BRB) is essential to maintain the normal structure and function of the retina. The destruction of the retina leads to the destruction of the BRB[5]. The BRB is composed of retinal vascular endothelial cells, adhesion zonules, occluded zonules, and retinal pigment epithelial cells[6]. Any damage to retinal vascular endothelial cells and retinal pigment epithelial cells, such as the damage observed in RD, retinitis pigmentosa, and diabetic retinopathy, can cause BRB destruction. Some authors have found that inhibiting NADPH oxidase 4 (NOX4) expression during myocardial infarction, hindlimb ischaemia, and stroke can specifically protect against stroke injury, mainly because the inhibition of NOX4 expression can effectively prevent the leakage of the BRB[7].
NOX is one of the main sources of ROS. Studies have shown that NOX may play an important role in regulating ROS production and apoptosis[8], and NOX4 is currently considered to be the main source of ROS production in the vascular endothelium[9-10]. Our group found that the expression of ROS increased after RD, and rapamycin treatment could reduce the expression of ROS and inhibit the necrotic apoptosis of photoreceptor cells[4]. Based on these findings, this study aims to examine the damage to the BRB, the expression of NOX4 and ROS after RD, and whether inhibiting NOX4 can exert a protective effect against BRB damage and improve visual function. It is hoped that this study will yield new discoveries for the protection and restoration of patients’ residual visual function and provide an important therapeutic target.
MATERIALS AND METHODS
Ethical ApprovalAll animal experiments were conducted with strict adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
AnimalsAdult, healthy, male SD rats weighing approximately 260 to 300 g were used, and they were provided by Anhui Medical University and the Provincial Hospital aきliated with Anhui Medical University. All the experimental rats were housed in a normal environment, and preoperative examination of the fundus was performed to ensure that the eyes of the experimental animals were normal.
Construction and Screening of VectorsNOX4 was inhibited by shRNA encoded by the green fluorescent proteinadeno-associated virus (GFP-AAV2) vector (Suzhou Gene Pharma Co., Ltd., Suzhou, China). The NOX4 sequence was blasted in PubMed (NM053524.1). Three AAV2-shRNAs were designed to target different regions of NOX4: NOX4-rat-321 5’-GCAGGAGAACAAGAAGATTGT-3’; NOX4-rat-578 5’-GCTTCTACCTATGCAATAAGA-3’, and NOX4-rat-1207 5’-GCCTCCATCAAGCCAAGATTC-3’. The sequence of the negative control AAV2-shRNA was 5’-TTCTCCGAACGTGTCACGT-3’. PC-12 cells were transfected with the four vectors described above. The expression level of NOX4 in transfected rats was detected using PCR.
Subretinal Injection of the AAV2 Virus VectorsPrior to initiating the different treatment methods, the experimental rats were anaesthetized with 10% chloral hydrate (400 mg/kg). The pupils were dilated with compound tropicamide eye drops before the operation, and the conjunctival sac was washed with levofloxacin eye drops. The vitreous cavity was punctured with a 50 G needle. After entering the vitreous cavity through the puncture hole, the needle slowly approached the retina, avoiding the retinal vascular, and 5 μL of the viral vector was injected into the subretinal space through the hole. The right eye of each rat was used as the experimental group, and the left eye was used as the control. After 3wk of subretinal injection of the AAV2 viruses, virus transfection was observed by frozen sectioning.
Establishment of the Experimental Retinal Detachment Model in RatsAfter 3wk of virus transfection, the rats were divided into the normal control group (attached group), non intervention group (untreated group), NOX4 intervention group (sh-NOX4 group), and negative intervention group (sh-neg group). The rat model of RD was established in the right eye of each experimental rat, and the left eye of each rat was used as a control. The rats were anaesthetized by intraperitoneal injection of chloral hydrate (10%, 400 mg/kg). The pupils were treated with compound tropicamide eye drops before the operation. The 23 G needle was used to penetrate the sclera vertically 2 mm after the corneoscleral margin. One percent sodium hyaluronate (50 mL) was injected into the periphery through the hole. Then, the needle was removed after the RD ridge was approximately 1/2 as observed under the microscope. A suture (10-0) was used to mark the approximate position of the range of RD, which rendered sampling more convenient. The treated eyes were treated with levofloxacin eye drops daily to prevent infection.
Observation of Structural Changes in the Rat Vascular Endothelial CellsThree days after the establishment of the RD model, the SD rats were anaesthetized by abdominal injection, and rat retinal tissue was harvested. The retinal tissue was cut into a size of 1 mm3and fixed in 2.5% glutaraldehyde for 4h at 4℃. Then, the tissue was washed three times with phosphate buあer saline (PBS) solution for 15min. The tissue was then removed and placed in 1% citric acid for 1h. The tissue was dehydrated by incubation in 30% and 50% ethanol for 15min each. Then, the tissue was removed and placed in a 70% saturated solution of uranyl acetate for 6h. Then, the tissue was dehydrated by incubation in 80% and 95% ethanol in sequence. After the epoxy resin was embedded, the tissue was cut into ultrathin sections (70-nm, LKB-NOVA type, Sweden). The retinal vascular endothelial structure and photoreceptor cell morphology were observed using a Nissan JEM-1230 microscope.
Western BlotRetinal tissue was harvested three days after the establishment of the RD model. The proteins were extracted in RIPA lysis buあer, and a 12% separation gel and a 4% concentrated gel were prepared for electrophoresis. After electrophoresis at a constant voltage, the proteins was separated, and the gel was removed from the apparatus. Then, the proteins were transferred for 2h at a constant voltage of 25 V, and the membrane was blocked with 5% skim milk for 1h. After that incubation, the blocking solution was removed by washing with Tris buあered saline Tween (TBST), and the diluted primary antibody against NOX4 (1:1500. Abcam, Cambridge, MA, USA) was added and incubated with a diluted primary antibody against GAPDH (1:2000. Sigma-Aldrich Chemie GmbH, Munich, Germany) at 4°C overnight. The primary antibodies were removed, and the membrane was washed three times with TBST for 10min each time and incubated with an HRP-labelled secondary antibody (1:5000 dilution in TBST, Santa Cruz Biotechnology) for 4h at 4°C. The secondary antibody was discarded, the membrane was washed three times with TBST for 10min each time, and the bands were visualized by the ECL chemiluminescent agent. The grey values of the electrophoresis bands were detected using Bandscan 4.3 software (Glyko, Canada). The NOX4 protein was compared to the internal reference GAPDH to determine its relative expression level.
Retinal Smear to Detect Retinal Vascular Leakage in RatsThree days after the establishment of the RD model, the rats were anaesthetized with chloral hydrate. The rats were exposed to a concentration of 30 g/L Evans blue (EB) solution (at a dose of 45 mg/kg) within 2min. The perfusion was successful, and the eye ring was placed in 4% paraformaldehyde for 2h in the dark. The eye ring was cut in PBS along the optic papilla into the shape of a cross petal. Then, the retina was bluntly separated with ophthalmic scissors, and the retina was completely removed and placed in PBS, divided into 4 quadrants, and the specimens were placed on the slides. Once it was removed, the translucent retina could be observed on the slide. An anti-fluorescence quencher was used to mount the slide, and the EB red fluorescence spot in the retina was observed by a confocal microscope at an excitation wavelength of 654 nm and photographed.
Morris Water Maze Assessment of Visual Function in RatsIn the modified Morris water maze (MWM) experiment[11], the rats were placed in the pool facing the wall, and the time required to move from four equidistant positions to the platform on the water surface (escape latency) was recorded. The rats were given 60s to arrive at the platform. If the rats did not arrive at the platform within the stipulated time, they were gently guided to the platform, they were allowed to stay on the platform for 10s, and their escape time was recorded as the maximum time (60s). The visual function of the rats was expressed as their escape latencies. Seven days after the RD model was established, the control eye of each rat was sutured. The MWM test was repeated once. All the measurements were recorded using a camera with an infrared source.
Rat Retinal ROS Intensity TestThree days after the establishment of the RD model, 50 mg of fresh retinal tissue from each group was washed with PBS, and 1 mL of homogenization buffer A was added. The tissue was homogenized and centrifuged at 1000 g for 10min at 4℃. The precipitate was discarded, and the supernatant was added to a 96-well plate. The probe was added and incubated at 37℃ for 30min in the dark. The fluorescence intensity was measured in a microplate reader by using an excitation wavelength of 530 nm and an emission wavelength of 610 nm. Another 50 mL of the homogenate supernatant was diluted 30 times with PBS, and the protein concentration in 100 mL of this solution was measured. The ROS intensity of the tissues is expressed as the fluorescence intensity per milligram of protein.
Statistical AnalysisAll the experimental results are expressed as the mean±standard deviation. Data analysis was performed using the SPSS 17.0 software package. ANOVA was used to compare the data between groups.P<0.05 was considered statistically significant.
RESULTS
Screening of Adeno-Associated VirusTo identify the viral interference sequences with the best interference eあect, PCR was performed to determine the relative expression level of NOX4 in rat PC-12 cells transfected with virus. As shown in Figure 1A, the expression ofNOX4was decreased in cells transfected with the NOX4-rat-321, NOX4-rat-578, and NOX4-rat-1207 viruses relative to that in the cells transfected with the blank control and negative interfering viruses, and the interference effect of NOX4-rat-1207 was the best. Therefore, NOX4-rat-1207 was used for the subsequent animal experiments. The final NOX4-rat-1207 virus titre selected for the experiments was 1.12×1013v.g./mL, and the AAV-NC virus titre was 3.99×1010v.g./mL.
Transfection Efficiency of the Subretinal Injection of Adeno-Associated VirusAs shown in Figure 1B, after 3wk of the injection of AAV2 virus into the subretinal space of the rats, the retinal tissues of the rats were harvested for frozen sectioning. The GFP fluorescence and DAPI staining were observed under an inverted fluorescence microscope using blue light and red light. It can be observed that most of the retina was eあectively transfected by the virus, the photoreceptor cell layer exhibited strong GFP fluorescence intensity, and only a small amount of GFP was expressed in the other layers.
NOX4 Interference Alleviated the Destruction of the Retinal Vascular EndotheliumThree days after the establishment of the RD model, the morphology of the vascular endothelial cells and the tight junctions between them were observed by electron microscopy. Figure 2A is a normal group (attached group) of rat retinal vascular endothelial cells, and it can be observed that the morphology of the endothelial cells was normal, the shuttle was performed, and the tight junctions between the cells were intact. Figure 2B is the untreated group, Figure 2C is the sh-NOX4 group, and Figure 2D is the shneg group. In these three groups, the vascular endothelial cells were swollen, and the tight junctions between the cells were incomplete. Compared with that in the untreated group, the degree of destruction of the connections between the cells in the sh-NOX4 group was significantly reduced.
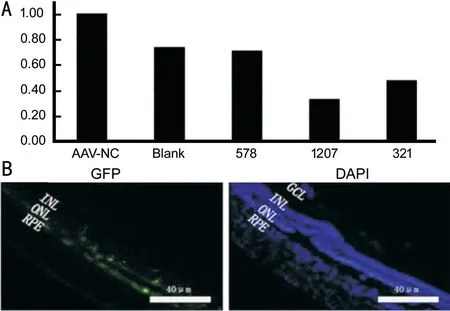
Figure 1 Screening and identification of NOX4 interfering virus A: The expression of NOX4 in cells transfected with the NOX4-rat-321, NOX4-rat-578, and NOX4-rat-1207 viruses was decreased relative to the blank control and transfected negative interference viruses, and the interference eあect of NOX4-rat-1207 was the best; B: Subretinal injection of the AAV2 virus vector expressing GFP could eあectively transfect the retinal tissue of rats in vivo, and the maximum transfection eきciency was achieved 3wk after subretinal injection.
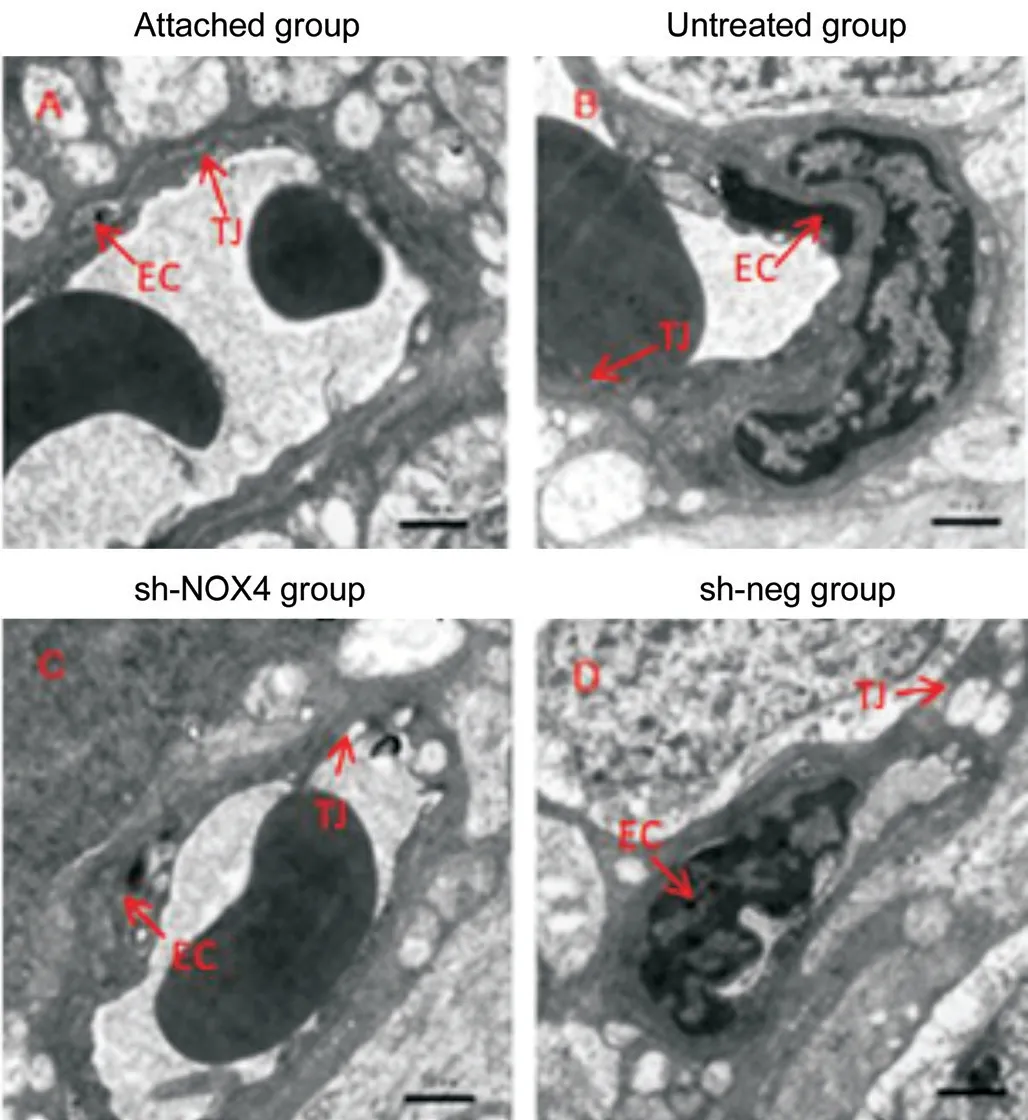
Figure 2 Compared with that in the untreated group, the degree of destruction of the connections between cells in the sh-NOX4 group was significantly reduced EC: Endothelial cell; TJ: Tight junction. Scale bar=1 μm.
NOX4 Interference Inhibited the Expression of the NOX4 Protein in Retinal TissueWestern blot was used to detect the expression of the NOX4 protein after transfection of the rat retinas. As shown in Figure 3, the Western blot results showed that the relative expression of the NOX4 protein in the sh-NOX4 group (1.03±0.14) was significantly lower than that in the normal control group (1.41±0.23), untreated group (1.94±0.36), and negative intervention group (1.71±0.18;n=6, per group,P<0.01).
NOX4 Interference Reduced the Retinal Vascular LeakageAs shown in Figure 4, the retinal vessels of the attached group had a clear vascular structure, good filling, uniform thickness, and no EB leakage. EB leakage occurred in the retinal vessels of the untreated group, sh-NOX4 group and sh-neg group, but retinal EB leakage was significantly reduced in the sh-NOX4 group compared with that in the untreated group and the shneg group. These results indicate that after interference, the BRB was eあectively protected.
NOX4 Interference Has a Protective Effect on the Visual Function in Rat RDThe rats that were unable to find the platform within the specified time during the training period were excluded. There were no significant differences in the time required to find the platform among 24 rats. These rats were divided into 4 groups, and there were 6 rats in each group. Three weeks after virus transfection and 7d after the establishment of the detachment model, the rats were subjected to the MWM test to assess their visual function. As shown in Figure 5A, the escape latency of the rats was used as a measurement of their visual function. The latency of the sh-NOX4 group (16.82±5.79s) was significantly reduced relative to that of the untreated group (48.54±8.15s) and the sh-neg group (45.46±16.00s;P<0.01). Therefore, after NOX4 interference, the time required for the rats to find the platform was significantly shortened, and the visual function of the rats was improved.
NOX4 Interference Reduced the Expression of ROS After RDAs shown in Figure 5B, after virus-mediated interference, the retinal expression of ROS in the sh-NOX4 group (8621.97±1064.57) was significantly lower than it was in the untreated group (15881.45±1770.43) and sh-neg group (14442.48±949.78;P<0.01). This result indicates that after NOX4 interference, the ROS intensity in the retinal tissue is reduced, thereby reducing photoreceptor cell damage and death.
DISCUSSION
In this study, a subretinal injection of an AAV2 vector carrying the NOX4 interference construct was used to establish a model of RD. It was found that inhibition of NOX4 expression after RD may have a protective effect on visual function. The mechanism by which this protection is achieved may be through the reduction of the damage to tight junctions between retinal vascular endothelial cells, the reduction of leakage of retinal blood vessels, and the inhibition of the production of ROS.

Figure 3 Western blot results showed the relative lower expression of the NOX4 protein in the sh-NOX4 group than that in the normal control group, untreated group, and negative intervention group.
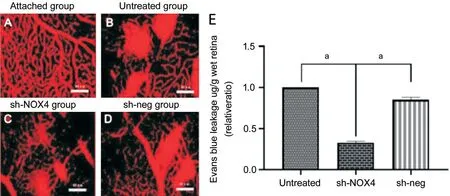
Figure 4 The leakage of EB after EB perfusion A-D: The retinal patch of the attached group (A), untreated group (B), sh-NOX4 group (C), and sh-neg group (D); E: EB leakage results showed the significantly reduced leakage in the sh-NOX4 group. aP<0.01. Scale bar=40 μm.

Figure 5 NOX4 interference reduced ROS expression and protected the visual function outcome after RD A: The escape latencies of the rats were used to assess the visual function of the rats. The latency of the sh-NOX4 group was significantly reduced relative to that of the untreated group and the sh-neg group. aP<0.01. B: The ROS expression test also showed the retinal expression of ROS in the sh-NOX4 group was significantly lower than that in the untreated group and sh-neg group. n=6, per group, aP<0.01.
The oxidative stress produced by NOX to produce excess ROS plays an important role in many ischaemic and hypoxic diseases. The excessive production of ROS and the resulting oxidative stress can lead to damage to the retina in retinal diseases, including diabetic retinopathy, retinopathy of prematurity, and RD. The protein of the NOX family is called “professional ROS-producing enzyme”, and this protein is one of the main sources of ROS. Therefore, therapy targeting NOX is an important direction for research on retinal diseases. Studies have shown that in animal models of retinal degeneration, the major causative factor leading to the loss of posterior cone cells and rod cells is oxidative damage. Therefore, the inhibition of NOX activation can reduce the production of ROS and effectively protect the structure and function of cone cells in mice with RD. It is thought that rod cell apoptosis and hyperoxia of the outer layer of the retina are activated due to genetic defects. NOX releases ROS in tissue cells, leading to the apoptosis of cone cells[12-13]. NOX4 is the main source of ROS in the vascular endothelium. Previous studies have shown that the reduction of ROS is closely associated with the neuroprotection of photoreceptors[14-15]. A large number of studies have shown that the inhibition of NOX4 expression in diabetic retinopathy can effectively protect the BRB[16]. Scioliet al[17]confirmed that NOX4 is a key factor in inducing oxidative stress and dysfunction in endothelial cells. Blocking NOX4 can effectively inhibit endothelial cell oxidative damage. The selective blockade of NOX with NOX1/NOX4 inhibitors reduces the production of ROS and the severity of neovascularization in rats housed in a hypertensive environment[18]. In addition, the inhibition of ROS production by NOX inhibitors or antioxidants can significantly reduce retinal vascular leakage in diabetic animals, suggesting that NOX activation promotes retinal ROS production and vascular injury in diabetic retinopathy[19]. The BRB is also mainly composed of vascular endothelial cells, so NOX4 was chosen as a target for studying BRB damage and photoreceptor cell death after RD in rats. The results of this experiment showed that after NOX4 interference, the expression of the NOX4 protein in the retina was decreased, and the expression of ROS in the rat retinas was decreased, indicating that the BRB damage and photoreceptor cell death were decreased and that the visual function of rats was protected.
The BRB limits the permeability between the blood and retina. The barrier has a clear anatomical basis and unique permeability characteristics and plays an important role in the pathophysiology and treatment of retinal diseases. There is evidence that BRB damage is closely associated with almost all retinal diseases, especially vascular retinopathy and pigment epithelial diseases. Retinal barrier damage is common in diabetic retinopathy, hypertensive retinopathy, retinal vein occlusion, ocular trauma or surgery, temporary arterial obstruction, perivascular inflammation, Coats’ disease, tumours, and retinal neovascularization. Choroidal ischaemia, pigment epithelial shedding, choroidal neovascularization, photocoagulation, RD, Koyanagi’s disease, central serous choroidal disease, multifocal endocarditis and acute placental pigment epithelial lesions can damage the extraretinal barrier. The results of this experiment showed that after NOX4 interference, the destruction of the tight junctions between the retinal vascular endothelial cells in RD rats was significantly decreased, as observed by electron microscopy, and the vascular leakage of RD rats was significantly reduced, as observed by EB retinal plaques. These results provide strong support for the eあective protection of the BRB against damage in RD rats by NOX4 interference. Therefore, we also expect that damage to the BRB is also the main cause of posterior function damage in RD. Alrashdiet al[20]found that endothelin-2 impairs the BRB, and this impairment is mediated through the interaction with the renin-angiotensin-aldosterone system and ROS derived from NOX. This observation also confirms our experimental results and provides a reference for our future study of NOX4-mediated mechanisms of BRB damage.
Finally, after NOX4 interference, the recovery of visual function in the eyes of RD rats was also supported by the behavioural data collected from the MWM test. The MWM test is a spatial learning task commonly used to assess cognitive function in a mouse model of neurological disorders[21]. A similar method was also used to assess the visual function of rats[11]. Panget al[22]found that under the same conditions, rd12 mice showed a performance similar to that of normal C57BL/6J mice when the rd12 mice were treated with AAV5-CBA-hRPE. This dramatic improvement in visual function is entirely due to the recovery of visual function after treatment because when light blocked from the treated eyes of rd12 mice, their performance was as poor as that of the untreated rd12 mice. This observation also demonstrates that monocular recombinant AAV treatment does not improve the visual function of the contralateral eye in this mouse model. In this experiment, the rats’ escape latency times were examined by the MWM test. The results showed that the visual function of
RD rats was significantly reduced, but the visual function of RD rats was significantly improved after NOX4 interference.
This finding is consistent with our expected experimental results, indicating that NOX4 is an important target for our study of the recovery of visual function in RD patients.
In conclusion, after RD surgery in rats treated with the AAV2 vector carrying the NOX4 interference construct, the expression level of NOX4 was decreased in the retinal cells, the leakage of the BRB was alleviated in the rats, and the visual function of the rats was significantly restored. This study further elucidated that NOX4 is one of the causes of photoreceptor cell death, since this death is mainly caused by large amounts of ROS, which damage the BRB of rats. NOX4 can be used as an eあective therapeutic target for RD-induced retinal damage.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81400407); Natural Science Foundation of Anhui Province (No.1408085QH159).
Conflicts of Interest: Dong K,None;Yang N,None;Ding J,None;Yan YY,None;Lu L,None;Wang YS,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
