Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
Rong Li, Lin Chen, Guo-Min Yao, Hong-Lin Yan, Li Wang
1Department of Ophthalmology, the First Aきliated Hospital of Xi’an Medical University, Xi’an 710077, Shaanxi Province, China
2Department of Pharmacy, the First Aきliated Hospital of Xi’an Medical University, Xi’an 710077, Shaanxi Province, China
3Department of Drug Clinical Trial Institution, the First
Aきliated Hospital of Xi’an Medical University, Xi’an 710077, Shaanxi Province, China
4Department of Scientific Research, the First Affiliated Hospital of Xi’an Medical University, Xi’an 710077, Shaanxi Province, China
Abstract
INTRODUCTION
The global incidence of diabetes increases rapidly year by year, and it has become the third chronic noninfectious disease endangering human health after cardiovascular, cerebrovascular diseases, and tumors. The morbidity and mortality of diabetic patients are usually caused by diabetic complications. As a common ocular complication of diabetes, diabetic retinopathy (DR) seriously threatens the eyesight of patients and causes blindness in most working-age people in developed countries[1-2]. Proliferative diabetic retinopathy (PDR), marked by the formation of retinal neovascularization (RNV), is particularly harmful to vision[3-4]. Given this, it is crucial to explore the mechanism and treatment strategy of DR, especially RNV in PDR.
In the study of diabetic macroangiopathy and microangiopathy, inflammation mechanism has become one of the focus and hot spots, and many inflammatory factors are involved in the development of DR. Among them, nucleotidebinding oligomerization domain-like receptors 3 (NLRP3) inflammasome consisting of NLRP3, apoptosis-associated speck-like protein (ASC), and cysteiny aspartate-specific protease-1 (Caspase-1), is an intracytoplasmic pattern recognition receptor attracting attention in recent years[5]. It has been shown that NLRP3 inflammasome signaling pathway may play an important role in the pathogenesis of DR, and interfering with this pathway may become a novel target for the treatment of DR[6]. Autophagy is a highly conserved intracellular degradation pathway, as an adaptive response under stress that guarantees the physiological turnover of senescent and damaged organelles, to control cell fate by various interacting signals[7]. Autophagy plays an important role in the maintenance of normal functions of the body and contributes to the pathophysiology of a variety of diseases[8-10], including DR[11].
Quercetin (3, 3’, 4’, 5, 7-pentahydroxy flavonoids) is a natural polyphenol existing in many fruits and vegetables, with a series of biological activities, such as inhibition of oxidation, inflammation, tumor, and angiogenesis[12-16]. It was speculated that quercetin may have therapeutic potential in RNV[16-17]. However, the quercetin’s therapeutic effect in DR and associated mechanisms have not been discussed yet. Previous studies suggest that both NLRP3 inflammasome and autophagy are involved in the pathological mechanism of DR, in view of this, we hypothesized that quercetin may exert a therapeutic influence on DR by inhibiting NLRP3 inflammasome and autophagy signaling pathway to inhibit retinal angiogenesis. We aimed to provide experimental basis for new treatment strategies for RNV in DR.
MATERIALS AND METHODS
Cell Culture and GroupingHuman retinal microvascular endothelial cells (HRMECs) were obtained (Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd., China) and cultured in M199 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Hyclone, Logan, UT), in 5% CO2at 37°C and 95% humidified room air. HRMECs were then randomly divided into five treatment groups, namely, control (5 mmol/L D-glucose in the medium), high-glucose (HG; adding 30 mmol/L D-glucose to the medium), HG+20 μmol/L quercetin (Aladdin, Shanghai, China; adding 30 mmol/L D-glucose and 20 μmol/L quercetin to the medium), HG+40 μmol/L quercetin (adding 30 mmol/L D-glucose and 40 μmol/L quercetin to the medium), and HG+80 μmol/L quercetin (adding 30 mmol/L D-glucose and 80 μmol/L quercetin to the medium). Finally, the plates in all groups were incubated for 48h.
Cell ViabilityThe cellular viability of HRMECs was analyzed using methyl tetrazolium (MTT) assay (Sigma, USA) according to the instructions. In brief, cells at a density of 5×104cells/mL were seeded onto a 96-well plate with 100 μL per well, while the well with 100 μL phosphate belanced solution (PBS) was set as the blank control. The cells were cultured at 37℃ overnight and then treated according to the above grouping method. The 10 μL MTT was added into each well and incubated at 37℃ for 4h. Then 150 μL dimethyl sulfoxide (DMSO; Amresco, Washington, USA) was added and the absorbance (A) of each well was estimated at 568 nm in a plate reader (Multiskan MK3, Thermo scientific, USA). The cell viability of each experimental group (%) was calculated as (Aexperimentgroup-Ablankgroup)/(Acontrolgroup-Ablankgroup)×100, where, Aexperimentgrouprefers to the absorbance value of treated cells, Ablankgrouprefers to the absorbance value of medium without cells, and Acontrolgrouprefers to the absorbance value of untreated cells.
Cell MigrationThe migration assay was performed by using a transwell chamber (BD Biosciences, USA). Briefly, 800 μL M199 medium containing 10% FBS was added to a 24-well plate, which was then placed in the transwell chamber. The 200 μL of HRMECs from different treatment groups at a density of 2×105cells/mL were plated into the upper compartment. At 48h of incubation, cells on the bottom of the transwell membrane were treated with 70% icy ethanol solution for 1h at 37℃ and then stained with 0.5% crystal violet for 20min at room temperature. The membranes were washed with PBS to clean those unmigrated cells on the side of the upper chamber and then photographed under a microscope. Three fields of view at ×200 magnification were randomly selected for each chamber, and the migrated cells were counted.
Tube FormationMatrigel (BD Biosciences, USA) was melt and 24-well plates and pipette tips were precooled at 4°C overnight. The 24-well plates were coated with 200 μL matrigel per well and maintained at 37℃ for 30min. Cells at a density of 2×105/well after treatment were seeded onto the 24-well plates covered with matrigel and then incubated at 37℃ overnight. The images were visualized and captured under a light microscope (IX51 Olympus, Japan). The number of mature vessel tubes was counted in three randomly selected fields of view at ×100 magnification by using Image J software (version 1.46, NIH, USA).
Western BlottingAfter sample preparation, the protein concentrations of the control and experimental groups were quantified using the bicinchoninic acid (BCA) assay (Pierce, IL) according to the manufacturer’s instructions. Then, a sample protein (40 μg) with equal amounts was loaded onto and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes. Then, the membranes were blocked with tris buffered saline containing 0.5% (v/v) Tween 20 (TBST) containing 5% non-fat dry milk for 2h at room temperature and incubated overnight at 4℃ with the corresponding diluted primary antibodies, namely, rabbit anti-NLRP3 (1:1000, Novus Biologicals, USA), rabbit anti-ASC (1:1000, Abcam, UK), rabbit anti-Caspase-1 (1:1000, Abcam, UK), rabbit LC3-II and LC3-I (1:1000, Aきnity, USA), rabbit Beclin-1 (1:2000, Abcam, UK); rabbit anti-GADPH (1:1000, Hangzhou Xianzhi Biotechnology Co., Ltd., China). Then, after washing thoroughly, the blots were incubated with HRP-conjugated secondary antibodies (1:50 000, Boster, USA) at 37℃ for 2h. The blots were visualized with an ECL reagent (Applygen Technologies Inc., China) and images were analyzed after scanning by using BandScan software (version 5.0, Glyko, Inc., Germany).
ELISAThe supernatant of treated cells was collected and added to the 96-well plate of ELISA kit for cytokine measurements. The concentrations of IL-1β (pg/mL) and IL-18 (pg/mL) in the collected culture media were measured with human IL-1β ELISA kits (Elabscience Biotechnology Co., Ltd., Wuhan, China) and human IL-18 ELISA kits (Elabscience Biotechnology Co., Ltd., China), respectively. The manufacturer’s instructions were strictly followed. Linear correlation regression analysis was performed with the concentration of IL-1β and IL-18 standard as the ordinate and the optical density as the abscissa, and the concentration of IL-1β and IL-18 was detected on the standard curve according to the optical density of the tested sample.
Cellular AutophagyAutophagy of HRMECs was estimated using Cyto-ID®autophagy detection kit (Enzo, USA) according to the instructions. Briefly, the control and experimental groups of HRMECs were washed twice with assay buあer and incubated with Cyto-ID stain or PBS (unstained control) and Hoechst 33342 (DNA staining dye) at 37℃ for 30min. After fixation with 4% paraformaldehyde at room temperature for 15min, the cells were washed again with assay buffer and then observed immediately using a laser confocal fluorescence microscope (C2, Nikon, Japan). The average fluorescent intensity was measured by Image J software (version 1.46, NIH, USA).
Statistical AnalysisThe statistical data were analyzed using the SPSS software (version 25.0IBM, USA).All values in this study were presented as mean±standard deviation (SD) of at least three independent experiments. Statistical differences between different experimental and control groups was estimated by pairwiset-test and one-way ANOVA followed by LSD post hoc test. Two-sidedP-values were calculated to determine statistical significance andP<0.05 was considered statistically significant.
RESULTS
Quercetin Inhibited Cell Viability of HRMECs Under a High-glucose ConditionMTT assay showed that statistically significant diあerences existed in cell viability among the five groups (P<0.05). Cell viability of the HG (163.56%±1.46%), HG+20 μmol/L quercetin (136.51%±1.89%), HG+40 μmol/L quercetin (129.35%±2.57%) and HG+80 μmol/L quercetin (122.72%±1.01%) groups were all higher than that of the control group (100.00%±0.92%; allP<0.05). Compared with the HG group, this rate in each quercetin treatment group was decreased (allP<0.05), and decreased with the increase of quercetin concentration. The differences in this rate among each quercetin concentration group were statistically significant (allP<0.05; Figure 1). These results indicated that the viability of HRMECs was increased under a HG condition, which can be inhibited by quercetin and its inhibitory effect increased with concentration.

Figure 2 Representative images of migration of HRMECs in transwell chamber in diあerent groups treated for 48h HG: High glucose; Q: Quercetin. aP<0.05 vs control; bP<0.05 vs HG; cP<0.05 vs HG+20 μmol/L Q; dP<0.05 vs HG+40 μmol/L Q. Bar=100 μm.
Quercetin Inhibited Migration of HRMECs Under a Highglucose ConditionTranswell assay showed that there were statistically significant differences in the number of migrated cells among the five groups (P<0.001). The number of migrated cells of the HG (117.33±7.23), HG+20 μmol/L quercetin (78.67±4.04), HG+40 μmol/L quercetin (66.67±4.16), and HG+80 μmol/L quercetin (52.67±6.81) groups were all higher than that of the control group (37.67±9.02; allP<0.05). Compared with the HG group, the number of migrated cells in each quercetin treatment group was decreased (allP<0.05), and decreased with the increase of quercetin concentration. The differences in the number of migrated cells among each quercetin concentration group were statistically significant (allP<0.05; Figure 2). These results indicated that the migration of HRMECs under the condition of high glucose was increased, and quercetin had an inhibitory effect on cell migration in the condition of high glucose. This eあect of quercetin on cell migration of HRMECs was dose dependent.
Quercetin Inhibited Tube Formation of HRMECs Under a High-glucose ConditionMatrigel assay showed that there were statistically significant differences in the number of migrated cells among the five groups (P<0.001). The number of tube formation of the HG (95.00±9.54), HG+20 μmol/L quercetin (84.67±1.53), HG+40 μmol/L quercetin (73.00±1.00) and HG+80 μmol/L quercetin (62.33±3.21) groups were all higher than that of the control group (45.00±4.58; allP<0.05). Compared with the HG group, the number of tube formation in each quercetin treatment group was decreased (allP<0.05), and decreased with the increase of quercetin concentration. The differences in the number of tube formation among each quercetin concentration group were statistically significant (allP<0.05). Similarly, the total branch length of the HG (14200.00±156.17), HG+20 μmol/L quercetin (13279.33±289.94), HG+40 μmol/L quercetin (12628.33±151.26), and HG+80 μmol/L quercetin (11488.00±403.28) groups were all higher than that of the control group (9614.00±499.81; allP<0.05). Compared with the HG group, the total branch length in each quercetin treatment group was decreased (allP<0.05), and decreased with the increase of quercetin concentration. The differences in the total branch length among each quercetin concentration group were statistically significant (allP<0.05; Figure 3). These results suggested that the tube formation ability of HRMECs under the condition of high glucose was increased, and quercetin had an inhibitory eあect on cell tube formation under the condition of high glucose. This eあect of quercetin on tube formation of HRMECs was dose dependent.
Quercetin Inhibited Expression of NLRP3 Inflammasome of HRMECs Under a High-glucose ConditionWestern blotting analysis demonstrated that there were statistically significant differences in the protein expressions of NLRP3 inflammasome signaling pathway including NLRP3, ASC, and Caspase-1 in HRMECs among the five groups (P<0.05). The relative expressions of NLRP3, ASC and Caspase-1 of the HG group and quercetin groups were all higher than that of the control group (allP<0.05). Compared with the HG group, these expressions in each quercetin treatment group was decreased (allP<0.05), and their values decreased with the increase of quercetin concentration. In addition, the diあerence in the expressions of NLRP3, ASC, and Caspase-1 among each quercetin concentration group was statistically significant (allP<0.05; Figure 4). ELISA showed that there were statistically significant diあerences in the expressions of IL-1β and IL-18 in HRMECs among the five groups (P<0.05). The expressions of IL-1β and IL-18 of the HG group and quercetin groups were all higher than that of the control group (allP<0.05). Compared with the HG group, both expressions of IL-1β and IL-18 in each quercetin treatment group was decreased (allP<0.05), and their values decreased with the increase of quercetin concentration. In addition, the diあerence in the expressions of IL-1β and IL-18 among each quercetin concentration group was statistically significant (allP<0.05; Figure 5). These results indicated that NLRP3 inflammasome signaling pathway was activated by a HG condition. Quercetin can inhibit this in HRMECs, and the eあect was dose dependent.
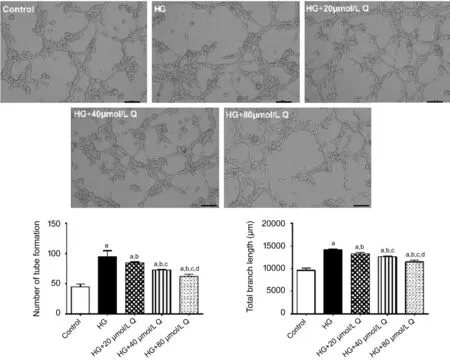
Figure 3 Representative images of tube formation of HRMECs in matrigel in diあerent groups treated for 48h HG: High glucose; Q: Quercetin. aP<0.05 vs control; bP<0.05 vs HG; cP<0.05 vs HG+20 μmol/L Q; dP<0.05 vs HG+40 μmol/L Q. Bar=100 μm.
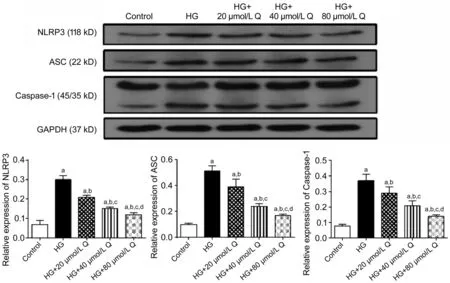
Figure 4 The relative expressions of NLP3, ASC, and Caspase-1 of HRMECs by Western blotting in diあerent groups treated for 48h HG: High glucose; Q: Quercetin. aP<0.05 vs control; bP<0.05 vs HG; cP<0.05 vs HG+20 μmol/L Q; dP<0.05 vs HG+40 μmol/L Q.

Figure 5 The expressions of IL-1β and IL-18 of HRMECs by ELISA in diあerent groups treated for 48h HG: High glucose; Q: Quercetin. aP<0.05 vs control; bP<0.05 vs HG; cP<0.05 vs HG+20 μmol/L Q; dP<0.05 vs HG+40 μmol/L Q.
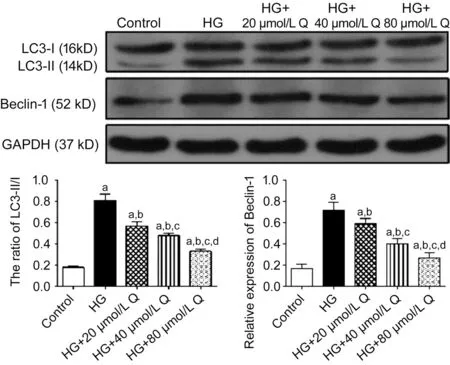
Figure 6 The relative expressions of LC3 and Beclin-1 of HRMECs by Western blotting in diあerent groups treated for 48h HG: High glucose, Q: Quercetin. aP<0.05 vs control; bP<0.05 vs HG; cP<0.05 vs HG+20 μmol/L Q; dP<0.05 vs HG+40 μmol/L Q.
Quercetin Inhibited Autophagy of HRMECs Under a Highglucose ConditionWestern blotting analysis demonstrated that there were statistically significant differences in the expressions of key autophagy proteins, including LC3 and Beclin-1 in HRMECs among the five groups (P<0.05). The relative expressions of LC3-II/I and Beclin-1 of the HG group and quercetin groups were all higher than that of the control group (allP<0.05). Compared with the HG group, these expressions in each quercetin treatment group was decreased (allP<0.05), and their values decreased with the increase of quercetin concentration (allP<0.05; Figure 6). Next, we analyzed the status of autophagy in the HRMECs by Cyto-ID fluorescence. Compared with the control, the average fluorescent intensity of autophagy in HRMECs in other four groups was increased. Compared with the HG group, the average fluorescent intensity of autophagy in each quercetin treatment group was decreased, and it decreased with the increase of quercetin concentration (Figure 7). These results indicated that autophagy was activated in HRMECs by a HG condition and quercetin can inhibit the activation of autophagy, and the inhibitory effect increased with the increase of quercetin concentration.
DISCUSSION
In view of the importance of RNV in the development of PDR, the focus of clinic control and basic research of DR is targeting at retinal vascular lesions all the time.Application of anti-vascular endothelial growth factor (VEGF) agents has been found to be beneficial in PDR to achieve regression of RNV[18], however, unsuccessful therapy due to the continuous growth of the RNV despite treatment with anti-VEGF is not rare[19], suggesting other factors except for VEGF also participate in the development of RNV in PDR. This study demonstrated that the expressions of NLRP3 inflammasome signaling pathway, including NLRP3, ASC, and Caspase-1 as well as the downstream factors IL-1, IL-18, were significantly enhanced under a high glucose condition, suggesting that high glucose stimulation can significantly activate inflammatory response in HRMECs. These findings were consistent with the results obtained from previous studies. As has been proved, DR is the result of interactions of multiple systemic and local factors, and the stimulation of various inflammatory factors and cytokines plays a regulatory role in DR and RNV[20]. Local inflammatory stimuli permeate the pathological process of DR and is crucial for the development of this disease. For example, the infiltration of inflammatory cytokines in the local retinal environment is related to the decline of retinal function, and the inhibition of inflammatory infiltration has a significant protective eあect on DR[21-22].

Figure 7 Detection of autophagy of HRMECs at 48h of different treatments under a laser confocal fluorescence microscope The autophagy vesicles labeled with Cyto-ID were shown as green fluorescence. HG: High glucose; Q: Quercetin. aP<0.05 vs control; bP<0.05 vs HG; cP<0.05 vs HG+20 μmol/L Q; dP<0.05 vs HG+40 μmol/L Q. Bar=20 μm.
The results of this study suggested that the viability, migration, and tube formation of HRMECs were significantly enhanced under the condition of high glucose, indicating that high glucose can induce RNV, which was similar to the results previously observed in other retinal endothelial cells[23]. Studies in retinal cells and DR animal models have found that NLRP3 inflammasome was associated with cell apoptosis and increased vascular permeability to participate in the development in RNV[6,24]. In addition, clinical studies found that NLRP3 protein was highly expressed in the anterior retinal membrane of PDR patients, and NLRP3, IL-1 and IL-18 were highly expressed in the vitreous body of PDR patients[25]. The gene and protein expressions of NLRP3, ASC, and Caspase-1 in peripheral monocytes of DR patients were significantly higher than that of normal controls. The expression of NLRP3 and ASC in fibrovascular membrane of PDR patients was also significantly higher than that of normal people, and IL-1 and IL-18 in peripheral monocytes as well as vitreous body of DR patients were significantly higher than that of normal people[26]. In addition, in this study, the autophagy level of HRMECs was observed and consistent with previous studies, we found that high glucose can up-regulate autophagy of retinal vascular endothelial cells, showing as enhanced expression of autophagy marker proteins LC3 and Beclin-1 as well as stronger autophagy fluorescence. Similarly, a large number of studies have confirmed that high glucose up-regulates autophagy, and autophagy dysfunction is closely related to the pathogenesis of DR and RNV[11,23,27-28]. Taken together, all these results suggested that the NLRP3 inflammasome and autophagy signaling pathway played an important role in the pathogenesis of DR, and interference with them may become a novel therapeutic target for RNV in PDR.
As the main active ingredient of Chinese herbal medicine, quercetin has shown great therapeutic potential in many diseases. The broad pharmacological action of quercetin makes it have a great medical prospect, however, the mechanism of the action of quercetin has not been fully elucidated. Moreover, there are few studies on its role in eye diseases, especially in retinal diseases. Given this, quercetin was applied to HRMECs with high glucose stimulation to observe its effects on retinal angiogenesis in this study. We found that quercetin significantly inhibited the viability, migration, and tube formation of HRMECs under high glucose conditions. Meanwhile, quercetin significantly inhibited NLRP3 inflammasome and autophagy signaling pathway which were activated by high glucose conditions. And this efficacy of quercetin was dose dependent. In view of the importance of NLRP3 inflammasome and autophagy in the pathological mechanism of DR, we speculated that inhibition of NLRP3 inflammasome and autophagy signaling pathway may be one of the pharmacological mechanisms of quercetin to inhibit angiogenesis of HRMECs induced by high glucose.
However, this study failed to observe whether inhibiting NLRP3 inflammasome or autophagy is directly associated with the effect of quercetin on HG induced retinal angiogenesis, and other possible mechanisms of quercetin’s inhibitory effect could not be ruled out, for example, the VEGF signaling pathway. Besides, the regulating relationship of NLRP3 inflammasome with autophagy was not clarified during the action of quercetin. It has been reported that the relationship of NLRP3 inflammasome with autophagy in diseases is very complex, and the influence between them is bidirectional and they may have a completely opposite eあect on each other due to the conditions in diあerent diseases[29-33]. In addition, the results from cell experimentin vitrocannot completely represent the real pathological process of DR. In order to further clarify the functional potential and molecular mechanism of quercetin in DR, studies with DR animal model and patients are required to provide a new direction for clinical treatment of DR.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81500726); the Matching Funds of the National Natural Science Foundation of China (No.XYFYPT-2020-01).
Conflicts of Interest:Li R,None;Chen L,None;Yao GM,None;Yan HL,None;Wang L,None.
REFERENCES
1 Cheung N, Mitchell P, Wong TY. Diabetic retinopathy.Lancet2010;376(9735):124-136.
2 Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss.Eye Vis (Lond)2015;2:17.
3 Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review.JAMA2007;298(8):902-916.
4 Chhablani J, Sambhana S, Mathai A, Gupta V, Arevalo JF, Kozak I. Clinical efficacy of navigated panretinal photocoagulation in proliferative diabetic retinopathy.Am J Ophthalmol2015;159(5):884-889.
5 Schroder K, Tschopp J. The inflammasomes.Cell2010;140(6):821-832.
6 Chaurasia SS, Lim RR, Parikh BH,et al. The NLRP3 inflammasome may contribute to pathologic neovascularization in the advanced stages of diabetic retinopathy.Sci Rep2018;12;8(1):2847.
7 Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation.Antioxid Redox Signal2014;20(3):460-473.
8 Mialet-Perez J, Vindis C. Autophagy in health and disease: focus on the cardiovascular system.Essays Biochem2017;61(6):721-732.
9 Lassen KG, Xavier RJ. Mechanisms and function of autophagy in intestinal disease.Autophagy2018;14(2):216-220.
10 Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease.Nat Rev Gastroenterol Hepatol2017;14(3):170-184.
11 Rosa MD, Distefano G, Gagliano C, Rusciano D, Malaguarnera L. Autophagy in diabetic retinopathy.Curr Neuropharmacol2016;14(8):810-825.
12 Cao XG, Liu M, Tuo JS, Shen DF, Chan CC. The eあects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice.Exp Eye Res2010;91(1):15-25.
13 Maurya AK, Vinayak M. Quercetin attenuates cell survival, inflammation, and angiogenesis via modulation of AKT signaling in murine T-cell lymphoma.Nutr Cancer2017;69(3):470-480.
14 Lan H, Hong W, Fan P, Qian D, Zhu J, Bai B. Quercetin inhibits cell migration and invasion in human osteosarcoma cells.Cell Physiol Biochem2017;43(2):553-567.
15 Song W, Zhao XF, Xu JR, Zhang H. Quercetin inhibits angiogenesismediated human retinoblastoma growth by targeting vascular endothelial growth factor receptor.Oncol Lett2017;14(3):3343-3348.
16 Zhao DX, Qin CJ, Fan XH, Li YC, Gu BH. Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells.Eur J Pharmacol2014;723:360-367.
17 Chen Y, Li XX, Xing NZ, Cao XG. Quercetin inhibits choroidal and retinal angiogenesis in vitro.Graefes Arch Clin Exp Ophthalmol2008;246(3):373-378.
18 Mehanna CJ, Abdul Fattah M, Haddad S, Tamim H, Ghazi N, Salti H. Anti-VEGF therapy for persistent neovascularization after complete panretinal photocoagulation in proliferative diabetic retinopathy.Ophthalmol Retina2019;3(6):473-477.
19 Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy.Eye (Lond)2014;28(5):510-520.
20 Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy.J Cell Biochem2016;117(11):2443-2453.
21 Semeraro F, Cancarini A, dell’Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic retinopathy: vascular and inflammatory disease.J Diabetes Res2015;2015:582060.
22 El-Asrar AMA. Role of inflammation in the pathogenesis of diabetic retinopathy.Middle East Afr J Ophthalmol2012;19(1):70-74.
23 Li R, Du JH, Yao Y, Yao GM, Wang XD. Adiponectin inhibits high glucose-induced angiogenesis via inhibiting autophagy in RF/6A cells.J Cell Physiol2019;234(11):20566-20576.
24 Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C, Lu L, Xu X, Guan H, Zheng Z, Qiu Q. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory eあect of minocycline.Inflamm Res2017;66(2):157-166.
25 Wei XF. The expression and significance of NLRP3 inflammatosome and its downstream factors IL-1 and IL-18 in diabetic retinopathy. Zhengzhou University. 2016.
26 Chen H, Zhang X, Liao N, Mi L, Peng Y, Liu B, Zhang S, Wen F. Enhanced expression of NLRP3 inflammasome-related inflammation in diabetic retinopathy.Invest Ophthalmol Vis Sci2018;59(2):978-985.
27 Du JH, Li X, Li R, Cheng BX, Kuerbanjiang M, Ma L. Role of autophagy in angiogenesis induced by a high-glucose condition in RF/6A cells.Ophthalmologica2017;237(2):85-95.
28 Lopes de Faria JM, Duarte DA, Montemurro C, Papadimitriou A, Consonni SR, Lopes de Faria JB. Defective autophagy in diabetic retinopathy.Invest Ophthalmol Vis Sci2016;57(10):4356-4366.
29 Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction.Nat Immunol2012;13(3):255-263.
30 Ahmed ME, Iyer S, Thangavel R, Kempuraj D, Selvakumar GP, Raikwar SP, Zaheer S, Zaheer A. Co-localization of glia maturation factor with NLRP3 inflammasome and autophagosome markers in human alzheimer’s disease brain.J Alzheimers Dis2017;60(3):1143-1160.
31 Jiang P, Guo Y, Dang R, Yang M, Liao D, Li H, Sun Z, Feng Q, Xu P. Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: involvement of autophagy and NLRP3 inflammasome.J Neuroinflammation2017;14(1):239.
32 Dupont N, Jiang SY, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β.EMBO J2011;30(23):4701-4711.
33 Zhou H, Feng L, Xu F,et al. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: a new mechanism linking berberine to insulin resistance improvement.Biomed Pharmacother2017;89:864-874.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- RNA interference targeting NOX4 protects visual function in an experimental model of retinal detachment by alleviating blood-retinal barrier damage
