Eきcacy of the WlNROP algorithm for retinopathy of prematurity screening in Southern China
Yi-Chen Bai, Rong Wu, Si-Zhe Chen, Shi-Yu Wei, Hui-Jie Chen, Yan-Chen Chen, Song-Fu Feng , Xiao-He Lu
1Department of Ophthalmology, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China
2Department of Ophthalmology, Liuzhou People’s Hospital, Liuzhou 545006, Guangxi Zhuang Autonomous Region, China
3Department of Pediatric, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China
Abstract
INTRODUCTION
R etinopathy of prematurity (ROP) is a retinal vasoproliferative disease which remains a leading cause of worldwide children blindness[1-2]. With the increasing survival rate of premature infants, the incidence of ROP especially sight-threatening ROP remains a crucial issue for ophthalmologists and neonatologists[3]. However, severe outcomes of ROP such as retinal detachment could be prevented by routinely fundus examination and timely treatment[4].
Serial examination was recommended by many ROP screening guidelines from different countries. Currently, the selection criteria for screening are mostly based on birth weight (BW) and gestational age (GA)[5-9], which means abundant of premature infants who reach the criteria should be examined, however, only less than 10% of the screened infants need treatment[10-11]. In addition, these examinations are laborintensive for ophthalmologists especially in countries with large population such as China. Meanwhile, it is also stressful for preterm infants which are susceptible to many postnatal complications[12-13]. Therefore, the combination of other predicting methods and ROP screening would contribute to the accuracy and eきciency for ROP diagnosis and treatment.
Extensive animal or clinical studies have demonstrated the relationship between poor postnatal weight gain and levels of serum insulin-like growth factor 1 (IGF-1) with the incidence of severe ROP[14-16]. The WINROP (weight, IGF-1, neonatal ROP) algorithm was developed in a Swedish cohort to evaluate the risk of proliferative ROP based on weekly postnatal weight gain[17]. This algorithm was widely validated and significantly reduced ROP screening according to various study cohorts[18-19].This study aims to evaluate the predictive value of the WINROP algorithm in a cohort of larger, more mature preterm infants in Southern China.
SUBJECTS AND METHODS
Ethical ApprovalInstitutional Review Board approved the protocol in accordance with the principles of the Declaration of Helsinki (revision of Edinburgh, 2008) and all parents or guardians of the recruited infants provided informed written consent before enrollment. This retrospective study was conducted by Department of Ophthalmology and Neonatal Intensive Care Unit (NICU) and was approved by the Ethics Committee of Zhujiang Hospital, Southern Medical University.
PatientsThe infants with a GA less than 32wk which were admitted to NICU in Zhujiang Hospital, Guangzhou, China from September 2015 to August 2019 were included in this study. Infants died before ROP screening or with genetic metabolic disease, hydrocephalus or weekly weight gain more than 450 g were excluded. Clinical data were collected included GA, BW, serial weight measurement, sex, multiple gestation, blood transfusion, oxygen administration and mechanical ventilation (MV). Systemic complications including necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), sepsis, patent ductus arteriosus (PDA) and periventricular leukomalacia (PVL) were also recorded.
Retinopathy of Prematurity Screening and ClassificationAll preterm infants which met the Chinese ROP Screening Criteria (GA≤34wk/BW≤2000 g or with other risk factors)[9]were examined weekly or biweekly from 4-6wk after birth.
The International Classification of Retinopathy of Prematurity 2005[20]was applied to determine the zone and stage of each case. Type 1 ROP (zone I, stage 1 or 2 with plus disease or stage 3 ROP without plus disease, or zone II, stage 2 or 3 with plus disease) and type 2 ROP (zone I, stage 1 or 2 without plus disease, or zone II, stage 3 without plus disease) were defined as described in the Early Treatment for Retinopathy of Prematurity (ETROP)[21]. Other ROP was defined as any type of ROP less than type 2. All screening examination were performed by two expert ophthalmologists using RetCam3 Imaging System (Clarity Medical System, Pleasanton, CA, USA) or binocular indirect ophthalmoscopy.
WINROP Algorithm ScreeningThe WINROP system is an algorithm that could predict high risk of ROP based on weekly weight gain and the level of IGF-1[17]. We entered GA, BW, weekly body weight of each included infant into the WINROP program (http://winrop.com) until an alarm was triggered or until 36wk of postmenstrual age (PMA)[18]. The infants were classified into two categories, alarm and no alarm, which represents a high or low risk for developing type 1 ROP, respectively.
Statistical AnalysisContinuous variables were presented as medians and interquartile ranges, and qualitative variables as numbers and percentages. Demographic clinical complication diあerences were compared by studentt-test or Mann-WhitneyUtest, when appropriate. The detecting accuracy of WINROP was evaluated by calculating the sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV). The 95% confidence intervals were calculated using Clopper-Pearson method. The statistical differences were defined asP<0.05. Statistical analysis was performed by SPSS 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patients and Retinopathy of Prematurity OutcomesIn this study, a total of 518 infants with GA<32wk were filtered from all the preterm infants which underwent ROP screening. For WINROP assessment, 86 infants were excluded, since 35 died before final ROP results were achieved, 13 missed weekly weights data or were lost to follow-up, 27 had a weekly weight gain more than 450 g and 11 were aあected by hydrocephalus. This resulted in 432 infants with 291 (67.4%) boys and 141 (32.6%) girls finally included in the study analysis. These infants had a median GA of 30.0 (24.0-31.9)wk and the median BW was 1360 (540-2700) g. Among these babies, 333 (77.1%) were single births and 99 (22.9%) were multiple births.
Of the 432 infants included in the study, 50 (11.6%) developed type 1 ROP, 16 (3.7%) were type 2 ROP, and 86 (19.9%) were other ROP. No ROP development were found in 280 (64.8%) babies. The total incidence of ROP was 35.2% (152/432) in these preterm infants. All the type 1 ROP were treated by laser or anti-VEGF treatment according to ETROP criteria[5,21]. There was a trend that some postnatal complications occurred more frequently in infants with severe ROP compared with those with no or mild ROP, such as NEC, BPD, IVH and PVL (Table 1).
WINROP Outcomes and Test CharacteristicsAs shown in Table 2, for all the 432 infants with GA<32wk, the low-risk alarm was triggered in 274 (63.4%) infants with a median GA of 30.3 (25.6-31.9)wk and a median BW of 1520 (810-2700) g. An alarm was signaled in 158 (36.6%) infants. 8 (5.1%) of these infants triggered alarm immediately after birth. Interestingly, all of them were born with a relative low BW (910±49 g, mean±SD) as they were not extremely preterm infants. The median time from birth to triggering alarm was 2 (0-6)wk. The median GA of the alarm group was 29.2 (24.0-31.9)wk, and the median BW was 1110 (540-1750) g.
Of the 158 high-risky infants, 28 developed type 1 ROP, 40 developed other types of ROP, and 90 infants had no ROP until 45 PMA. Totally 22 infants with type 1 ROP were omittedby the WINROP algorithm, the sensitivity and specificity were 56.0% (28/50) and 65.4% (252/382), respectively. However, when we tested the very low-BW (BW<1000 g) or extremely preterm (GA<28wk) infants, the sensitivity improved apparently. In the BW<1000 g group, the sensitivity was 93.8% (15/16) and the specificity was 31% (9/29). For the GA<28wk group, these data were 93.3% (14/15) and 60.6% (20/33), respectively. However, for infants with BW≥1000 g or GA≥28wk, the prediction sensitivities of WINROP algorithm were relatively low (Tables 3 and 4).
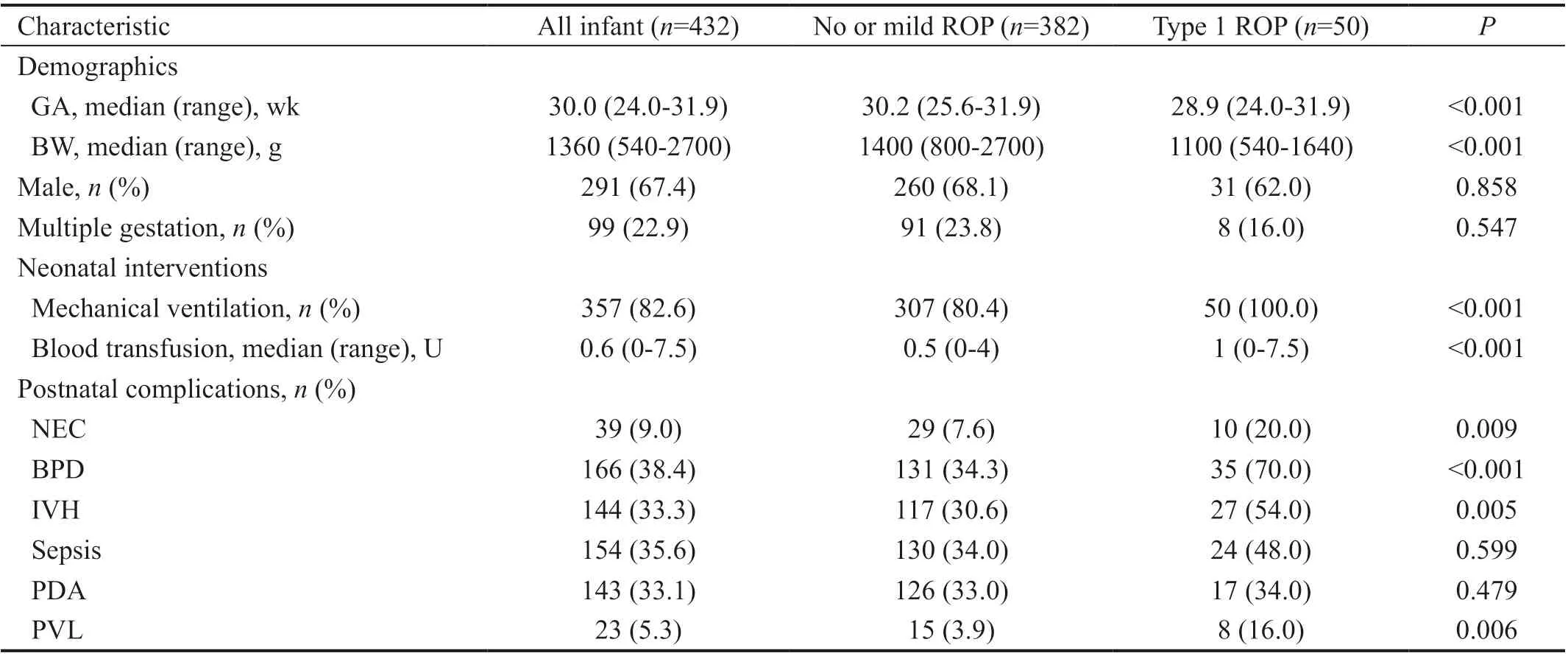
Table 1 Clinical characteristics of the infants in this study

Table 2 Predictive performances in detecting ROP using the WINROP algorithm
For the overall 22 patients with type 1 ROP who were missed by WINROP algorithm, we also tried to improve the test accuracy by combining WINROP with additional risk factors associated with ROP, such as BPD, PVL, IVH, and NEC. We found that the sensitivity improved to 96.0% (48/50). The characteristics of the 22 missed patients and the modified sensitivities were listed in Tables 5 and 6, respectively.
DISCUSSION
ROP remains a leading cause of worldwide childhood blindness[1,22]. The screening and treatment of proliferative ROP are vitally important. The WINROP algorithm mainly depends on postnatal weekly weight gains which is widely verified[16,23-24]. Various studies have validated the predictive performance of this algorithm[10,25-27]. A study in Swedish cohort demonstrated 100% sensitivity to detect stage-3 ROP and reduced 75% of ROP examination[18]. Similarly, a study in North America also reported a sensitivity of 100% to identify severe ROP and partially reduced the total number of ROP examinations[19].
In this study, we retrospectively tested 432 preterm infants with the WINROP algorithm, the overall sensitivity for detecting type 1 ROP was 56.0%, which was not as effective as other studies[10,19,25]. Totally 22 infants with type 1 ROP and receiving treatment were missed by the algorithm, most of which had a BW more than 1000 g. Our results were similar to some studies based on infants from developing areas[28-29]. A retrospective study of 148 infants in Taiwan reported a sensitivity of 64.7% when identifying treatment-demanding ROP by WINROP algorithm[28]. Another study of 278 premature infants in Japan using WINROP demonstrated 72.2% of sensitivity to detectingtype 1 ROP[30]. There are probably heterogeneous causes of this discrepancy.It was reported that severe ROP happens frequently in larger and more mature infants in China when compared with developed countries[9,31-32]. There are several causes of this discrepancy, first, exposing more frequently to risk factors such as unmonitored oxygen supplement. Furthermore, lower survival rate of extremely preterm infants caused by the limitation of prenatal and postnatal care. Finally, the imbalance of medical resources results in the omission of ROP screening for premature infants[22,33-34]. Our study also demonstrated this tendency, the median GA of the included infants was 30.0 (24.0-31.9)wk, and median BW was 1360 (540-2700) g. In a North American cohort study with 98.6% sensitivity on detecting type 1 ROP by WINROP, the included infants had a median GA of 28 (22-31)wk, and a BW of 1016 (378-2240) g, which was considerably lower than our study[25]. The WINROP algorithm was invented by a Swedish study group, thus it was mostly built up on Swedish population, in which severe ROP occurs mostly in extremely premature infants[8,17-18]. In the EXPRESS study, the sensitivity was 95.7% for infants with a median GA of 25+4(23-26+6)wk and BW of 784 (348-1315) g, no type 1 ROP was developed in infants with a GA of more than 29wk[35]. It was also reported that in high-income countries such as United States and Canada, most infants with a GA more than 32wk only develop mild ROP, which does not require treatment[10]. However, in our study, 50.0% (25/50) of the infants had GA of more than 29wk in the type 1 ROP group. When we focused on extremely small (BW<1000 g) or preterm infants (GA<28wk), the sensitivities of the algorithmwere 93.8% and 93.3%, respectively, which means WINROP was comparatively eあective for these infants. There were only one infants with GA<28wk developed type 1 ROP but not been detected by WINROP system. The mother of this patient was diagnosed as gestational diabetes mellitus, this may cause a heavier BW as this GA, thus diきcult to be recognized by the algorithm.
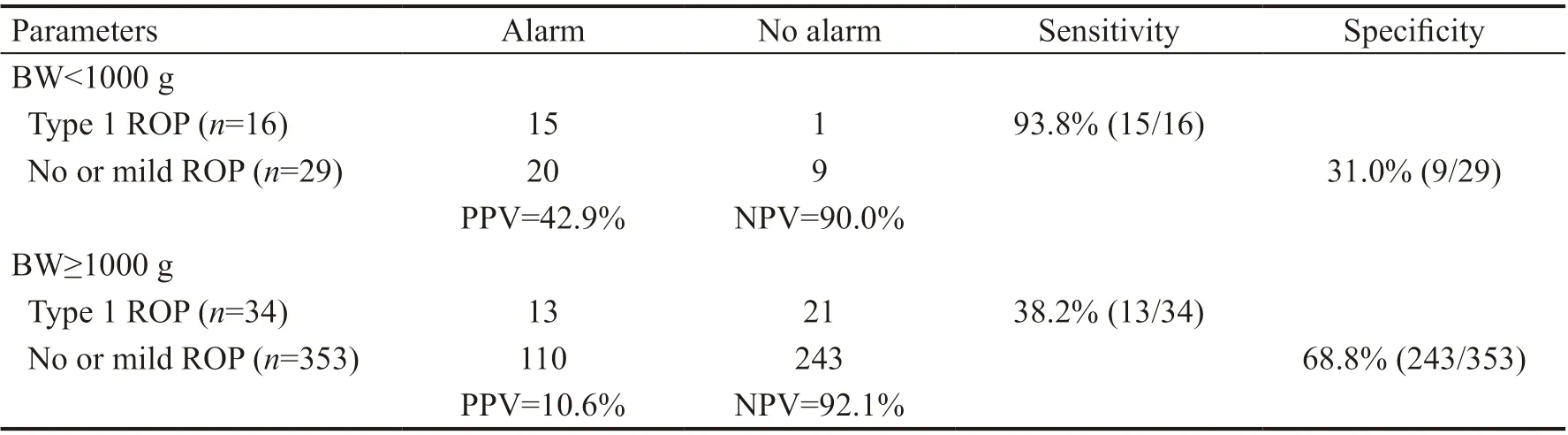
Table 3 Predictive performances in detecting ROP using the WINROP algorithm in infants with diあerent BWs

Table 4 Predictive performances in detecting ROP using the WINROP algorithm in infants with diあerent GAs
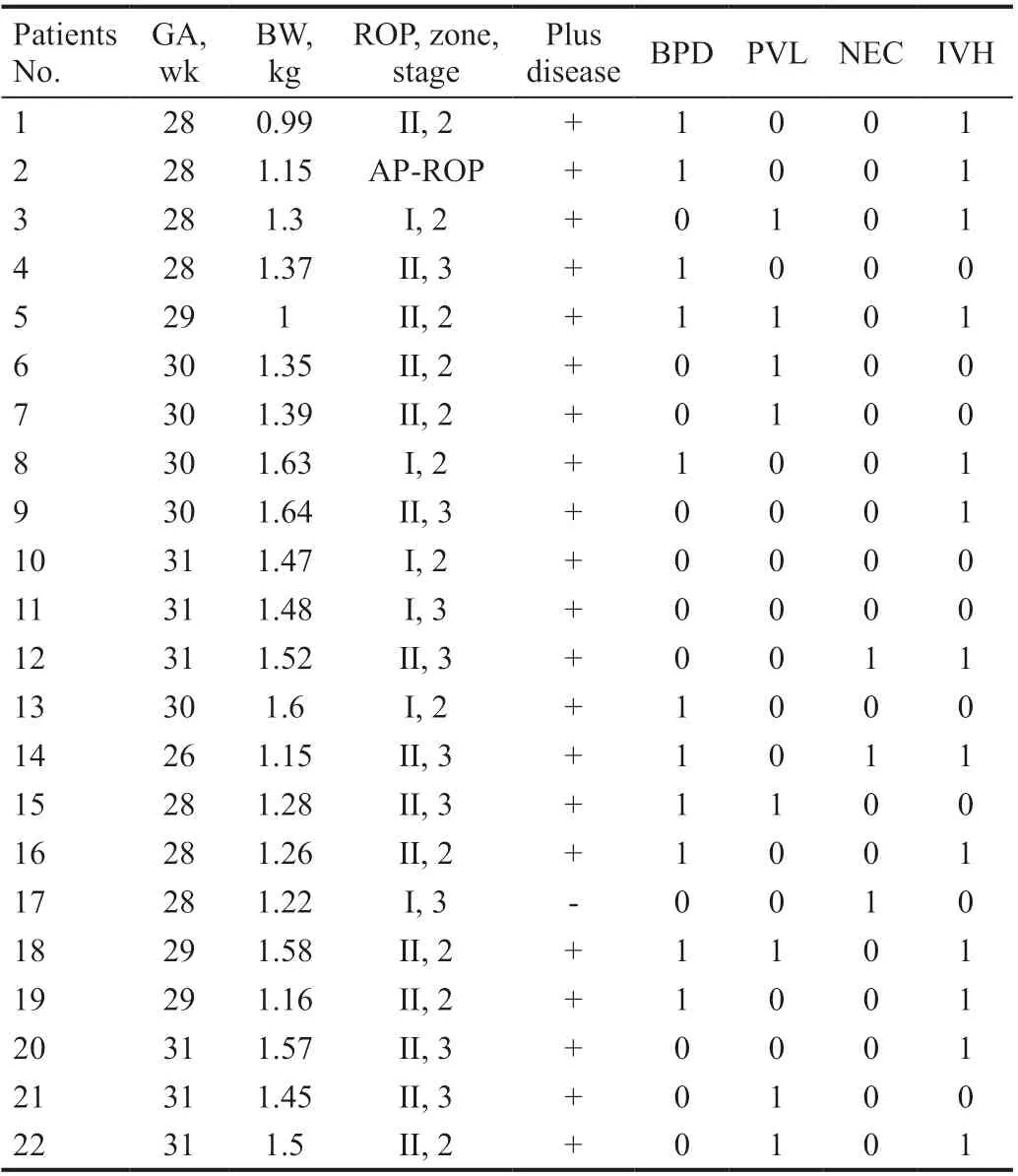
Table 5 Characteristics of 22 infants who developed type 1 ROP in no alarm group
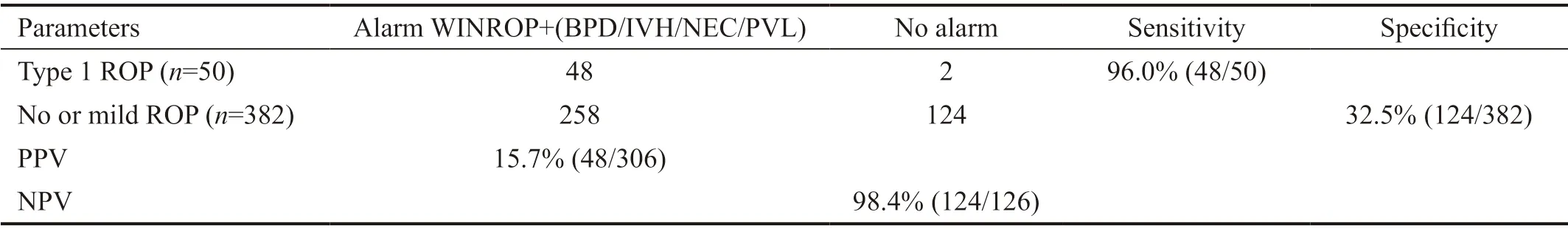
Table 6 Predictive performances in detecting ROP using the WINROP algorithm combined with risk complications
However, for infants with a GA more than 28wk or BW more than 1000 g, it was less effective which should be modified with other ROP risk factors. In a study carried out in Korea, several clinical complications were combined with the WINROP algorithm, such as BPD, IVH, NEC and sepsis, which were proved to be risk factors associated to ROP, and the sensitivity was improved obviously[36]. Another study based on a Polish cohort combined surfactant treatment as a risk factor with WINROP, the sensitivity of detecting type 1 ROP were also improved[37]. In this study, we have found that postnatal complications such as BPD, IVH, NEC and PVL, happened more frequently in infants with type 1 ROP compared with the no or mild ROP group. Thus, we tried to modify the predicting accuracy through combining these postnatal complications with WINROP, and not surprisingly, the sensitivity was improved to 96.0% (48/50). It demonstrated that WINROP was not as eあective in Chinese people as that in developed countries, thus, more adjustment methods should be added for the detecting accuracy.
There are some limitations of this study. First, this is single center study with a relatively small sample size. Second, WINROP could be only applied for infants less than 32wk, thus, those with larger GA may be missed by the algorithm. More modified algorithms with higher predicting accuracy remain to be developed particularly for Chinese population.
ACKNOWLEDGEMENTS
Authors’ contributions:Concept and design: Feng SF, Lu XH. Acquisition, analysis, or interpretation of data: Bai YC, Wu R, Chen SZ, Wei SY, Chen HJ. Statistical analysis: Wu R. Administrative, technical, or material support: Chen YC, Chen SZ. Drafting of the manuscript: Bai YC. Critical revision of the manuscript for important intellectual content: Feng SF. Study supervision: Lu XH. Final approval of the manuscript: All authors.
Foundation:Supported by the National Nature Science Foundation of China (No.81500722).
Conflicts of Interest:Bai YC,None;Wu R,None;Chen SZ,None;Wei SY,None;Chen HJ,None;Chen YC,None;Feng SF,None;Lu XH,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
