Endogenous fungal endophthalmitis: risk factors, clinical course, and visual outcome in 13 patients
Jamel Corredores, Itzhak Hemo, Tareq Jaouni, Zohar Habot-Wilner, Michal Kramer, Shiri Shulman, Haneen Jabaly-Habib, Alaa Al-Talbishi, Michael Halpert, Edward Averbukh, Jaime Levy, Iris Deitch-Harel, Radgonde Amer,7
1Department of Ophthalmology, Hadassah Medical Center, Jerusalem 91240, Israel
2Sackler Faculty of Medicine, Tel Aviv University, Tel Avi 6997801, Israel
3Division of Ophthalmology, Tel Aviv Sourasky Medical Center, Tel Aviv 64239, Israel
4Division of Ophthalmology, Rabin Medical Center, Petah Tikva 49100, Israel
5Department of Ophthalmology, Poriya Medical Center, Tiberias 1528001, Israel
6St John Eye Hospital, Jerusalem 91198, Israel
7Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem 9112102, Israel
Abstract
INTRODUCTION
Endogenous fungal endophthalmitis (EFE) represents a rare severe ocular infection, which can lead to irreversible vision loss. Candida species are the most common organisms that cause EFE in all age populations. Predisposing conditions include recent hospitalization, history of gastrointestinal surgery, indwelling catheters, systemic antibiotic use, bacterial sepsis, diabetes mellitus (DM), liver disease, renal failure and immunosuppressive therapy among others[1-2]. EFE may rarely occur in healthy immunocompetent patients without any risk factors[3-4].
Candida reaches the eye hematogenously through the choroid after an episode of fungemia, for which it first manifests in the posterior segment in the form of chorioretinitis. Slow progression in a subacute form is suggestive of candida endophthalmitis, as the infection may remain imperceptible until significant vitritis has developed. Funduscopic evaluation is characterized by multiple, fluあy, white chorioretinal lesions, usually located in the posterior pole, with overlying vitreous inflammation and vitreous exudates assuming a “string of pearls” appearance[1-2].
In this multicenter retrospective study, we aimed to analyze the risk factors, ophthalmological features, treatment modalities with their eあect on visual outcome and ocular complications in 13 patients who presented with EFE.
SUBJECTS AND METHODS
Ethical ApprovalThe medical records of patients with EFE between 2006 and 2018 were extracted from the databases of the uveitis clinics in 5 tertiary referral centers. The Institutional Review Boards of the hospitals approved the study, including waiver of informed consent for this chart review study. The study was conducted adhering to the tenets of the Declaration of Helsinki.
EFE was diagnosed clinically and diagnosis was confirmed by positive vitreous and/or blood and urine cultures. Cases graded II-IV according to Ishibashi’s proposed classification of the stages of EFE were included in the study [stage I: appearance of inflammatory cells in anterior chamber and vitreous, stage II: appearance of white round lesions in posterior fundus, stage IIIa: appearance of mild opacity in vitreous, stage IIIb: moderate or severe opacity in vitreous, stage IV: retinal detachment (RD) or totally opaque vitreous][5-6].
From 2007 to 2012, identification of Candida and filamentous fungi was based on microscopic appearance and the use of CHROMagar culture medium and API ID 32C (bioMérieux, France). From 2012 and on, isolates were identified mainly by Matrix-Assisted Laser Desorption Ionization Time-of-Flight mass spectrometry (MALDi TOF-MS, VITEK MS, bioMérieux, France). Susceptibility testing was performed using the E-test method according to manufacturer’s instructions (bioMérieux, France). Data retrieved from the medical files included age at presentation to the uveitis clinic, gender, ocular symptoms and their duration before presentation, history of fever, eye aあected, anatomical diagnosis according to the standardization of uveitis nomenclature (SUN)[7], laboratory evidence of fungal infection in ocular fluids, blood or other bodily fluids, predisposing underlying conditions, time interval between preceding procedure/operation or underlying illness and onset of EFE and other co-morbidities.
Data of ophthalmologic examination at presentation and at last follow-up was collected and included best-corrected Snellen’s visual acuity (VA), clinical findings on slit-lamp biomicroscopy, intraocular pressure by applanation tonometry and fundoscopy. B-scan echography was performed whenever indicated. Log of the minimum angle of resolution (logMAR) notation was used to compute the change in VA. VA of ≤6/60 was defined as severe visual loss, VA of >6/60 -<6/12 was defined as moderate visual loss and VA of ≥6/12 was defined as good VA.
Medical therapy recorded included systemic antifungal therapy and its duration, use of intravitreal antifungal agents and oral/intravitreal steroids. Surgical procedures were also recorded. Treatment and management decisions were dictated by the individual treating physician without a predefined study protocol. Duration of follow-up in weeks, ocular complications, time to resolution of EFE, and recurrences were also documented.
Statistical AnalysisStatistical analyses were performed using SPSS (version 25.0; IBM Corp., Armonk, NY, USA). Tests for normality of data were first performed using the Kolmogorov-Smirnov and Shapiro-Wilk analysis. Measurements of logMAR VA were summarized using mean, standard deviation (SD), and range. As the data were normally distributed, the comparison between initial and final logMAR VA was performed using Student’st-test.Pvalue <0.05 was considered as statistically significant.
RESULTS
DemographicsThirteen patients (10 females, 77%) with EFE were included in the study (Table 1). The mean age at diagnosis was 58y (median 63, range: 22-82y) and ten patients (77%) were in their 5thdecade and above. Mean follow-up time was 46wk (median: 40wk, range: 8-128wk).
Underlying Predisposing Conditions and Other ComorbiditiesTen patients presented after gastrointestinal or urological invasive procedures/operations and two patients presented after organ transplantation (both being <50 years of age; Table 1). In one patient, there was no recent or remote history of previous procedure/operation. Mean time interval between the onset of symptoms and previous intervention/operation was 4wk (median: 4wk, range: 3d-10wk). Six of the 10 patients who underwent previous gastrointestinal or urological intervention had fever and none of the 2 patients who underwent organ transplantation had fever.
All patients were either overtly or occultly immunocompromised. Seven patients were clearly immunocompromised because of liver cirrhosis (3 patients), underlying malignancy (3 patients)and cystic fibrosis and lung transplantation (one patient). Six patients on the other hand were occultly immunocompromised [five had diabetes mellitus type 2 (DM2) and one presented with acute inflammatory bowel disease; Table 1].
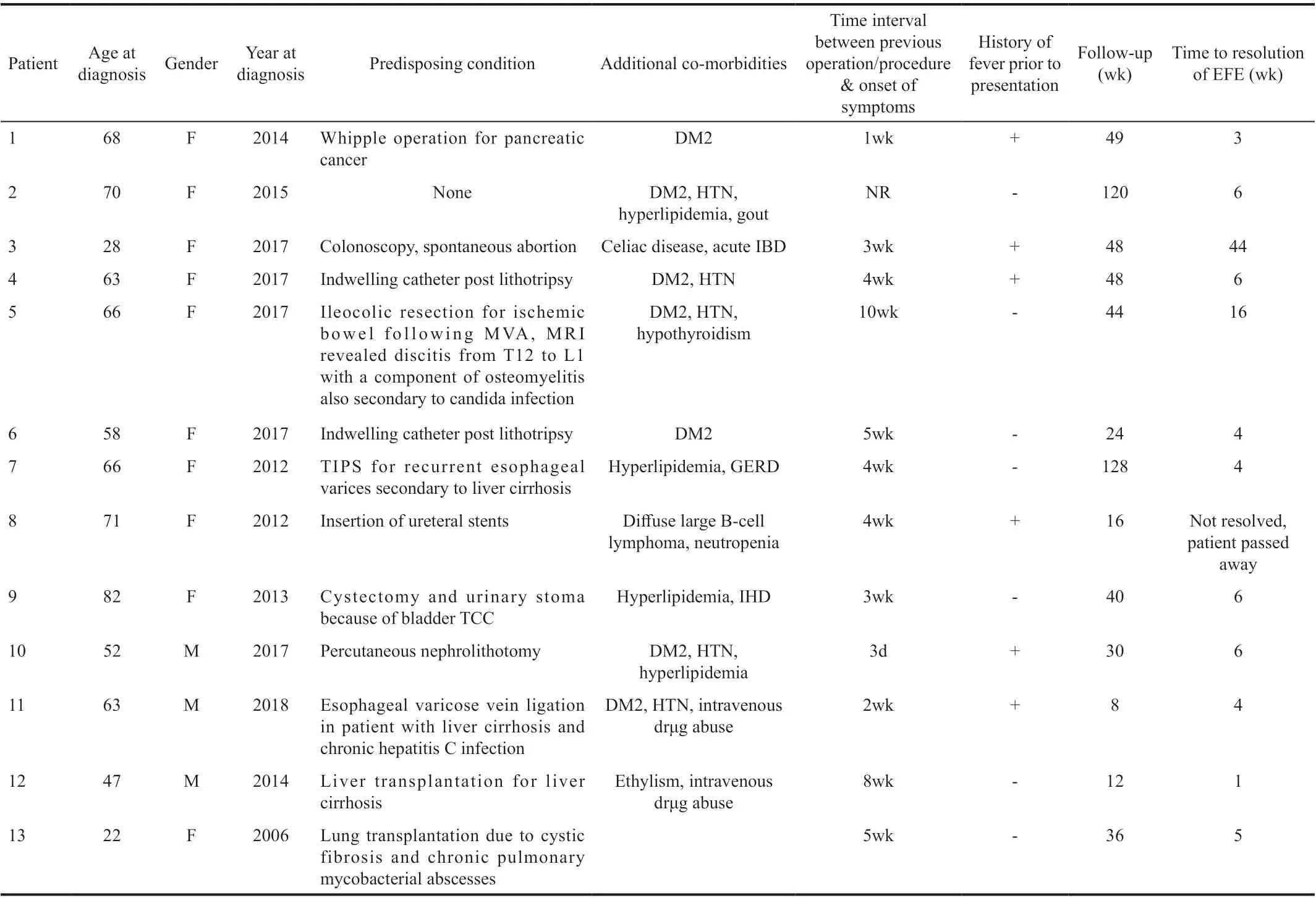
Table 1 Demographic features, predisposing conditions, and co-morbidities
Ocular Signs and Symptoms, VA at Presentation and at Last Follow-up and Ocular ComplicationsSeven patients had bilateral involvement (54%), with a total of 20 affected eyes. The most common ocular symptoms at presentation were reduced vision (10 eyes) and eye pain (7 eyes). Other symptoms included floaters (5 eyes) and eye redness (3 eyes). In four patients with unilateral symptoms, involvement of the fellow eye was diagnosed at presentation. In one asymptomatic patient, bilateral EFE was diagnosed as he was referred for examination because of candidemia (patient 12). Thus, EFE was asymptomatic and diagnosed on eye examination in 6 eyes (30% of eyes).
Fifteen eyes (75%) presented with stage III (11 had IIIb, and 4 IIIa) according to Ishibashi’s proposed classification, 4 eyes (20%) with stage II and one eye (5%) presented with stage IV with no view of the fundus (Table 2). Peripheral and macular retinal infiltrates (Figure 1) were seen in 8 and 6 eyes respectively (40%, 30%). Anterior uveitis was observed in 11 eyes (55%) and hypopyon was seen in 3 eyes (15%).
At presentation, VA was good in 9 eyes (45%), poor in 9 eyes (45%) and moderate in 2 eyes (10%). At last follow-up, 14 eyes had good VA (70%), 3 eyes had poor VA (15%) and one eye had moderate VA (5%; Table 2). In patient 8, VA could not be assessed due to the poor general condition. VA improved from 0.9±0.9 (6/48) at initial exam to 0.5±0.8 logMAR (6/20) at last follow-up (P=0.03, pairedt-test). The mean diあerence in logMAR VA between presentation and last follow-up was greater for eyes treated with oral steroids (±intravitreal dexamethasone) than eyes that were not treated with steroids, however, this difference did not reach statistical significance (P=0.1,t-test). In the former, VA improved from 1.2±1.2 at presentation to 0.6±0.7 logMAR at last follow-up while in the latter VA improved from 0.7±0.7 at presentation to 0.4±0.9 at last follow-up.
The most common complications on last follow-up included epiretinal membrane (ERM) and cystoid macular edema (CME) in 2 eyes, macular scar in 2 eyes, ERM in one eye (Figure 2), bilateral posterior subcapsular cataracts in 2 eyesand tractional RD, proliferative vitreoretinopathy, hypotony and band keratopathy in one eye. Twelve eyes (60%) showed no complications on last follow-up (Table 2).

Table 2 Clinical data at presentation and last follow up, complications, and staging of the disease

Figure 2 Follow-up photographs of the left eye (patient 1) 13mo following presentation A: Wide-angle fundus photograph showing clear vitreous with normal looking optic disc, macula, and blood vessels. An atrophic retinal lesion is seen in the superotemporal periphery. B: Spectral-domain optical coherence tomography showing preserved foveal contour with fine ERM in the papillomacular bundle.
Mean time to resolution of EFE was 9wk (median: 5.5wk, range: 1-44wk). In one patient (patient 8), EFE did not resolve as she had peripheral retinal infiltrates at the last follow-up and passed away after 16wk because of lymphoma complications.
DiagnosisDiagnostic vitrectomy was performed in 16 eyes of 12 patients (in four patients it was performed in both eyes). Only one patient (patient 10) did not undergo diagnostic vitrectomy as both blood and urine cultures were positive.
Of these 16 eyes, culture result was positive in 10 eyes (62.5%) of 8 patients (vitreous cultures were positive in both eyes of patients 4 and 11; Table 2). In the remaining four patients, two had positive urine or blood cultures (patients 6 and 12 respectively) and 2 patients (patients 3 and 9) had all cultures negative (vitreous, blood, and urine samples). In the latter 2 patients (15%), diagnosis was presumed based on clinical picture (Figure 3) in the presence of suggestive risk factors and resolution with antifungal systemic and local therapy.

Figure 3 Color fundus photograph of the right eye (patient 3) at presentation (A) shows fluffy white retinal lesion along the inferotemporal arcade with preretinal extension and hazy view because of associated vitritis. Spectral-domain optical coherence tomography shows a hyperreflective dense retinal elevated lesion in the area of the white retinal infiltrate with vitreous infiltration and CME. Color fundus photograph of the right eye 10d later shows clear vitreous with marked regression of the white retinal lesion along the inferotemporal arcade (B). Spectral-domain optical coherence tomography shows resolution of the CME with fine hard exudates in the outer retinal layers and resolving retinal infiltrate.
Blood samples were tested for fungi in all patients. In four patients only (31%) were the blood cultures positive. In two of them the vitreous cultures were also positive (patients 5 and 8; Table 2).
Candida albicans was the most common pathogen detected in 7 patients. Candida tropicalis, Candidaglabrata, and Aspergillus terreus were detected in one patient each (Table 2).
TreatmentAll patients received systemic antifungal therapy (Table 3): the most commonly used was fluconazole in 9 patients (69%); voriconazole in 3 patients (23%) and amphotericin in one patient (7.7%). Mean duration of systemic treatment was 12.6wk (median 6wk, range 2-48wk).
Sixteen eyes (80%) received intravitreal antifungal agent with voriconazole being the most commonly used. Four eyes (patients 7, 9, 10) did not receive intravitreal antifungal agent. However, one eye of those 4 eyes underwent vitrectomy (patient 7) and one eye (left eye of patient 10) did eventually receive intravitreal amphotericin because of recurrence of EFE after discontinuation of systemic therapy.
Diagnostic vitrectomy was performed in 16 eyes (80%). The remaining 4 eyes were treated as follows: patient 10 who presented with bilateral involvement was initially treated with systemic antifungal therapy alone; however, following cessation of therapy, the patient suffered from recurrence of EFE and systemic antifungal therapy was re-instituted together with left eye intravitreal amphotericin injection, one eye (left eye of patient 8) received intravitreal amphotericin and one eye (left eye of patient 9) did not undergo intravitreal injection or vitrectomy but resolved with systemic antifungal therapy.

Table 3 Medical therapy and surgical intervention
Oral steroids were used in 5 patients (3, 4, 5, 11, and 13) with a mean duration of 27wk (median 36, range 5-48wk), of whom two patients (3 eyes) were also treated with intravitreal dexamethasone injections (0.4 mg/0.1 mL).
Two patients (3 and 10) sustained recurrence of EFE after cessation of therapy. Patient 3 sustained two recurrences, 4wk and 3mo after cessation of therapy. She was subsequently maintained on oral fluconazole till the last follow-up. Patient 10 sustained a recurrence 11wk following cessation of therapy. Antifungal local and systemic therapy was re-instituted for a period of 5mo with complete regression of EFE.
DISCUSSION
The present study highlights the importance of recognizing the clinical features and risk factors of EFE in light of low to moderate yield of laboratory tests. First, the most common predisposing risk factors in the present study were previous gastrointestinal and urological interventions. In almost half of the patients there was no clear indication of a possible immunosuppressive condition; most of them however, were diabetics. Second, screening for EFE in patients at risk is important since patients may be asymptomatic and in our study 30% of eyes were asymptomatic. Third, vitreous samples represented a better approach for confirming diagnosis than blood cultures since they yielded positive culture results in almost 63% of the eyes, twice the yield obtained with blood samples. This fact confirms previous knowledge of EFE occurring after transient fungemia and is emphasized in the present cohort as a preceding episode of fever was documented in only half of the patients. Fourth, adding steroid therapy to the systemic and local antifungal therapy was associated with a trend of a bigger gain in VA than eyes treated solely with antifungal therapy. Fifth, avoidance of ocular complications is possible with prompt medical and surgical intervention and in the present cohort 60% of eyes showed no ocular complications by the last follow-up. Several studies reported on low diagnostic yield from ocular cultures. Tirpacket al[8]reported on negative ocular cultures in 7 out of 10 patients. Authors described this low yield to be characteristic of Candidaendophthalmitis since Candida preferentially sequesters within the inflammatory nodules limiting the yield of culturing techniques[9].
In only 4 patients (31%), blood cultures were positive. The sensitivity of blood culture in invasive candidiasis[10]is estimated to be 50% and culture is likely to miss deep-seated candidiasis in the absence of candidemia. Binderet al[11]reported that positive blood cultures were less frequent in patients with yeast endophthalmitis (20%) compared to those with bacterial endophthalmitis (50%).
Although candidemia is the most common manifestation of invasive candidiasis, deep-seated infections of organs such as the liver or eye, might occur after a bloodstream infection and persist after clearance of fungi from the bloodstream[12-14]. It is therefore imperative to consider EFE even when blood cultures are non-yielding since patients may present following transient fungemia such as after an outpatient endoscopy. It is subsequent to this fact that patients do not frequently present with overt constitutional symptoms. In the index study, fever was reported by only half of the patients. Leiet al[15]reported that 60% of the patients had history of fever before their eye symptoms. Tirpacket al[8]reported that nine out of ten patients were clinically well, lacking systemic signs of infection.
Mean age at presentation in the index study was 58y. Similarly, Sridharet al[16]and Lingappanet al[17]reported a mean age at diagnosis of 51 and 50y in their cohorts respectively. Leiet al[15]reported a mean age at presentation of 44y after genitourinary procedures and Shen and Xu[18]reported 25 patients who presented at a mean age of 43y. Tirpacket al[8]reported 10 patients with I.V. drμg use who presented at a mean age of 34y. Variability in the age at presentation reflects the different underlying predisposing events leading to the inciting systemic fungal infection.
Only one patient in our cohort had no apparent inciting recent risk factor (patient 2). She was diabetic and she did not develop any form of immune deficiency over a period of 2.5y. Candidaendophthalmitis was described in immunocompetent women during pregnancy or after vaginal delivery or post-partum period or secondary to surgical abortion[19-24]. Half of the patients in the present cohort were diabetic. Diabetic patients are more susceptible to fungal infections, particularly with Candida albicans. Lamichhaneet al[25]reported an increased frequency of oral Candida carriage in diabetic patients related to poor metabolic control, high glucose concentrations in the blood and saliva and a deficient immune response. Also, diabetic females are at risk of vulvovaginal candidiasis[26]. It is possible therefore to speculate that the patient had a subclinical well-contained candida infection which resulted in transient candidemia with subsequent hematogenous ocular seeding. Unfortunately, visual outcome was the worst in this patient owing to the prolonged delay till diagnosis.
The only patient with mold infection had cystic fibrosis and presented with EFE after lung transplantation. Sridharet al[16]reported that mold-associated EFE patients were more likely to have history of whole-organ transplantation than patients with yeast-associated EFE. Lung transplant recipients have a higher risk of fungal infections than other solid-organ recipients[27].
Twelve patients (92%) in the current study received systemic fluconazole or voriconazole. According to the IDSA guideline[28], fluconazole or voriconazole are recommended as first-line therapy for Candida endophthalmitis because of broad spectrum activity and superior ocular penetration. Voriconazole is a second-generation derivative of fluconazole with a 96% bioavailability. It achieves vitreous levels that are approximately 40% of serum levels even in uninflamed eyes[29]. One added advantage of voriconazole over fluconazole is that it has activity against Aspergillus and fluconazole-resistant Candida species[30]. Gaoet al[31]postulated that voriconazole was safer than amphotericin B because very low doses of intravitreal amphotericin B (4.1-8.3 μg/mL) caused focal retinal necrosis[32]. Gaoet al[31]sμggested that voriconazole should be considered the first-line intravitreal agent for treatment of fungal endophthalmitis.
Five patients were treated with oral steroids (of whom 2 also received intravitreal dexamethasone). A trend of greater visual improvement was noted in favor of eyes treated with oral steroids (±intravitreal dexamethasone) than eyes that were not treated with steroids. In the endophthalmitis vitrectomy study[33], all patients received intravitreal antibiotics, oral and topical steroids but no patient received intravitreal steroids. The role of intravitreal corticosteroids in fungal endophthalmitis is controversial. Coats and Peyman[34]in their rabbit model of exogenous Candida albicans endophthalmitis concluded that corticosteroids did not impair antifungal activity or enhance fungal proliferation. Similarly, Majjiet al[35]showed in patients with exogenous fungal endophthalmitis that intravitreal dexamethasone promoted faster clearance of inflammation. Bayramet al[36]showed for the first time that adjuvant corticosteroids, added to antifungal therapy, were beneficial for chronic disseminated candidiasis (CDC).
No ocular complications were observed in the majority of eyes (60%). The most common complications were ERM, CME, and macular scar. RD was observed in one eye only (5%). Lingappanet al[17]reported that RD developed in a third of their eyes, mostly after one month.
Pars plana vitrectomy was performed in 80% of eyes in the present cohort. Visual rehabilitation is faster and more complete by early and full vitrectomy[37]. This has also been reflected in the significant visual improvement in the present study with 70% of eyes attaining good vision at the last follow-up. Recurrence of EFE remains a challenge[38-39]since patients usually present following complete recovery. Two patients in the present cohort sustained recurrent EFE and were promptly treated for extended period of time with excellent visual outcomes.
The study limitations include its retrospective nature and the variability of follow-up and management schedules between the centers. Despite these limitations, we believe that this cohort represents real-life experience of EFE in the country.
In conclusion, EFE is a medical emergency with potentially devastating ocular consequences. High index of suspicion in patients with inciting risk factors is essential because of low yield of blood cultures and the good general condition of patients at presentation. Visual prognosis is improved with the prompt institution of the most potent systemic and intravitreal pharmacotherapy and the immediate surgical intervention when indicated. Oral±local steroids could be considered in cases of prolonged or marked inflammatory responses in order to hasten control of inflammation and limit ocular complications.
ACKNOWLEDGEMENTS
Conflicts of Interest: Corredores J,None;Hemo I,None;Jaouni T,None;Habot-Wilner Z,None;Kramer M,None;Shulman S,None;Jabaly-Habib H,None;Al-Talbishi A,None;Halpert M,None;Averbukh E,None;Levy J,None;Deitch-Harel I,None;Amer R,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
