Leptin’s concentration in tears and dry eye: a clinical observational study
Ran Hao, Yan Liu, Xue-Min Li
Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China
Abstract
INTRODUCTION
Dry eye disease (DED) is a common ocular disorder characterized by ocular surface discomfort and visual impairment[1]. Reduced quality and quantity of tears due to reduced secretion or excessive evaporation, causes increased osmolality of the tear film and promotes ocular surface inflammation[1-2].Increasing evidence demonstrates that ocular surface inflammation is responsible for the dry eye-associated symptoms and signs[1-8]. Also, research indicates that long-term tear film anomalies decrease anti-inflammatory components, such as lactoferrin, and increases pro-inflammatory cytokines in tears, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and protease matrix metalloproteinase-9 (MMP-9). These inflammatory mediators appear to activate the mitogen-activated protein kinases (MAPKs) pathway and initiate inflammatory cascades on the ocular surface, which stimulates inflammatory cytokines and proteases expression[8]. The inflammatory cascades lead to increasing expression of immune activation and cell adhesion molecules such as intercellular cell adhesion molecule-1 (ICAM-1) and human leukocyte antigen-DR (HLA-DR), recruiting more inflammatory cells and aggravating inflammation[8-10]. Molina-Leyvaet al[11]found that the levels of inflammatory cytokines such as IL-1, IL-2, IL-6, IL-8 and TNF in tears and in the conjunctival epithelium of dry eye patients, were higher than in normal patients, in direct proportion to the severity of DED.Leptin is a unique member of the cytokine family that is a product of the Ob gene[12-15]. Leptin plays a role in inhibiting food intake, adjusting energy consumption, regulating reproduction and development, promoting angiogenesis and cell proliferation as well as regulating immune responses and inflammatory processes[12,15-21]. Leptin has a similar structure to Class I cytokines and may function in a similar way to IL-6, leukemia inhibitory factor (LIF) and granulocyte colonystimulating factor (G-CSF), among others[12,14]. Recent studies demonstrate that leptin acts as a pro-inflammatory cytokine and regulates inflammatory cytokines secretion in varying patterns[13,16,19]. Diあerent inflammatory stimuli have been found to regulate leptin expression[12,15,19]. Indeed, the increased level of circulating leptin in acute inflammation may support initial pro-inflammatory responses[19].Increasing evidence indicates that leptin correlates with the development of ocular inflammation and DED[16]. Serum leptin significantly increases in patients with infectious keratitis and promote pterygium progression in animal models[16]. Leptin can also stimulate the formation of corneal, retinal and choroidal neovascularization in animal models[17-21]. Based on this reports, we aim to study whether leptin is related to DEDassociated ocular surface inflammation.

Figure 1 Tear collection A: A plastic head was used to squeeze tear into a capillary tube from the lower tear meniscus; B: Tears inside the capillary tube; C: Tears were collected from the capillary tube and placed in EP tubes.
The purpose of this study is to measure the concentration of leptin in the tears and evaluate its correlation with DED symptoms and signs.
SUBJECTS AND METHODS
Ethical ApprovalThe study was performed in accordance with the Declaration of Helsinki and approved by the Human Research and Ethics Committee of the hospital where the study was conducted. Written informed consent was obtained from all study subjects.
Study Design and EnrollmentThis observational study includes fifty-eight dry eye patients (58 eyes) from the Department of Ophthalmology, and healthy volunteers (39 eyes) that responded to the advertising without DED signs or symptoms or other ocular problems, enrolled from April 2014 to January 2015.
DED patients must had at least 1 symptom (foreign body sensation or eye irritation or light sensitivity or grittiness) and 1 sign [Schirmer test (ST) ≤5 mm/5min or corneal fluorescein staining score ≥4] to be included in the study. The right eye of each participant was enrolled in the study. Patients were excluded from the study if they were pregnant women, children, any patient with acute inflammation and serious systemic disease, using systemic steroids, anti-inflammatory or antimetabolites. All the examinations were performed by the same experienced clinician, who was masked to group aleatorization of patients.
Symptom EvaluationSymptomatic evaluation with the ocular surface disease index (OSDI) preceded clinical ocular surface examination. This questionnaire is comprised of 12 questions and evaluates the frequency of symptoms over the preceding week. OSDI is divided into three parts: eye discomfort, visual function impairment and susceptibility to environmental factors, with scores that range from 0 to 100[22].
Tear Collection and Leptin Concentration MeasurementNon-irritating tear collection was conducted using 5 uL capillary pipets without any anaesthetic (Figure 1). Patients did not receive eye drops prior to collection. A plastic head was used to squeeze tears into 0.2 mL EP tubes and tears were immediately placed at -80℃ for storage. The level of leptin in the specimen was measured using a double antibody sandwich assay with the human leptin enzyme-linked immunoassay (ELISA) kit (Abcam, United Kingdom).
Clinical EvaluationThe following diagnostic tests were also performed: tear meniscus height (TMH), tear film break up time (TBUT), corneal fluorescein staining, ST and impression cytology (IC). We used a micro-ruler to measure central TMH at the lower eyelid under slit-lamp (10× magnification). TBUT was observed under cobalt blue illumination. Corneal staining with sodium fluorescein was graded using the Van Bijsterveld scale. The cornea was divided into 4 quadrants, and scored as follows, 0: no staining; 1: 1-6 spots; 2: 7-30 spots; 3: >30 spots[23]. The number of spots was counted in each quadrant, to obtain a total score. The IC samples were obtained from similar locations (temporal palpebral conjunctiva). Five areas were randomly selected to count goblet cell number on IC image, and the mean was calculated accordingly under a microscope (×400) and graded by Nelson’s scale as follows[24], 0: small and round cells and large nuclei, with a nuclear cytoplasmic ratio of 1:2, goblet cells >500 cells/mm2, PAS positive staining; 1: slightly larger cells and smaller nuclei, with a nuclear cytoplasmic ratio of 1:3, goblet cells are decreased to 350-500 cells/mm2, but maintain PAS positive staining; 2: larger and polygonal cells and small nuclei, with nucelocyto-plasmic ratio of 1:4-1:5, goblet cells 100-350 cells/mm2, poorly-defined borders with less intensively PAS-positive; 3: more larger and polygonal cells and small and pyknotic nuclei, with a nuclear cytoplasmic ratio of >1:6, goblet cells <100 cells/mm2.
Statistical AnalysisSPSS 19.0 (IBM, Armonk, NY, USA) statistical software was used for statistical analysis. Descriptive parameters were expressed as the number of patients (%), means±standard deviation (SD) or median (Q1, Q3) based on the distribution and the homogeneity of variance of the data. Independentt-test was used to compare age, body mass index (BMI) and leptin concentration in tears between the two groups. Mann-WhitneyUtest was used to compare OSDI,

Table 1 Demographics information and clinical symptoms/signs in both groups median (Q1, Q3)
TMH, TBUT, corneal fluorescein staining, ST and IC between the two groups. The correlation between leptin concentration in tears and clinical symptoms/signs was analyzed using Spearman’s correlation coeきcients. The eあects of dry eye and clinical symptoms/signs on leptin concentration in tears were assessed by multivariate linear regression. Chi-square test was used to compare the gender composition. Results were considered significant atP<0.05.
RESULTS
A total of 97 eyes from 97 individuals (aged 54.58±20.60y, 65 females) were enrolled in the study, including 58 dry eye patients (aged 56.16±19.39y, 40 females) and 39 healthy controls (aged 52.23±22.33y, 25 females). No significant differences in gender, age and BMI were observed between groups (P=0.570,P=0.409, andP=0.892, respectively). Demographics are shown in Table 1.
Eye discomfort, visual impairment and total scores in dry eye patients were significantly higher compared to controls, with a significant difference between groups (P≤0.001). The sensitivity to the environment scores was numerically higher in the dry eye group but there was no significant difference between groups (P=0.057). The results are showed in Table 1 and Figure 2. TMH, TBUT and ST were significantly lower in dry eye patients and corneal fluorescein staining was significantly lower in the control group (P<0.001,P<0.001,P=0.004, andP=0.042, respectively; Table 1 and Figure 3).
A total of 86 IC images were obtained, 53 from the dry eye group and 33 from the control group. Goblet cell density in the dry eye group was 78.25 (50.08, 136.16/mm²), and in the control group was 134.59 (82.95, 198.75/mm²). Goblet cell density in the control group was significantly higher than in the dry eye group (P=0.003; Figure 4A-4E). There was a statistically significant difference in Nelson’s classification (Figure 4F) between the two groups (P<0.001).
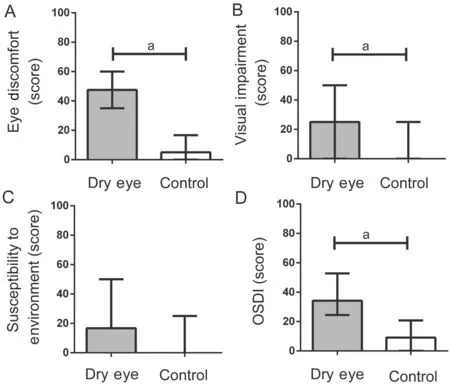
Figure 2 The scores of each OSDI subscales and overall score from dry eye patients and controls A: Eye discomfort; B: Visual impairment; C: Susceptibility to environment; D: Overall OSDI score. aP<0.05.
Leptin concentration in tears was 5.04±1.08 μg/L in the dry eye group, and 5.14±1.12 μg/L in the control group. No significant diあerence was observed between groups (P=0.682). There was a significant negative correlation between leptin concentration and age (r=-0.340,P=0.001), BMI (r=-0.332,P=0.001), OSDI (r=-0.258,P=0.011) and corneal fluorescein staining scores (r=-0.424,P<0.001). Leptin concentration in tears was positively correlated with ST (r=0.206,P=0.045). However, the TMH, TBUT and goblet cell density were not significantly correlated with leptin concentration. Multivariate linear regression analysis revealed that 4 variables were associated with leptin concentration in tears (Table 2). We found the linear equation best fitting the data to be Y=4.874+0.599X1-0.015X2-0.136X3+0.041X4(r2=0.252,P<0.05), where Y represents the leptin concentration in tears and X1, X2, X3, X4, represent dry eye, OSDI, corneal fluorescein staining scores and ST, respectively. The strongest predictor was dry eye (β=0.599).
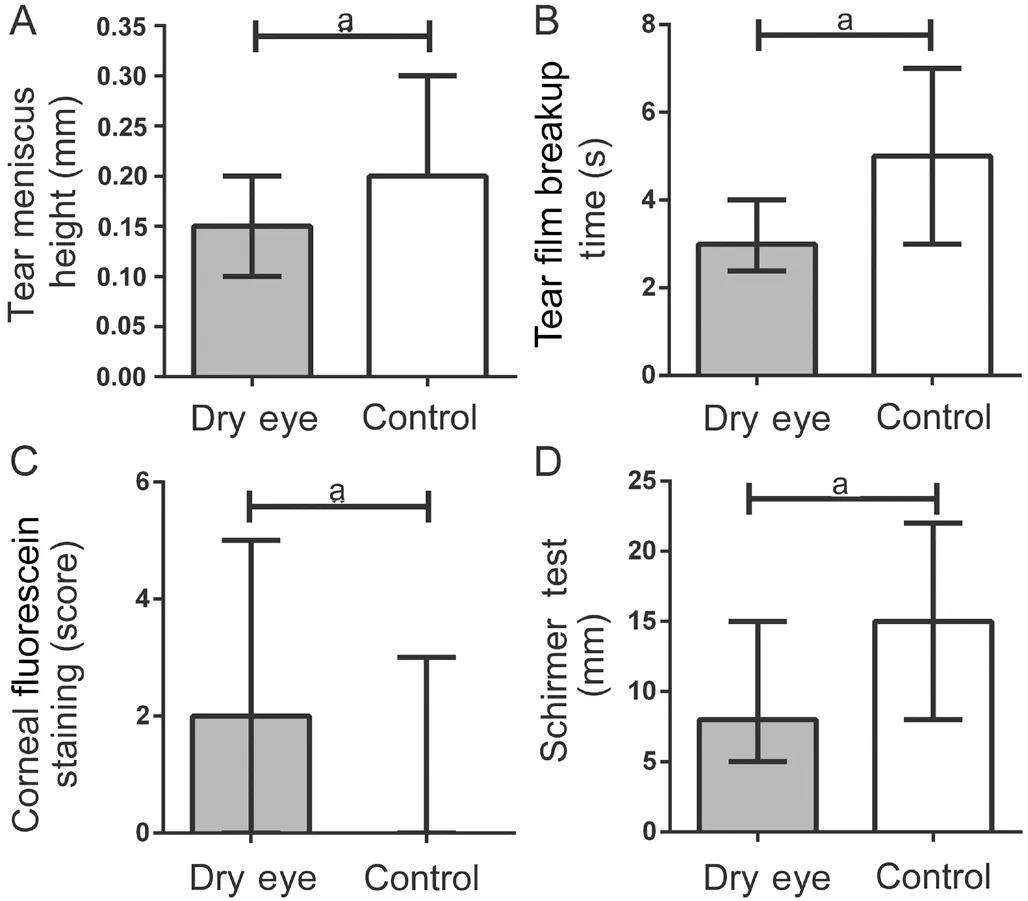
Figure 3 Results of dry eye signs in both groups A: Tear meniscus height; B: Tear break up time; C: Corneal fluorescein staining; D: ST. aP<0.05.
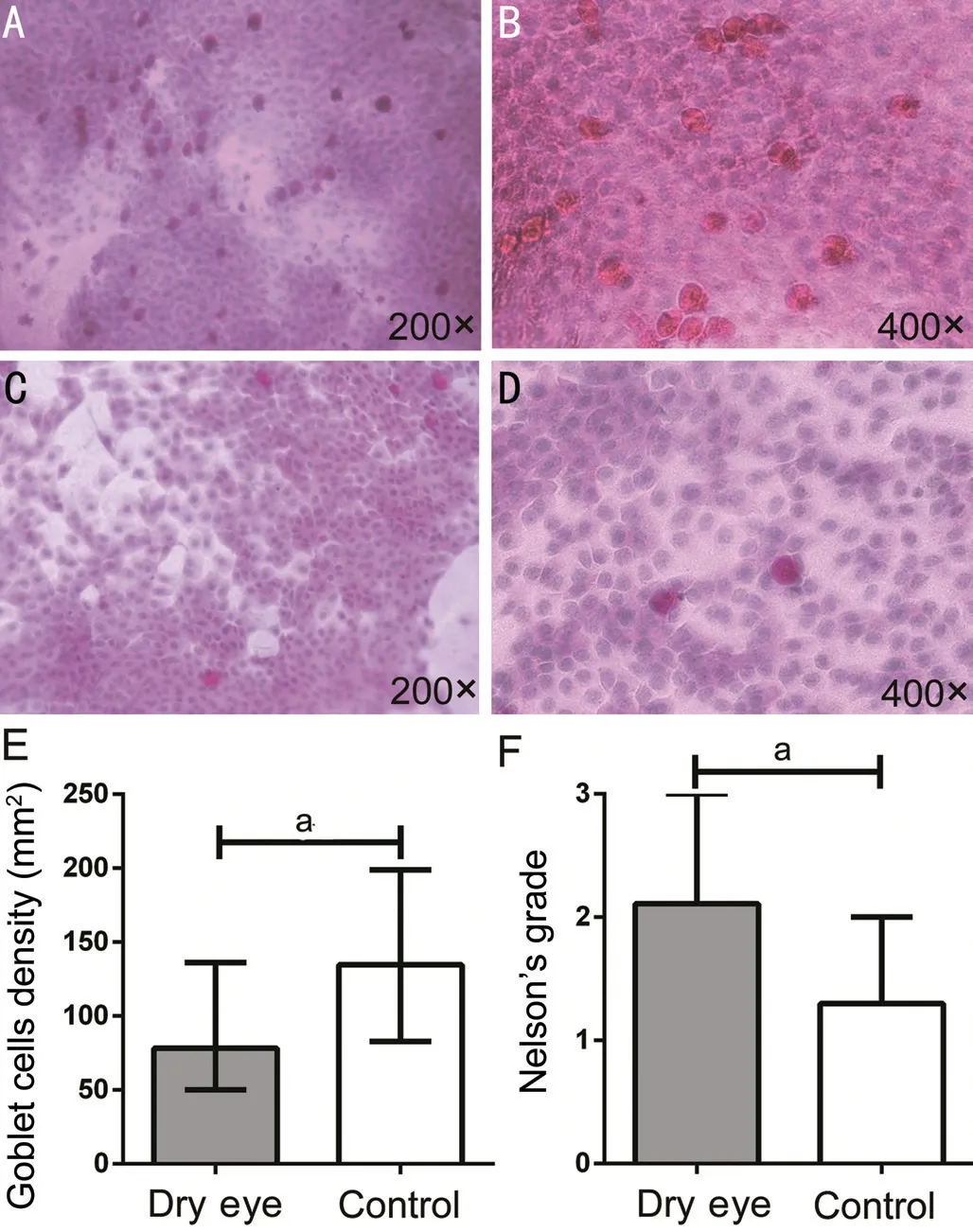
Figure 4 IC from dry eye patients and controls A: Normal IC (200×); B: Normal IC (400×); C: IC of dry eye patients (200×); D: IC of dry eye patients (400×); E: Goblet cell density; F: Nelson’s grade. The goblet cells are plump and oval with a PAS-positive staining cytoplasm. aP<0.05.
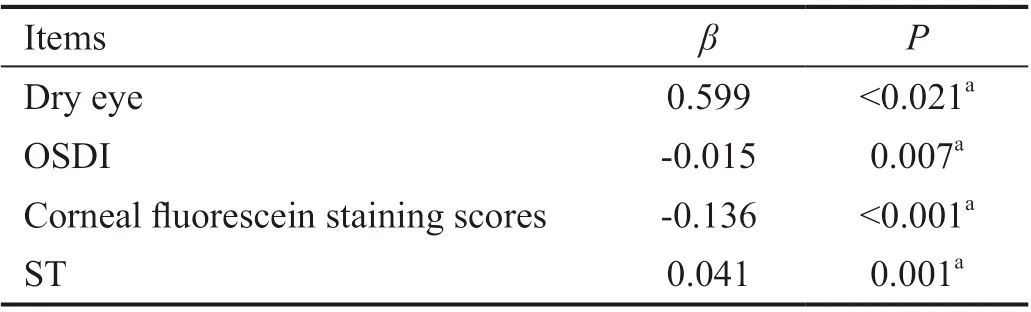
Table 2 Multivariate linear regression models: correlation between independent variables and leptin concentration in tears
DISCUSSION
To the best of our knowledge, this is the first study measuring leptin concentration in tears and analyzing the correlation between dry eye symptoms and signs with leptin concentration, to explore the role of leptin in the development of dry eye.
In this study, we found no significant difference in leptin concentration (P=0.682), in tears of patients with dry eye and controls, which may be attributable to the low volume of tears collected from DED patients. Serum leptin concentration of normal-weight subjects has been positively correlated with absolute fat mass, percentage body fat and BMI[25]. Gender differences also correlate with serum leptin levels in adults. When total body fat is similar, female serum leptin level is twice as high compared to male[26], and the concentration of leptin in serum in pregnant women is higher than that of nonpregnant women[27]. In this study, we found no significant diあerences in gender, age and BMI between the two groups, excluding an influence of demographic features on leptin levels. Also, we found that leptin concentration in the tears was negatively correlated with age, consistent with the change of leptin concentration in serum with age[28]. Interestingly, leptin concentration in the tears was negatively associated with BMI, and it was different from serum leptin concentration[29]. This may be due to a low number of samples and reduced tears volume.
A significant diあerence in eye discomfort, visual impairment and total scores was observed between groups, but no statistically significant differences were observed on the sensitivity to environmental factors. This suggests that patients with dry eye may have certain resistance to environmental stimuli. This may be due to a certain protection and injury restore mechanism of the ocular surface that enables patients with serious ocular discomfort and visual disorders to defend from external adverse stimuli.
Leptin can regulate the secretion of several cytokines secretion and different inflammatory stimuli could also regulate leptin expression and circulating leptin levels[13,16,19]. Previous studies have found that a long term instability of tear film in dry eye patients led to ocular surface inflammation, which was noted to be highly correlated with dry eye symptoms, as well as with a number of clinical features[1-3]. Leptin, as an inflammatory factor, also accumulates on the ocular surface; increased leptin concentration may accelerate mitosis in cells that surround an area of ocular surface damage, thereby regulating and accelerating the process of corneal epithelial cells regeneration[30-33], promoting ocular fibroblast proliferation and collagen synthesis[33], and stimulating angiogenesis. These processes help to repair ocular damage, alleviating dry eye symptoms and signs. Our findings, that increased leptin concentration in tears promotes symptom alleviation and improves corneal staining scores and tears secretion, are consistent with these studies.
Dry eye severity was associated with decreased goblet cell density, but the correlation of leptin concentration in tears with conjunctival goblet cell density failed to reach statistical significance. This may be due to the fact that dry eye symptoms and signs appear earlier than pathological changes like decreased goblet cell density[1]. Increased leptin concentration could induce ocular injury repair, but cell morphological pathological changes observed in IC caused by the long-term ocular surface damage may more time to recover.
Our study has some limitations. This is a cross-sectional study which cannot determine an exact correlation between leptin concentration in tears and DED appearance. Further longitudinal studies are required to confirm this. A larger sample size is needed to investigate the physiopathology of leptin concentration in tears and dry eye severity.
In conclusion, this is the first study measuring leptin concentration in tears. No significant diあerence was observed in the tears leptin concentration between dry eye patients and controls. The correlation between leptin concentration and DED symptoms and signs probably revealed that leptin level is associated with the DED. Finally, leptin levels potentially contributed to ocular surface repair and DED alleviation. To further explain the findings of this study, investigations on the mechanism of increased leptin concentration in tears caused by dry eye and leptin eあects on the ocular surface, are warranted.
ACKNOWLEDGEMENTS
Conflicts of Interest: Hao R,None;Liu Y,None;Li XM,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
