Long-term follow-up of cataract surgery in eyes filled with silicone oil
Piotr Kanclerz, Christoph Leisser, Andrzej Grzybowski, Pawe Lipowski
1Hygeia Clinic, Gdańsk 80-286, Poland
2VIROS-Vienna Institute for Research in Ocular Surgery, a Karl Landsteiner Institute, Vienna 1140, Austria
3Hanusch Hospital, Vienna 1140, Austria
4Department of Ophthalmology, University of Warmia and Mazury, Olsztyn 10-082, Poland
5Institute for Research in Ophthalmology, Foundation for Ophthalmology Development, Poznan 60-554, Poland
6Department of Ophthalmology, Medical University of Gdańsk, Gdańsk 80-952, Poland
Abstract
INTRODUCTION
Silicone oil (SO) is used mainly when managing complex retinal detachments, particularly with proliferative vitreoretinopathy, and as a hemostatic agent in proliferative diabetic retinopathy[1]. Many surgeons prefer phacoemulsification cataract surgery (PCS) and pars plana vitrectomy even for cataracts that are not clinically significant. However, combined surgery has disadvantages, including that it is more diきcult to perform, has a longer operating time, and involves a possible loss of corneal transparency[2]. Moreover, PCS can lead to zonular weakening with consecutive SO displacement into the anterior chamber. An advantage of sequential surgery is that it induces less postoperative anterior chamber inflammation; thus it may be recommended in proliferative diabetic retinopathy or retinal detachment[2-3]. A recent study in France found that combined surgeries accounted for only 15.8% of vitreoretinal procedures performed in 2005-2014[4]. Cataract development is common in phakic eyes filled with SO, necessitating subsequent cataract removal. PCS is commonly performed with concomitant SO removal[5-7]; however, in most cases, PCS can be performed while leaving the SO in place[8]. This study evaluated the refractive outcome of biometry in eyes filled with SO undergoing PCS.
SUBJECTS AND METHODS
Ethical ApprovalThis retrospective study included patients with 1000 cS or 5000 cS SO tamponade who were scheduled for cataract surgery at the Department of Ophthalmology, Medical University of Gdańsk, Poland between January 2012 and December 2017. We included individuals with nuclear and/or cortical cataracts who have underwent uncomplicated PCS with in-the-bag intraocular lens (IOL) placement and had follow-up data for a minimum of 3mo after surgery. Subjects with immeasurable refraction before or just after PCS, with a cataract due to previous capsular touch, or with macular detachment were excluded. The study complied with the principles of the Declaration of Helsinki, and was approved by the Clinic Ethics Committee.Preoperatively, all patients underwent a standard ophthalmological examination. Experienced examiners performed the biometry in an upright position using Alcon Ocuscan RxP (Alcon®, Fort Worth, Texas, USA) according to the departmental standards by a single trained observer. The IOL power was calculated based on axial length (AL) using the SRK/T, Hoffer, or Holladay formula, as recommended by Hoあer[9]. The ultrasound velocity for the vitreous compartment was set at 986 m/s[10], and the desired target was plano refraction. Surgeries were conducted under topical anesthesia and all patients received an acrylic one-piece IOL with a hydrophobic surface, the Acriva UDB 625 (VSY Biotechnologies, Amsterdam, Netherlands). The refractive error was assessed objectively using the Nidek ARK-530A (Nidek Co., Ltd., Aichi, Japan). Each measurement was deemed reliable if three subsequent measurements were obtained, and if the patient maintained fixation during the examination.
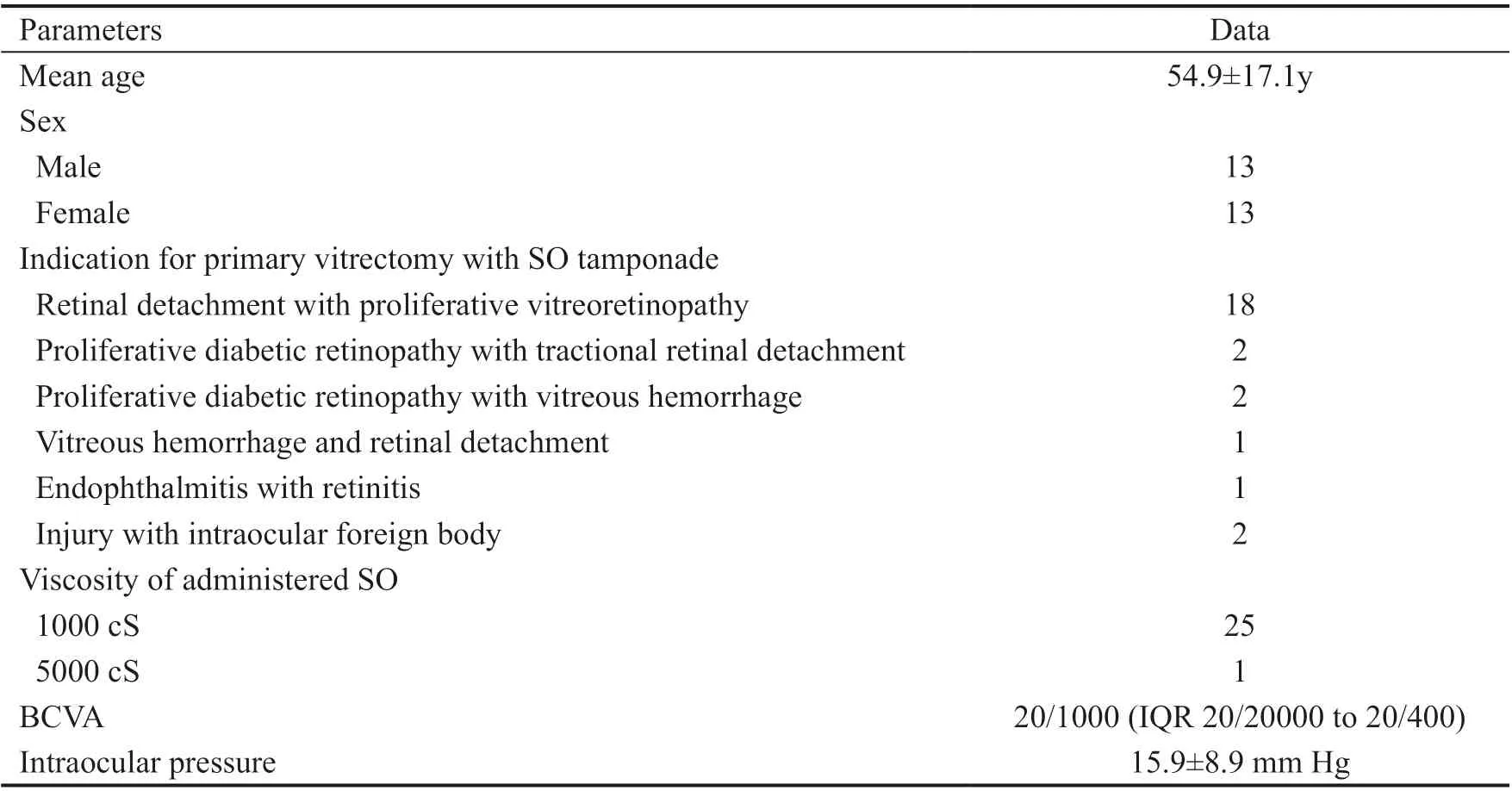
Table 1 Patient data
The Shapiro-Wilk test was applied to assess the distribution of the analyzed data. Variables following a Gaussian distribution are described using means and standard deviations; otherwise, the median and interquartile range (IQR) are stated. The diあerences in the results at consecutive time points in normally distributed data were calculated using the pairedt-test; otherwise, the Skillings-Mack test was applied. Results withP<0.05 were considered statistically significant. Best corrected visual acuity (BCVA) is presented in US equivalent form (Snellen). Counting fingers at 60 cm was assigned to a visual acuity of 20/2000, while hand movement was converted to 20/20000[11].
RESULTS
Thirty-two eyes of 32 patients underwent cataract surgery within the observation period. Six patients were excluded

Table 2 Long-term follow-up in patients with SO that have undergone cataract surgery n (%)
from the analysis because they were lost to follow-up due to unavailable medical records (2 patients), immeasurable autorefractometry just after cataract surgery (3 patients), or death (1 patient). Therefore, outcomes were analyzed for 26 eyes of 26 patients. In all cases, the preceding vitrectomy was performed at the same clinic, and the mean interval between vitrectomy with SO tamponade and PCS was 9.1±8.1mo. The median spherical equivalent refraction (SER) before PCS was +5.3 (IQR +2.9 to +6.7), and the mean cylinder was -1.9 D (IQR -2.8 to -0.8). Table 1 summarizes patients’ data.
The median SER changed after cataract surgery (P<0.01)and was +3.4 D (IQR +2.0 to +4.4), while the median postoperative refractive cylinder was -1.8 D (IQR -2.8 to -0.8). After PCS, the BCVA was 20/500 (IQR 20/2000 to 20/200), and the intraocular pressure was 17.6±3.6 mm Hg; not statistically different from the preoperative results (P=0.10 andP=0.79, respectively).
Subjects were followed for 29.5±13.9mo after cataract surgery (Table 2). Within this period, retinal reattachment was achieved after SO removal in 15 of 26 eyes (57.7%). Of these, 12 eyes (46.1%) required only one surgical procedure. Patients who developed retinal detachment after SO removal had anadditional vitrectomy and retinectomy with SO reintroduced; in two eyes, SO was reintroduced once, and in 1 eye, SO was reintroduced twice. The mean time between PCS and a successful SO removal was 7.2±5.4mo. In four eyes, SO was injected directly after removal, with no attempts to remove postoperatively. In seven eyes, no attempt was made to remove SO because of a poor prognosis.

Table 3 SER and VA before and after cataract surgery, as well as after SO removal
An objective assessment of refraction after SO removal was possible in 13 eyes, while in two eyes it wasn’t due to low visual acuity and poor fixation. The refraction after SO removal showed a myopic shift of -4.6 D (IQR -2.9 to -7.3;P<0.01); and the SER was equal to -0.9 D (IQR -2.1 to 0.6), and the refractive cylinder was -1.5 D (IQR -2.3 to -1.0). The cylinder did not diあer at any time point (P>0.05). The median BCVA did not change after SO removal (P=0.81). After removing SO, 5 of 13 eyes (38.5%) were within ±1.0 D of the target refraction, while 9 of 13 eyes (69.2%) were within ±2.0 D. After successful SO removal, one eye developed open-angle glaucoma and required implantation of an Ex-press glaucoma drainage device (Alcon®, Fort Worth, Texas, USA).
DISCUSSION
Until 2016, the Polish National Health System reimbursed only sequential surgeries and not phacovitrectomies. Thus, in our study all patients underwent sequential PCS and silicone oil removal as the method of choice. Although the refractive changes associated with the presence of SO have already been studied, our investigation additionally presents long-term follow-up in eyes filled with SO undergoing PCS[12-15]. In 7 out of 26 eyes (26.9%) no attempts were made to remove SO due to poor prognosis. Thus, it should be questioned whether these patients should have undergone PCS, which did not influence the BCVA (Table 3). In seven other eyes, SO was removed, but had to be reintroduced. Despite modern vitreoretinal techniques, SO removal is unfeasible in some cases, and the SO must remain in the eye indefinitely. The rate of these cases is likely underreported[16]. In some studies, the retinal reattachment rate was reported to be as low as 30.0%[17-18]; in our cohort, 42.3% of eyes required long-term SO tamponade. It is known that biometry in eyes filled with SO is less accurate than in normal eyes[13]. The main limitation of this study is the application of contact A-mode scans for biometry, as optical biometry is known to be more accurate that the applanation ultrasound technique[19]. However, some eyes cannot be measured using optical methods (e.g., dense cataracts); therefore, immersion A-scan biometry has an important role in these cases. Moreover, even if partial coherence interferometry can be employed, the refractive outcome in eyes filled with SO remains much worse than in normal eyes: in a study by Al-Habboubiet al[12], only 33.7% of eyes achieved the planned postoperative refraction of -2.0 D to +0.25 D[12-13]. In eyes filled with SO, AL measurements can be significantly biased by limited vitreous base removal during the vitrectomy; partial filling of the vitreous chamber with SO resulting in retrosilicone space[5]; macular edema or detachment; or incorrect parameters for AL adjustment[20]. Thus, one could consider performing biometry before vitrectomy when possible; this may be limited by a macula-off retinal detachment, prior scleral buckling, or surgery performed at another center[13]. Biometry of the contralateral eye is another alternative, although this is impossible in monocular patients and inaccurate in patients with anisometropia, after scleral buckling and after vitrectomy with SO tamponade. Although this study does not employ a control group, the refractive outcome in eyes without SO tamponade is well known. For example, in a recent study, 79.1% of cases reached ≤0.5 D of the refractive target, while 97.2% of eyes were within ≤1.0 D of target refraction[21].
We have also noted that in out cohort the myopic shift after SO removal was not predictable. Previous studies reported a refractive shift associated with SO removal in pseudophakic eyes from -3.9 D to -4.5 D with a large standard deviation (reaching 1.8 D)[14-15]; in our investigation, the change in refraction was similar (-4.6 D, with a wide IQR of -2.9 D to -7.3 D). A significant limitation of the current study is the group size: although this is the main vitreoretinal surgical department in the region, we were able to collect only 26 patients within the 5-year period. This could also be due to the fact that SO is less commonly used due to attempts to employ a temporary gas tamponade. Additionally, not all patients having undergone vitrectomy with SO could present for PCS at this department.
We conclude that the refraction after PCS for eyes filled with SO manifests low predictability, as does the myopic shift following successful SO removal. A significant percentage of the eyes undergoing SO administration required a long-term tamponade.
ACKNOWLEDGEMENTS
Conflicts of Interest:Kanclerz P,None;Leisser C,None;Grzybowski A,None;Lipowski P,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Response of L V Prasad Eye Institute to COVID-19 outbreak in India: experience at its tertiary eye care centre and adoption to its Eye Health Pyramid
- Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix
- Therapeutic potential of Rho-associated kinase inhibitor Y27632 in corneal endothelial dysfunction: an in vitro and in vivo study
- Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits
- A multi-omics study on cutaneous and uveal melanoma
- Eあects of quercetin on diabetic retinopathy and its association with NLRP3 inflammasome and autophagy
