Toxic response of aquatic organisms to guide application of artemisinin sustained-release granule algaecide
Li-xiao Ni *, Na Wang Xuan-yu Liu Fei-fei Yue Yi-fei Wang Shi-yin Li , Pei-fang Wang
a Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Ministry of Education, Hohai University, Nanjing 210098, China
b College of Environment, Hohai University, Nanjing 210098, China
c School of Environment, Nanjing Normal University, Nanjing 210097, China
Abstract In our previous study, we prepared the granules by embedding artemisinin into alginate-chitosan using microcapsule technology. These granules can release artemisinin sustainably and have a strong inhibitory effect on the growth of both single Microcystis aeruginosa and mixed algae.To safely and effectively use artemisinin sustained-release granules to control algal blooms,the ecotoxicity was studied by assessing their acute and chronic toxicity to Daphnia magna(D.magna)and Danio rerio(D.rerio),along with their antioxidant activities.The results showed that the 48-h median effective concentration(EC50)of pure artemisinin to D.magna was 24.54 mg/L and the 96-h median lethal concentration(LC50) of pure artemisinin to D. rerio was 68.08 mg/L. Both values were classified as intermediate toxicity according to the Organization for Economic Co-operation and Development(OECD).The optimal algae inhibitory concentration of artemisinin sustained-release granules(1 g/L)had low acute toxicity to both D.magna and D.rerio.The sustained-release granules had higher chronic toxicity to D.magna than to D.rerio.Partial indices of D. magna were inhibited by granules when the concentrations were larger than 0.1 g/L. Low granule concentration had an inductive effect on antioxidant enzyme activities in D.magna and D.rerio.With the increase of the exposure concentration and time,the enzyme activity presented a trend of first increasing and then decreasing,and the overall changes were significant.The change trend and range of enzyme activity indicated that the granules could cause serious oxidative stress to D.magna and D.rerio,and the changes were consistent with the results of toxicity experimentation.
© 2020 Hohai University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords: Artemisinin sustained-release granules; Aquatic organisms; Toxicity assessment; Median lethal concentration; Antioxidant enzyme activity
1. Introduction
With universal water eutrophication and harmful algal blooms, more attention has been paid to algal inhibition. The allelopathic effect of aquatic and terrestrial macrophytes is considered an environmentally friendly and promising approach to control algal blooms (Chiang et al., 2010;Fabrowska et al., 2018). Berberine has an allelopathic inhibition effect onMicrocystis aeruginosa(M. aeruginosa),which can significantly increase the content of extracellular microcystin by killing and lysing algae cells (Zhang et al.,2013). It was found thatAilanthus altissimaextracts could effectively inhibit the cell density ofM. aeruginosa, decrease the content of extracellular cyanotoxinM. aeruginosa, and destroy the photosynthesis-related structure of algae cells. It has certain potential in controllingM.aeruginosa(Meng et al.,2015).
In the process of algal inhibition, these allelopathic materials may influence other organisms and even the whole ecosystem (Cheng and Cheng, 2015). Thus, it is important to evaluate the allopathic algaecide by testing their toxicity to non-target aquatic organisms. Some allelochemicals, which present acute toxicity against animals,have been examined in previous studies (Griffiths and Saker, 2003; Lopes et al.,2017). The barley straw used for algal inhibition was found to significantly influence zooplankton community structure(Murray et al., 2010). The thiazolidinedione derivatives TD49 and TD53 have been used in the marine ecological system as new algaecides, and toxicity assessments showed that the application concentration for treating red tide blooms was higher than the corresponding predicted no-effect concentration (PNEC) values (Kim et al., 2011). In addition, an integrative analysis for evaluating the potential of 14 kinds of algaecidal materials as algaecides was made by comparing the 24-h median lethal concentration(LC50)and ratio of efficiency to safety (RES) of each material, the results showed that the toxic effects of an algaecidal material differed with the species of aquatic organisms involved (Zhou et al., 2010).
In recent years, in addition to algae toxicological studies,aquatic organism tests including fish and invertebrates have gradually become an important method in aquatic ecotoxicological research. Both acute and chronic toxicity experiments are necessary when exploring the toxicological effects of water to aquatic organisms.The purposes of acute toxicity tests are to investigate the toxicity levels of chemical substances, the relationship between dosage and biological response, and the mode of toxicity function; and to provide theoretical basis for further/other toxicity tests. Acute toxicity tests often concentrate on a single acute effect like mortality after short-term exposure (in the 48-h range) with adult or mature animals(Braunbeck et al., 2005). The chronic response of animals towards the gradually accumulated poisons is called chronic toxicity. Chronic toxicity tests require young females to be individually exposed to test compounds over 21 d (OECD,2004a,2012).Two typical aquatic organisms,the zooplanktonDaphnia magna(D. magna) and fishDanio rerio(D. rerio),have been widely used as test organisms in a variety of ecotoxicological studies(Jung Collard et al.,2013;Wu et al.,2014).D. magnacan maintain populations at a high density in a relatively small living space in laboratories with a short life cycle, and they can show up a variety of physiological and behavioral characteristics, such as swimming, phototactic behavior,and reproduction(Adam et al.,2015).D.reriogrow rapidly,have a short reproductive cycle,are easy to breed,and have transparent embryos.They are amenable to toxicological analysis(Wu et al.,2014).The gills of fish are in direct contact with other substances in the water environment during gas exchange. Toxic substances can easily enter the blood through contact with gill filaments.The liver is the detoxification center of the body and the target organ of many foreign compounds(Van Veld et al., 1988). The physiological and biochemical characteristics ofD. reriowill change before death due to poison. Thus, we can select indices of molecular and cellular level to determine the toxicity of algaecides(Qian et al.,2010).
A biomarker is defined as a change in a biological response(ranging from molecular through cellular and physiological responses to behavioral changes), which can be related to exposure to or toxic effects of environmental chemicals(Sabullah et al., 2015). Biochemical and physiological changes often involve the changes in protein levels and enzyme activity. For biomarker investigation, the activities of two antioxidant enzymes (superoxide dismutase (SOD) and catalase(CAT)),which are associated with the defense system to oxidative stress(Ribeiro et al.,2015),were evaluated in this study. The two enzymes are both considered to play key roles in detoxification (Malar et al., 2016).
In our previous study, artemisinin, which is extracted fromArtemisia annua, and artemisinin sustained-release granules were found to have a very strong inhibition effect onM.aeruginosaand mixed algae(Ni et al.,2012,2015).However,the toxicological research of allelopathic substances on aquatic animals is still in a preliminary stage in the algaecidal process(Zhang et al.,2009).In order to evaluate the safety of artemisinin sustained-release granules in the practical application to algal inhibition,this study aimed to(1)investigating the toxicity of artemisinin sustained-release granules at the optimal algal inhibiting concentration toD. magnaandD.rerioin organism level through acute and chronic toxicity tests, and (2) determining the response of antioxidant enzyme(SOD and CAT) activities in biochemical level.
2. Materials and methods
2.1.Preparationofartemisininsustained-releasegranules
The artemisinin (with a purity higher than 99%) was purchased from Nanjing Zelang Medical Technology Co., Ltd.The optimal preparation of artemisinin sustained-release granules was based on our previous study (Ni et al., 2013).The anti-algal sustained-release granules were prepared with artemisinin using the alginate-chitosan microcapsule technology. The optimum capsule preparation consisted of 2.5% sodium alginate, 0.25% chitosan, 0.6% artemisinin,2% calcium chloride, and 1.5 mL of the cross-linking agent, glutaraldehyde. According to the optimum preparation conditions, the encapsulation efficiency of artemisinin sustained-release granules could reach 68%, and the release content could reach about 4 mg/L in distilled water daily with an additional dosage of 10 g/L(Ni et al.,2013).In addition,the artemisinin released from granules accumulated in the water day by day.
2.2. Experimental animals and pre-incubation
D.magnaandD.reriowere obtained from the Nanjing Institute of Environmental Sciences.Aquaculture water forD.magnaandD.reriowas tap water,which was naturally aerated over three days.During the period of pre-incubation,D.magnawere fed on freshScnedesmus obliquus(S. obliquus) with the density of 2×105to 3×105cells/mL.D.reriowere fed on commercial fish food oncea day,and thefeces anduneatenfoodwere siphonedout in time. The culture condition maintained at (20 ± 1)°C under 40-60 μmol·m-2·s-1photons (12 h light/12 h dark). Furthermore,to reduce the amounts of excreted products in the test tanks,D. magnaandD. reriowere quarantined for 2 h and 24 h,respectively,without feeding before the experiment.HealthyD.magnaneonates about 6-24 h after birth,which shared the same parent fleas with parthenogenesis and were cultured for at least three generations,were used as test animals.The experiment forD.magnawas based on the guidance of Ma et al.(2016)and the acute toxicity was evaluated by calculating 24-h median effective concentration(EC50)values.Potassium dichromate was used as poison to assess the sensitivity ofD.magna,and the 24-h EC50valuewas1.2mg/L,whichwascompliedwiththerequirementsof sensitivity(0.6-1.7 mg/L)(Barata et al.,2016).D.rerioinsimilar body size, with a mortality of less than 5% after preincubation were selected for the test.
2.3. Toxicity assays of artemisinin sustained-release granules
According to the previous studies (OECD, 2004b), acute toxicity tests of pure artemisinin and artemisinin sustainedrelease granules toD. magnawere performed to derive 48-h EC50and LC50values. The test solution forD. magnawas reconstituted hard water (made by adding 123.25 mg MgSO4·2H2O,64.75 mg NaHCO3,294 mg CaCl2·2H2O,and 6.25 mg KCl into 1 L distilled/deionized water) with an electrical conductivity lower than 10 μS/cm, a hardness of(250±25)mg/L,a dissolved oxygen(DO)concentration over 2 mg/L,and a pH value of 7.8±0.2.According to the results of preliminary experiments, pure artemisinin (with concentrations of 0, 10, 20, 50, 100, and 120 mg/L) and artemisinin sustained-release granule groups (with concentrations of 0, 1,2, 4, 8, and 10 g/L) were used in the acute toxicity test toD.magna. The acute bioassay was performed under static nonrenewal conditions for 48 h, and five neonates younger than 24 h were exposed to 25 mL test solution with four replicates.It was recorded whether theD. magnawere survival or inhibited to grow every 12 h.TheD.magna,which swam less than three times of their body length within 15 s after gentle agitation, was considered to be immobile. In order to determine the EC50and LC50,the percentages of immobile and deadD. magnawere analyzed with probit analysis, and EC50and LC50were compared with the control group.
Acute toxicity testing forD. reriowas performed in accordance with several studies (Griffitt et al., 2007;Krishnaraj et al., 2015). About 100D. reriowere divided evenly into ten groups, which were separately exposed to 0,20, 50, 80, 100, and 120 mg/L pure artemisinin and 0, 5, 10,15, 20, and 25 g/L artemisinin sustained-release ganules in glass beakers with 2 L test solution according to the results of preliminary experiments. Three replicates were used for each concentration and a blank group was served as control. The experiments were performed at(27 ± 2)°C and pH 8.2 ± 1.It was considered a sign of death when the fish had no response to a tweezer clamping its tail. Poisoning symptoms, such as swimming stroke and body color, along with the number of dead fish were registered and recorded after 24, 48, 72, and 96 h. Mortality and LC50were calculated after the experiment.
Chronic toxicity testing of artemisinin sustained-release granules forD. magnawas conducted in accordance with the OECD guideline (OECD, 2012). Ten percent of the 48-h LC50value was set as the safety concentration under the stress of artemisinin sustained-release granules.The experimental groups included a blank group and four different concentrations of artemisinin granule groups with 0.05,0.1(safety concentration),0.2, and 0.5 g/L. Some neonateD. magnaabout 6 to 24-h old was placed in a 100 mL beaker with 20 mL culture solution.Three replicates were set up for each treatment.D.magnawere fed with 0.5 × 106cells per mL ofS. obliquusevery day. The test solution was refreshed every 24 h and the pre-released artemisinin was replenished in time. Neonates were removed promptly once they began to breed. The survival, growth, and reproduction ofD. magnawere monitored and the following parameters were recorded: survival time, date of first reproduction,number of first reproduction,fetus and neonate numbers per female,and death number.Body length(from top of its head to the base of its tail) of each femaleD. magnawas measured under the microscope at the end of the experiment.The intrinsic growth rate (r), representing the growth rate of an increasing population, was calculated using the Euler-Lotka equation(Cort´es, 2016):

wherelxis the proportion of individuals surviving to agex(d),andmxis the age-specific fecundity (number of neonates produced per surviving female between agesxandx+1).AsrofD. magnais indistinguishable fromrestimated for the entire lifespan, due to the importance of early reproduction(Villarroel et al.,2013),all calculations were based on the 21-d experiment.In addition,the changes of enzyme activity were measured after 3, 9, 15, and 21 days of exposure.
Taking ten percent of the 96-h LC50as safety concentration in chronic toxicity tests,D. reriowas exposed to four different concentrations of artemisinin sustainedrelease granules: 0.5, 1.0, 1.5 (safety concentration), and 2.0 g/L for 28 d. Each treatment was composed of four beakers containing 2 L test solution and tenD. rerio. Living conditions, including swimming velocity, were recorded every day and fourD. reriowere chosen randomly from each group at 7, 14, 21, and 28 d for further biomarker assessment (enzyme activity). The artemisinin content in the system was determined using the method proposed by Chadha et al. (2012).
2.4. Determination of enzyme activity
The 48-h enzyme activity ofD. magnawas measured with the pure artemisinin concentrations of 0, 5, 10, 15, and 20 mg/L and the artemisinin sustained-release granule concentrations of 0, 0.2, 0.4, 0.8, and 1.6 g/L. In the process of enzyme activity determination, 10-15 livingD.magnawere transferred into eppendorf tubes and rinsed three times with 2 mL phosphate buffer (50 mmol/L,pH 7.0). 500 μL cold homogenate (50 mmol/L, pH 8.6),which contained 250 mmol/L saccharose and 100 mmol/L Tris-HCl, was added into the tube and broken by an ultrasonic cell pulverizer (JY88-IIN) for 10 min. The homogenate was then centrifuged at 14 390g(Mini-10K) for 10 min at 4°C, wheregis the gravitational acceleration.The supernatant was used for biomarker investigations.D.reriowas quickly frozen on an ice desk, its head and body were separated, and the liver and gill tissues were taken out. The liver and gill from every four fish were sampled and washed with 0.86% normal saline solution. After drying and weighing, 50 mmol/L of phosphate buffer (with a weight-to-volume ratio of 1/8 and pH of 7.0) was added to homogenize the sample in ice bath. Then, the homogenate was transferred into a centrifugal tube and centrifuged at 14 390g(Mini-10K) for 20 min at 4°C. The supernatant was isolated for biomarker investigations.
The SOD activity was determined by means of the nitroblue tetrazolium (NBT) (Beauchamp and Fridovich, 1971; Cheng et al., 2015). The reaction mixture contained 0.8 mL phosphate buffer (50 mmol/L, pH 7.8), 0.3 mL methionine solution(130 mmol/L),0.3 mL Na2EDTA solution(100 μmol/L),0.3 mL riboflavin solution (20 μmol/L), 0.3 mL NBT solution(750 μmol/L),and 1 mL enzyme extract.The total volume was 3 mL.As SOD has the ability to inhibit photochemical reduction of NBT, this assay utilized negative controls (silver paper wrapped around the test tube to mimic fully dark conditions without any photochemical reduction of NBT),positive controls(deficiency of SOD activity in light with complete photochemical reduction of NBT), and treatment groups (under light with SOD inhibition of photochemical reduction of NBT). The absorbency of all experimental tubes was measured at a wavelength of 560 nm after a 20-min irradiance of 40-60 mmol·m-2·s-1photons. One unit of SOD activity was defined as the amount of enzyme that inhibited 50% of photochemical reduction of NBT.CAT activity was assayed according to the previous studies (Giannopolitis and Ries, 1977;Abdelgawad et al., 2014). The reaction mixture contained 1 mL H2O2, 1.9 mL H2O, and 1 mL crude enzyme solution.Reaction started when crude enzyme solution was added. The absorbance was measured at a wavelength of 240 nm.
2.5. Statistical analysis
The values of EC50and LC50in acute tests were determined by SPSS 22.0 based on the probit analysis method, and the differences between groups were analyzed using the one-way analysis of variance (ANOVA).
3. Results and discussion
3.1. Acute toxicity of artemisinin sustained-release granules to D. magna and D. rerio
In the acute toxicity tests of pure artemisinin and artemisinin sustained-release granules,D. rerioshowed analogous poison symptoms:they swam faster,with body and fins speeding up in the initial period of poisoning. After a period of time, the poisoned fish slowed down,and showed poor balance ability and dull response to external stimuli until ultimately death.The body color of deadD. reriobecame darker, and their gills and chest turned into deep red.
The testing results of acute toxicity toD.magnaandD.rerioare presented in Table 1. It shows that the 48-h EC50of pure artemisinin toD. magnawas 24.54 mg/L and the 96-h LC50toD. reriowas 68.08 mg/L, both of which could be classified as intermediate toxicity (with a range of 10-100 mg/L) (OECD,2004a, 2004b). Pure artemisinin had an excellent inhibition effect (60%-70%) on the growth ofS. obliquus(exposed to 12-20 mg/L pure artemisinin) and mixed algae (exposed to 16 mg/L pure artemisinin)(Ni et al.,2015),but this dosage also inhibited the activities ofD.magnaandD.rerio.Conversely,the values of 48-h LC50toD. magnaandD. rerio(2.51 and 22.57 g/L, respectively) of artemisinin sustained-release granules were both higher than the optimal concentration on algal inhibition (1 g/L) (Ni et al., 2015), which indicated that the granules had less effect onD. magnaandD. rerio(48 h) than pure artemisinin with the optimal dosage of algal inhibition. In comparison, the 48-h LC50ofD. reriowas much higher than that ofD. magna, which indicated that the acute toxicity toD.reriowas much lower and the granules were safer to organisms with a higher trophic level. The granules release artemisinin every day, but the toxicity of artemisinin sustained-release granules to other aquatic organisms is not clear and thesubsequent toxicology research has been in progress. The preliminary results showed that the optimal algal inhibition concentration (1 g/L) of artemisinin sustained-release granules had little acute toxicity toD. magnaandD. rerio.
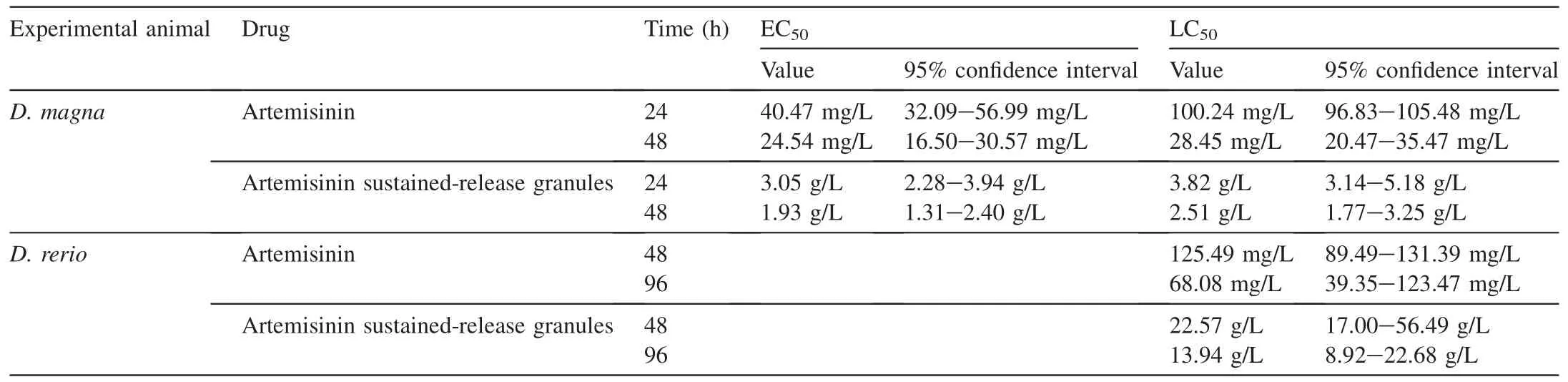
Table 1 Acute toxicity of artemisinin and artemisinin sustained-release granules to D. magna and D. rerio.
3.2. Effects of concentration on chronic toxicity of artemisinin sustained-release granules to D. magna and D. rerio
The testing results of chronic toxicity toD. magnaare shown in Table 2.With the increase of exposure concentration,artemisinin sustained-release granules had some negative effects onD. magna. First reproduction time, first reproduction number, and neonate number per femaleD. magnashowed obvious changes(p<0.05)in the 0.1 g/L concentration group,where four femaleD.magnadied.In the 0.2 g/L concentration group of artemisinin sustained-release granules, almost all indices of testedD. magnaexhibited significantly different results (p<0.01), which meant that the growth and reproduction ofD. magnawere inhibited. Especially in the 0.5 g/L concentration group,there was no reproduction and the survival time of femaleD. magnawas very short ((9.1 ± 0.01) d).
The intrinsic growth rate (r) is shown in Fig. 1. With the increase of exposure concentration, the intrinsic growth rate had a significant decline (p<0.01), which indicated that the testedD. magnagrew slowly under the stress of artemisinin granules.Combining the results shown in Table 2 and Fig.1,it could be concluded that the higher concentration of artemisinin granules would have larger effects on the survival, total number of neonate, and intrinsic growth rate. The sensitivity of indices can be ranked in the following order: first reproduction time; first reproduction number, neonate number, and intrinsic growth rate in parallel;fetus number;and body length of femaleD. magna. Taking the first reproduction time as an indicator,through the various research onD.magna,it can be concluded that the no-observed-effect concentration (NOEC)of artemisinin sustained-release granules is 0.05 g/L and the lowest-observed-effect concentration (LOEC) is 0.1 g/L. The optimal algal inhibition concentration (1 g/L) of granules is higher than LOEC, which means that the growth and reproduction ofD. magnawill be inhibited under this condition.
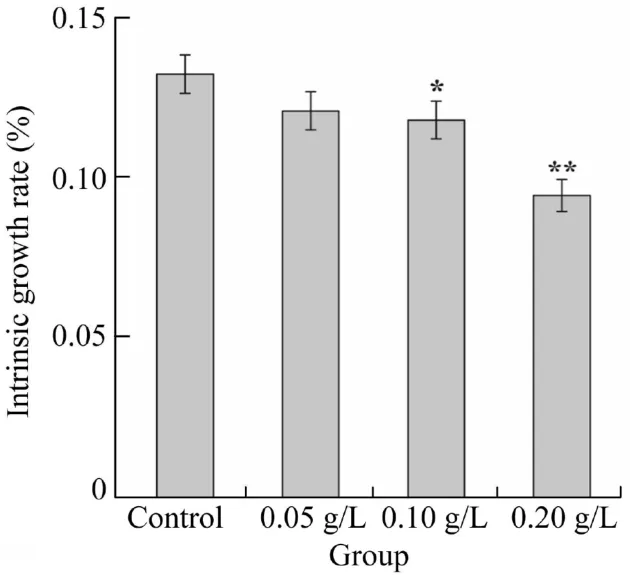
Fig. 1. Effect of concentration of artemisinin sustained-release granules on intrinsic growth rate of D. magna (*p < 0.05,**p <0.01).
In the chronic toxicity test ofD. rerio,we found that some fish exposed to a high concentration of artemisinin sustainedrelease granules(2 g/L)died after three days and the poisoning symptoms were similar to those in acute toxicity tests. Fig. 2 shows the chronic fatality rate of artemisinin sustained-release granules onD. rerio. The higher dosage of artemisinin sustained-release granules led to the higher mortality ofD.rerioover time. However, a definite correlation between the dosage of granules and mortality ofD. reriowas unapparent.After a 28-d treatment,the mortalities ofD.reriowere around 55% and 10%, respectively, when exposed to 2 g/L and 1 g/L concentrations of artemisinin sustained-release granules. The granules have little chronic toxicity toD. reriounder the optimal algal inhibition concentration (1 g/L), because the LC50toD. reriowas 1.974 g/L after 28 d (data not shown).
3.3. Effect of artemisinin sustained-release granules on antioxidant enzyme activity of D. magna
Fig. 3 shows the oxidative stress response ofD. magnaexposed to pure artemisinin and artemisinin sustained-release granules. The activity of SOD and CAT in the pure artemisinin group was lower than the control group when the concentration was higher than 20 mg/L and 15 mg/L, respectively. In addition, the SOD activity in the group of its granules was always higher than the control group,but the CAT activity in the 1.6 g/L group was lower (p<0.01). Organisms can remove reactive oxygen species (ROS) and free radicals with antioxidant enzymes and some small reducing molecules to protect themselves (Imlay, 2013). SOD and CAT are key antioxidant enzymes inD.magna,as well as a wide variety of other aquatic invertebrates (including otherDaphniaspecies), and their relatively high enzyme activity may help these organisms maintain internal oxidative balance and efficiently protect themselves against potential production or increase of ROS(Feng et al., 2013).

Table 2 Results of chronic toxicity test of artemisinin sustained-release granules on F0 generation of D. magna.
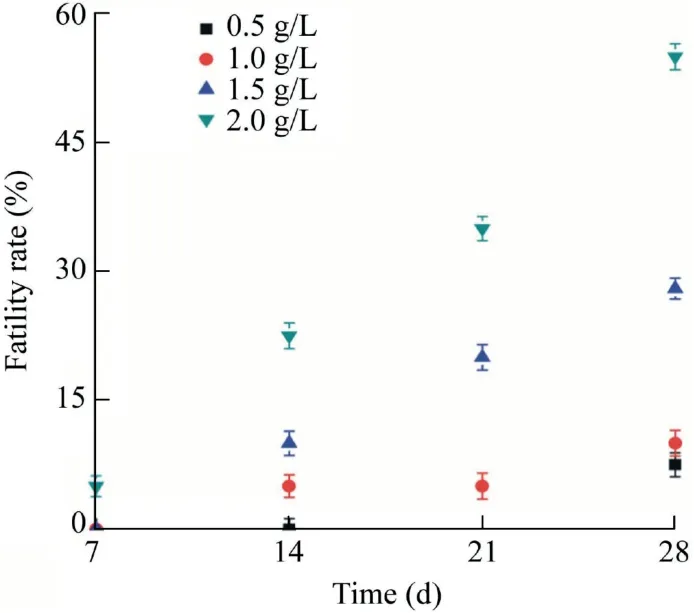
Fig. 2. Chronic fatality rate of artemisinin sustained-release granules on D. rerio.
In this study,the SOD and CAT activity increased whenD.magnawas exposed to low stress levels, but began to reduce when they were exposed to higher levels of pure artemisinin(>15 mg/L) and granules (>1.6 g/L,p< 0.01). This may indicate that both pure artemisinin and its granules can cause oxidative stress response ofD.magnaand activate antioxidant systems,so as to defend external damage.The oxygen radical concentration increased whenD. magnawere exposed to low concentration of artemisinin, enhancing the activity of SOD and CAT against this damage. However, with the increase of concentration, SOD and CAT were consumed in large amounts. When the demand exceeded synthesis, the activity was inhibited. Compared with pure artemisinin, artemisinin sustained-release granules have less effect on the antioxidant enzyme activity ofD. magna.
The effect of artemisinin sustained-release granules on antioxidant enzyme activity ofD. magnaexposed for longer time (21 d) is presented in Fig. 4. Higher-concentration artemisinin granules (0.2 and 0.5 g/L) could lead to a significant increase in SOD activity(p<0.01)and other groups had little variation after a 3-d exposure. When exposed for 9 d, SOD activity began to decline, especially in the 0.5 g/L granule group (p<0.01). After a 15-d exposure, SOD activity still presented a significant decline,even lower than the detection limit in the 0.5 g/L granule group. At the end of chronic toxicity tests, the SOD activity of all test concentration groups declined (p<0.01), which indicated that artemisinin granules could affect the SOD activity ofD.magnagradually. At the early stage of a test, CAT activity in higher-concentration (0.2 and 0.5 g/L) groups had a higher growth rate compared to the control group, which was similar to SOD, while after a 21-d exposure, CAT activity in all groups declined, especially in higher-concentration groups (p<0.01). Throughout the changes of SOD and CAT activity in each exposure concentration, the antioxidant enzyme activity was induced first and then inhibited.Comparing these results to the results of acute toxicity tests,we can speculate that the oxidative stressin vivocaused by the stress of artemisinin granules damaged the organism. To protect itself, the organism increased the antioxidant enzyme activity to defend this stress (Qian et al., 2010; Almeselmani et al., 2015). When artemisinin granules released artemisinin into water continuously, the amount of oxygen radicals was higher and exceeded the amount that antioxidant enzyme could timely remove. In the group of 0.5 g/L artemisinin granules, the SOD activity was much lower than the control group on the 9th day,and someD.magnadied at that time in this group. This shows that the biomarker of antioxidant enzyme activity can be considered an index to assess ecotoxicity of drugs.
3.4. Effect of artemisinin sustained-release granules on antioxidant enzyme activity of D. rerio
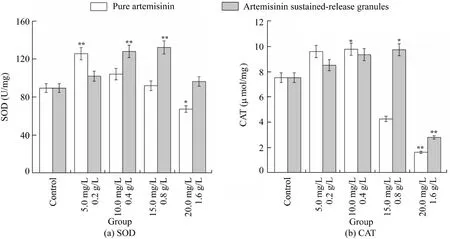
Fig. 3. Antioxidant enzyme activity of SOD and CAT in D. magna exposed to artemisinin and artemisinin sustained-release granules for 48 h(*p < 0.05, **p < 0.01).

Fig. 4. Effect of different concentrations of artemisinin sustained-release granules on SOD and CAT in D. magna for 21 d (*p <0.05,**p <0.01).
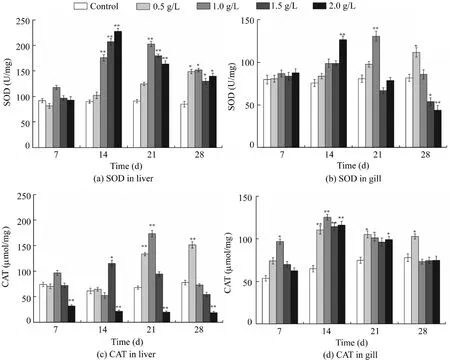
Fig. 5. Effect of different concentrations of artemisinin sustained-release granules on SOD and CAT activity in liver and gill tissue of D. rerio(*p < 0.05, **p < 0.01).
Fig. 5 shows the effect of different concentrations of artemisinin sustained-release granules on SOD and CAT activity in the liver and gill tissues ofD. rerio. There was no significant change in SOD activity after a 7-d exposure in liver tissue. Compared with the control group, SOD activity showed a significant rise in higher-concentration groups(1.5 g/L and 2 g/L) until the 14th day (p<0.01). After that,SOD activity in the liver in both the 1.5 g/L and 2 g/L groups declined persistently. Nevertheless, it began to rise observably in lower-concentration groups(0.5 g/L and 1 g/L),and it was still higher than that in the control group (p<0.05) till the end. Except for the 0.5 g/L group, the activity of SOD in the liver tissue of each treatment group showed an upward and downward trend. This reflects the excitatory effect of granules onD. rerio, which means that this new algaecide may be stimulating or even more beneficial at a low dose(Levente and Bud, 2010).
The change trend in gill tissue was consistent with that in liver tissue. There was almost no variation in SOD activity after a 7-d exposure, compared to the control. Only the SOD activity in the group of 2 g/L artemisinin granules was higher on the 14th day(p<0.01).However,a reversal took place on the 28th day and the SOD activity in gill tissue in the higherconcentration group (1.5 g/L and 2 g/L) was significantly lower than the control group (p<0.05). Poison may produce plenty of reactive oxygen accompanied by metabolism in the liver and gill, leading to a rise in SOD production (Du et al.,2012). Meanwhile, there was relatively less damage to the liver, since the reactive oxygen created by stress could be removed timely owing to the detoxification function of the liver (Camargo and Martinez, 2007; Lee et al., 2013), but the gill could not be detoxified. Some scholars have also pointed out that SOD activity in the liver ofD. reriowas higher than that in the gill after exposured to colchicine. From Fig. 5(c)and(d),it can be determined that,as exposure time passed,the CAT activity of liver tissue in the 0.5 g/L group increased and was significantly higher than the control group by the end of the test(p<0.01).Both the 1 g/L and 1.5 g/L groups followed the trend of first increasing and then decreasing. Thus, the differences between the control group and these two groups were non-significant at the end of this experiment(p<0.01).However, the CAT activity of the 2 g/L group was suppressed from the beginning to the end and was significantly lower than the control group (p<0.01). This indicated that the CAT activity was inhibited by artemisinin sustained-release granules, and suggested that fish exposed to higher-concentration groups had suffered higher oxidative stress (Xiong et al.,2011). At the same time, CAT activity of gill tissue in all groups showed a downward trend after the first rise,increased significantly in each group (p< 0.01), and reached a maximum after a 14-d exposure.Until the 28th day,except for the 0.5 g/L group,each treatment group returned to the level of the control group. It was revealed that artemisinin sustainedrelease granules had little effect on CAT activity in gill tissue.
The SOD and CAT activity ofD. rerioexhibited different responses after exposured to artemisinin sustained-release granules in this study. This revealed that only the enzyme activity in the group of 2 g/L granules was significantly inhibited, while others were all higher than or similar to the control group (p<0.01), because the antioxidant enzyme could remove free radicals effectively and decrease the damage in low-concentration groups (below 2 g/L). Linking the role ofD. rerioin chronic toxicity tests, we found that,when the granule concentration was lower than 2 g/L, the mortality rate decreased, indicating lower toxicity. Furthermore,when it was higher than or equal to 2 g/L,the granules would induce theD. rerioto produce an amount of active oxygen that exceeded the threshold that the antioxidant enzyme system can remove, and lead to mortality of at least half of the fish.
4. Conclusions
Compared with pure artemisinin, artemisinin sustainedrelease granules had a slight acute toxicity toD. magnaandD. rerio. In the chronic toxicity tests, the LC50of granules (1.974 g/L) toD. reriowas higher than the optimal algal inhibition concentration (1 g/L). Therefore, the artemisinin granules of 1 g/L were less toxic toD. rerioand safer for advanced aquatic organisms. However, the artemisinin granules (with a concentration greater than 0.1 g/L)caused significant inhibition of first reproduction time, first reproduction number, fetus number per female, neonate number per female, body length, and intrinsic growth rate ofD. magnain chronic toxicity tests. This indicated that the granules are much safer than pure artemisinin and the toxicity is much lower to aquatic organisms of higher trophic level.
In addition, artemisinin sustained-release granules also affected enzyme activity related to the antioxidant processes.In the short-term exposure to pure artemisinin and its granules,there was a significant induction of SOD and CAT activity inD. magna, but these enzymes were repressed, with exposure time prolonged, especially in higher-concentration groups.SOD and CAT activity inD. reriopresented a similar trend,both increasing first and then decreasing.Only the antioxidant enzyme activity in the group of 2 g/L granules was significantly inhibited. Due to the differences of detoxication function, the enzyme activity in the liver tissue was much higher than that in the gill. The changes of antioxidant enzyme activity were in agreement with the results of toxicity tests, and this biomarker can be considered an index for assessment of the ecotoxicity of drugs.Further investigations will be focused on the toxicity of artemisinin to the more advanced aquatic organisms and toxicological mechanisms.
Declaration of competing interest
The authors declare no conflicts of interest.
 Water Science and Engineering2020年2期
Water Science and Engineering2020年2期
- Water Science and Engineering的其它文章
- Hydrological response to climate change and human activities:A case studyof Taihu Basin,China
- Possibilities of urban flood reduction through distributed-scale rainwater harvesting
- Effects of water application intensity of micro-sprinkler irrigation and soil salinity on environment of coastal saline soils
- Responses of river bed evolution to flow-sediment process changes after Three Gorges Project in middle Yangtze River: A case study of Yaojian reach
- PIV analysis and high-speed photographic observation of cavitating flow field behind circular multi-orifice plates
- Multi-objective reservoir operation using particle swarm optimization with adaptive random inertia weights
